Abstract
Secretory leukocyte protease inhibitor (SLPI) is an anti-inflammatory protein that is observed at high levels in asthma patients. Resiquimod, a TLR7/8 ligand, is protective against acute and chronic asthma, and it increases SLPI expression of macrophages in vitro. However, the protective role played by SLPI and the interactions between the SLPI and resiquimod pathways in the immune response occurring in allergic asthma have not been fully elucidated. To evaluate the role of SLPI in the development of asthma phenotypes and the effect of resiquimod treatment on SLPI, we assessed airway resistance and inflammatory parameters in the lungs of OVA-induced asthmatic SLPI transgenic and knockout mice and in mice treated with resiquimod. Compared with wild-type mice, allergic SLPI transgenic mice showed a decrease in lung resistance (p < 0.001), airway eosinophilia (p < 0.001), goblet cell hyperplasia (p < 0.001), and plasma IgE levels (p < 0.001). Allergic SLPI knockout mice displayed phenotype changes significantly more severe compared with wild-type mice. These phenotypes included lung resistance (p < 0.001), airway eosinophilia (p < 0.001), goblet cell hyperplasia (p < 0.001), cytokine levels in the lungs (p < 0.05), and plasma IgE levels (p < 0.001). Treatment of asthmatic transgenic mice with resiquimod increased the expression of SLPI and decreased inflammation in the lungs; resiquimod treatment was still effective in asthmatic SLPI knockout mice. Taken together, our study showed that the expression of SLPI protects against allergic asthma phenotypes, and treatment by resiquimod is independent of SLPI expression, displayed through the use of transgenic and knockout SLPI mice.
Asthma is a complex, multifactorial (1), and inflammatory disease whose symptoms include cough, wheezing, and shortness of breath (2). It is one of the most common chronic diseases among children and adolescents (3). It is a major public health problem (4) because of its high and increasing worldwide morbidity and mortality (5) and because of its associated health care costs (6). Pathophysiology associated with asthma is characterized by an increase in plasma IgE levels and acute and chronic inflammation of the airways caused by activation of immune cells (2, 7). Bronchial inflammation, in turn, leads to airway hyperresponsiveness (AHR), airflow obstruction, goblet cell hyperplasia, and airway remodeling (8). A better understanding of the roles of various effector molecules regulating allergic asthma will aid in the development of new therapies for the associated symptoms.
Secretory leukocyte protease inhibitor (SLPI; 11.7 kDa) is a serine protease constitutively expressed in mucosal tissues and immune cells, including monocytes, macrophages, and neutrophils (9, 10); it can be found in bronchial secretions, seminal fluid, saliva, and breast milk (11). It exhibits anti-inflammatory, antifungal (12), and antimicrobial functions (13). SLPI also modulates the activity of matrix metalloproteinases (14), improves cutaneous wound healing (15, 16), and prevents HIV-1 infection (17–19). The protective effect of SLPI as an anti-inflammatory mediator has been documented in chronic obstructive pulmonary disease (20) and cystic fibrosis (21). The potential therapeutic effect of recombinant SLPI was shown when administered by aerosol to Ascaris-sensitized sheep and OVA-sensitized guinea pigs (2). However, the role of SLPI in immunomodulating the response during allergic asthma has not been fully elucidated.
Despite the broad spectrum of strategies for the treatment of asthma, there is a need for new effective therapeutic options with fewer side effects. One strategy is to treat through TLRs, which are a family of widely expressed homologous proteins that play a crucial role in innate and adaptive immunity (22, 23). They are some of the first molecules to trigger the immune system to respond to environmental stimuli, to activate immune cells, and to produce cytokines (24). Pathogen-derived molecular patterns are the common ligands for TLRs; these receptors and their ligands have been associated with the development of asthma phenotypes. Some TLRs initiate a conserved intracellular-signaling cascade to activate NF-κB and to induce expression of NF-κB–regulated genes (25).
TLR7 and TLR8 have been associated with the recognition of single-stranded viral RNA; however, these receptors have been also linked to a pharmaceutical compound called resiquimod (RES) (26, 27). RES, also known as S28463, is a member of the imidazoquinoline family and was demonstrated to be a TLR7/8 ligand. Our group showed the protective effect of RES against acute and chronic allergic asthma in mice (28) and rats (29).
Previous studies demonstrated that asthmatic patients express significantly higher levels of SLPI compared with healthy controls (30). In addition, preliminary studies demonstrated a significant increase in SLPI expression at the mRNA and protein levels in macrophages after in vitro treatment with RES. We hypothesized that increased expression of SLPI during the development of allergic asthma protects the lungs from allergic asthma-induced inflammation and, thereby, improves lung physiology. Using an acute asthma model in SLPI gene transgenic (TG) and SLPI knockout (KO) mice, our findings document the importance of SLPI in the regulation of allergic asthma. Furthermore, SLPI may play a role, directly or indirectly, in the treatment of allergic asthma by RES.
Materials and Methods
Animals
Eight to ten-week-old SLPI KO [generously donated by Dr. Sharon M. Wahl (15)], SLPI KO wild-type (WTKO) littermates, SLPI TG, SLPI TG wild-type (WTTG) littermates, and C57BL/6 male mice were used in all experiments. SLPI TG mice were generated as previously described (31). Mice were bred in the pathogen-free facility of the Montreal General Hospital, Research Institute at the McGill University Health Center, Montreal, Quebec, Canada. The McGill University Animal Care Committee, in compliance with the Canadian Council of Animal Care guidelines, approved all of the procedures performed on the animals.
Sensitization and challenge
Sensitization and challenge were performed as previously described (29). Briefly, mice were injected i.p. with 100 µg OVA (Sigma-Aldrich, St. Louis, MO) adsorbed to 1.5 mg of aluminum hydroxide (Imject Alum; Pierce, Rockford, IL) in a total volume of 0.2 ml sterile PBS on days 0, 7, and 14. Mice were challenged on days 21, 22, and 23 by aerosol exposure to 1% OVA solution or to PBS for 30 min. One group of mice was injected i.p. with 100 µg RES on 3 consecutive days, starting 1 d before the first OVA challenge.
The animals were organized into 10 groups: SLPI TG mice challenged with PBS (TG-PBS) or OVA (TG-OVA), SLPI TG mice wild-type (WT) littermates challenged with PBS (WTTG-PBS) or OVA (WTTG-OVA), SLPI KO mice challenged with PBS (KO-PBS) or OVA (KO-OVA), and SLPI KO WT littermates challenged with PBS (WTKO-PBS) or OVA (WTKO-OVA). SLPI KO mice treated with RES and challenged with OVA were identified as KO-RES-OVA and SLPI KO WT littermates treated with RES and challenged with OVA as WTKO-RES-OVA, respectively.
Assessment of respiratory resistance
Forty-eight hours after the last challenge, mice were anesthetized with ketamine (70 mg/kg) and xylazine (10 mg/kg). After the depth of anesthesia was verified, mice were tracheotomized, endotracheally intubated (with an 18-gauge stainless steel cannula), injected i.p. with 10 µl/g pancuronium (1 mg/ml), and connected to the MiniVent (type 845) small animal ventilator (Harvard Apparatus, Saint-Laurent, Quebec, Canada). Tidal volume was adjusted to 10 µl/g and a respiratory frequency of 180 strokes/min. The peak respiratory system resistance was measured with the Buxco resistance system (Buxco Electronics, Wilmington, NC) after administering increasing doses of aerosolized methacholine (Mch; 10 µl of 0–80 µg/ml) (Sigma-Aldrich, Oakville, Ontario, Canada).
Histological analysis of lung inflammation
Forty-eight hours after the last challenge, the left lung was dissected, inflated with 10% buffered formalin at a pressure of 25 cm H2O, kept in a 50-ml tube filled with 15 ml 10% formalin overnight, and then embedded in paraffin. Lungs were cut into 3-µm sections for histological analysis. Deparaffinized and hydrated sections were stained with H&E, Congo red (CR), or periodic acid-Schiff (PAS).
Inflammation of the lungs was assessed by histological analysis of H&E- or CR-stained lung sections. Quantification of the infiltrating cells was performed by counting 300 infiltrating cells in the peribronchial space, and the percentage of eosinophils was calculated based on the nuclear morphology and the presence of eosin staining in the cytoplasm. The percentage of eosinophils was corroborated with CR-stained lung sections. Goblet cell hyperplasia of the airways was assessed by histological analysis of PAS-stained sections. Goblet cell hyperplasia was quantified by determining the percentage of PAS-stained epithelial cells in at least five airway cross-sections per slide. Five independent experiments were performed, and 15 animals were included in each group.
Plasma IgE levels
Mice were euthanized with CO2 48 h after the final challenge, and blood was collected by cardiac puncture in 0.05 M EDTA-coated tubes. Plasma was isolated from blood by centrifuging for 7 min at 2000 × g. Total IgE levels were measured using a commercial ELISA kit, following the manufacturer’s instructions (BD Biosciences, San Diego, CA). Briefly, 96-well ELISA plates (Thermo Labsystems, Franklin, MA) were coated with anti-mouse IgE mAb and blocked with 10% FBS (Winsent Inc., Saint-Bruno, Quebec, Canada) in PBS. Standards and plasma samples were incubated for 2 h at room temperature, and mouse IgE was detected with biotinylated anti-mouse IgE and streptavidin-HRP conjugate using ABTS reagent mixture (Roche Diagnostics, Laval, Quebec, Canada). The plate was read at 450 nm in an ELISA plate reader (Biorad, Hercules, CA). Purified mouse IgE, provided with the kit, was used as a standard.
RNA extraction
Total RNA was extracted from half lung homogenized with a Mixer Mill type MM 301 (Retsch GmbH & Co. KG, Haan, Germany), using TRIzol reagent and RNA extraction protocol (Invitrogen, Burlington, Ontario, Canada). RNA quality was tested by electrophoresis in 2.2 M formaldehyde-1.2% agarose gel and with a 2100 Bioanalyser using the RNA 6000 Nano LabChip Kit (Agilent Technologies, Böblingen, Germany). Total RNA was used for gene-expression analysis by real-time quantitative PCR.
Quantitative real-time PCR
A DNA-free Kit (Ambion, Austin, TX) was used to digest any residual DNA from 1 µg RNA obtained from the lungs. A reverse-transcription reaction was performed using the QuantiTect reverse transcription kit (Qiagen, Mississauga, Ontario, Canada), according to manufacturer’s instructions. Next, 2 µl the 25-µl reverse-transcriptase reaction was added to 50 µl Brilliant II SYBR Green quantitative PCR Master Mix (Stratagene, Cedar Creek, TX), and the Stratagene MX-4000 apparatus was used to amplify the target cDNA to assess SLPI expression by real-time quantitative PCR. The following primers were used to amplify murine SLPI cDNA: 5′-CTCAGGCAAGATGTATGATG-3′ (sense) and 5′-TTTCCCACATATACCCTCAC-3′ (antisense). The amount of cDNA was calculated based on the threshold cycle (CT) value and was standardized by the amount of the housekeeping gene Gapdh using the 2−ΔΔCT method (32):
where “target” represents the gene of interest and “calibrator” represents KO-PBS mice. SLPI gene expression was standardized against the expression of Gapdh. Melting-curve analysis and agarose gel electrophoresis were also performed to confirm that a single product of the expected length was amplified.
The amplification program consisted of an enzyme-activation step at 95°C for 10 min, followed by 40 cycles of denaturing at 95°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 30 s. Gapdh was used as the normalizing gene to compensate for potential differences in total cDNA amounts. The primer sequences were all designed based on the National Center for Biotechnology Information GenBank mRNA sequence, using the PrimerQuest Web-based software, Integrated DNA Technologies (http://www.idtdna.com/Scitools/Applications/Primerquest/).
Effect of RES in mRNA expression of SLPI
C57BL/6 mice were injected i.p. with PBS or 0.1 mg RES (generously provided by Dr. T.C. Meng, Graceway Pharmaceuticals LLC, Exton, PA). Three or six hours following i.p. injection, the mice were euthanized by CO2, and their lung RNA was extracted as described above.
Immunoprecipitation
Mice were euthanized by CO2 48 h after the last challenge; the right lung was dissected and homogenized in 500 µl PBS containing a protease inhibitor mixture (Complete Inhibitor; Roche Diagnostics). One microgram of rabbit anti-mouse SLPI Ab, generated as previously described (33), was added to 500 µl the homogenate and incubated at 4°C for 1 h. A mix of 20 µl protein A/G plus agarose beads (Santa Cruz Biotechnologies, Santa Cruz, CA) was added, and the solution was gently mixed at 4°C overnight. Supernatants were removed after centrifugation at 1000 rpm for 5 min and washed three times with 500 µl immunoprecipitation buffer (2% Triton X-100, 100 and 300 mM NaCl). Samples were concentrated using Microcon YM-3 centrifugal filters (Millipore, Billerica, MA), and total protein concentration was measured using the Bio-Rad protein assay dye (Biorad).
Western blot
Protein concentration was adjusted, and 30 µg each sample was mixed with SDS-PAGE sample buffer containing 2.5% 2-ME (Fisher, Fairlawn, NJ), heated at 95°C, and loaded in a 4–12% Bis-Tris NuPAGE gel (Invitrogen) for acrylamide electrophoresis. Human recombinant SLPI protein was used as a positive control, and SeeBlue Plus2 (Invitrogen) molecular weight marker as reference. Next, proteins were transferred onto Immobilon transfer membranes (Millipore) by semidry transfer. Membranes were blocked with 5% nonfat skim milk at 4°C overnight; they were incubated with rabbit anti-mouse SLPI (33) at a concentration of 1 µg/ml in 5% nonfat skim milk for 1 h at room temperature and subsequently with a solution of anti-mouse IgG HRP-conjugated Ab (1:5000; Santa Cruz Biotechnologies) for 1 h at room temperature. The signal was visualized using Western Lightning Plus-ECL reagent (GE Healthcare Bio-Sciences Corp., Piscataway, NJ).
Western blot analysis for NF-κB was performed by detection of IκB-β using an anti–IκB-β Ab (Santa Cruz Biotechnology) and following the protocol described above.
Cytokine analysis
Mice were euthanized 3 h after the last challenge, and the lungs were dissected and homogenized in 500 µl PBS containing a protease inhibitor mixture (Complete Inhibitor; Roche Diagnostics). Using Microcon YM-3 centrifugal filters (Millipore), the samples were concentrated 20-fold, and total protein concentration was adjusted using the Bio-Rad protein assay dye (Bio-Rad) for analysis using a custom Lincoplex mouse cytokine kit (Millipore) on a Luminex 100 LS apparatus with software version 2.3 (Luminex Corporation, Austin, TX). The minimum detectable concentrations for the Lincoplex kit using the Luminex 100 LS apparatus are as follows: IL-4, 0.3 pg/ml; IL-5, 0.6 pg/ml; IL-6, 0.7 pg/ml; IFN-γ, 0.7 pg/ml; IL-2, 0.8 pg/ml; RANTES, 0.7 pg/ml; TNF-α, 0.9 pg/ml; and MCP-1, 6.3 pg/ml.
Statistical analysis
Data were analyzed using a nonparametric one-way ANOVA, followed by the Bonferroni multiple-comparison posttest and correlation coefficient using 95% confidence intervals (GraphPad Prism 4, version 4.03; GraphPad Software, San Diego, CA). Differences were considered significant at p < 0.05.
Results
SLPI mRNA and protein expression in the lungs of SLPI TG mice
Because TG mice frequently show a highly variable expression of the transgene, we first evaluated SLPI mRNA and protein levels in the lungs. OVA-sensitized SLPI TG mice clearly expressed higher levels of SLPI mRNA following PBS and OVA aerosol challenge compared with similarly treated WT controls (Supplemental Fig. 1A). Furthermore, the expression of SLPI mRNA was induced by allergen challenge in SLPI TG mice (304 ± 91 in TG-OVA versus 91 ± 17 in TG-PBS; p < 0.001) and WT mice (85 ± 7 in WTTG-OVA versus 12 ± 3 in WTTG-PBS; p < 0.01). Compared with SLPI WTTG animals, SLPI constitutive-protein expression was greater in lungs of TG mice following PBS challenge, and it was increased in the lungs of OVA-challenged TG animals following allergen challenge (Supplemental Fig. 1B). Taken together, SLPI protein is overexpressed in OVA-sensitized and OVA-challenged TG mice (Supplemental Fig. 1).
SLPI overexpression improves AHR and influences IgE levels in plasma
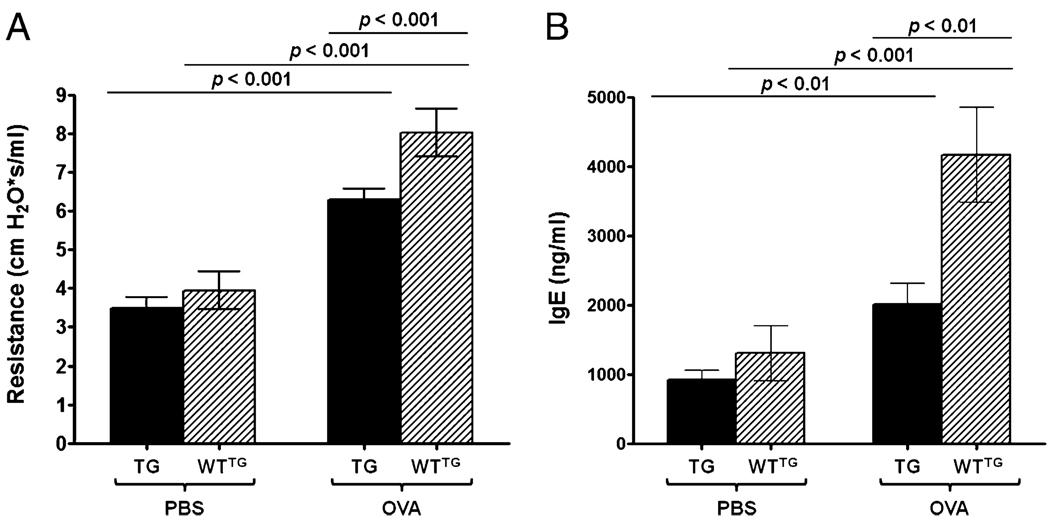
Previous studies suggested that SLPI modified the airway response in guinea pigs induced with a chronic model of allergic asthma (2). We evaluated whether the expression of SLPI was able to influence AHR and levels of plasma IgE in sensitized and challenged SLPI TG or KO mice. As expected, allergen challenge increased airway resistance in response to Mch in SLPI TG mice (6.3 ± 1.1 cm H2O × s/ml) and WT mice (8.0 ± 2.1 cm H2O × s/ml) (Fig. 1A). TG (3.6 ± 0.3 cm H2O × s/ml) and WT (4.3 ± 0.5 cm H2O × s/ml) mice demonstrated similar levels of airway responsiveness in the absence of OVA challenge. However, compared with WT mice, SLPI overexpression (TG-OVA) significantly (p < 0.001) prevented the increase in airway responsiveness associated with OVA challenge (Fig. 1A). The same protective effect of SLPI overexpression was observed for plasma IgE levels (Fig. 1B). Significantly lower IgE levels were observed in OVA-challenged TG mice (2014.1 ± 309.2 ng/ml) compared with OVA WT mice (2014.1 ± 309.2 ng/ml; p < 0.01), and a significant difference was observed compared with the TG-PBS group (925.3 ± 136.4 ng/ml; p < 0.01). WTTG-PBS (3308 ± 396.3 ng/ml) control group displayed lower (p < 0.001) IgE levels than did WTTG-OVA mice. Overall, greater SLPI expression was associated with lower lung resistance and plasma IgE.
FIGURE 1.
Airway resistance and IgE levels in plasma from SLPI TG and WTTG mice. Bars represent the mean ± SEM of five independent experiments (n = 15 per group). A, Airway resistance. B, IgE levels in plasma. TG-OVA mice showed statistically significantly lower lung resistance and IgE levels in plasma compared with WTTG-OVA mice.
SLPI decreases allergic inflammation in SLPI TG mice
OVA-challenged SLPI TG mice displayed a lower degree of inflammatory cell infiltration in the lungs compared with WTTG mice under the same conditions (Fig. 2). Consistent with allergic inflammation, the inflammatory cells found in the lungs of OVA-challenged TG mice contained a significantly lower (p < 0.05) proportion of eosinophils (24 ± 1.1%) compared with WTTG OVA-challenged mice (29 ± 2.3%) (Supplemental Fig. 2A). Almost no eosinophilic infiltration was observed in the control groups, which were sensitized with OVA and challenged with PBS (TG-PBS [1 ± 0.2%] and WTTG-PBS [1 ± 0.3%]) (Supplemental Fig. 2A). Again, SLPI overexpression exerted a protective effect against the development of allergic asthma by significantly reducing the inflammatory cell influx and the proportion of eosinophils into the peribronchial space.
FIGURE 2.
H&E-stained lung sections from SLPI TG and WTTG mice. Lungs from TG-PBS (A), TG-OVA (B), WTTG-PBS (C), and WTTG-OVA (D) mice. The lungs from TG-OVA mice show a decrease in cellular infiltration after OVA challenge compared with the lungs of similarly treated WTTG-OVA mice.
SLPI decreases goblet cell hyperplasia in SLPI TG mice
The development of goblet cell hyperplasia and mucus production are other histological changes that characterize allergic asthma (34). OVA challenge caused a significant and marked increase (p < 0.001) in the number of airway goblet cells in WT and TG animals (Supplemental Fig. 2B). WT OVA-challenged mice showed a significantly higher (p < 0.001) percentage of goblet cells compared with the control group challenged with PBS (8 ± 1.9%). However, TG OVA-challenged mice had a significantly lower (p < 0.05) percentage of goblet cells (25 ± 2.4%) compared with WT mice under the same conditions (36 ± 4.0%) or TG mice (p < 0.001) challenged with PBS (6 ± 1.4%), and it correlated with the percentage of eosinophils (r = 0.72) under each condition. These results demonstrated that the expression of SLPI plays a protective role against the induction of goblet cell hyperplasia in our model of allergic asthma.
SLPI mRNA and protein expression in the lungs of SLPI KO mice
We used an SLPI-KO mouse model to further corroborate our findings regarding the biological importance of SLPI in allergic asthma. As anticipated, SLPI mRNA was not expressed in the lungs of any KO mice (Supplemental Fig. 3A), regardless of the Ag challenge. SLPI mRNA levels in the pulmonary tissue from WTKO-OVA mice (73 ± 8.7) demonstrated a significant increase following OVA challenge compared with WTKO-PBS (26 ± 3.1; p < 0.01) and KO-OVA (0 ± 0.0l; p < 0.001) animals. Western blot analysis was used to confirm the expression of SLPI at the protein level. As expected, SLPI protein expression was not detectable in the KO-PBS and KO-OVA mice, whereas WTKO-OVA mice showed higher protein levels compared with WTKO-PBS mice (Supplemental Fig. 3B). These results demonstrated the absence of SLPI in KO mice and confirmed the SLPI constitutive expression in OVA-sensitized and -challenged WTKO mice.
SLPI ablation increases AHR and IgE levels in plasma
Our results demonstrated that the overexpression of SLPI protein prevented the development of AHR following allergen challenge, and it prevented the increase in plasma IgE levels. We then hypothesized that ablation of the SLPI gene would generate the opposite effect.
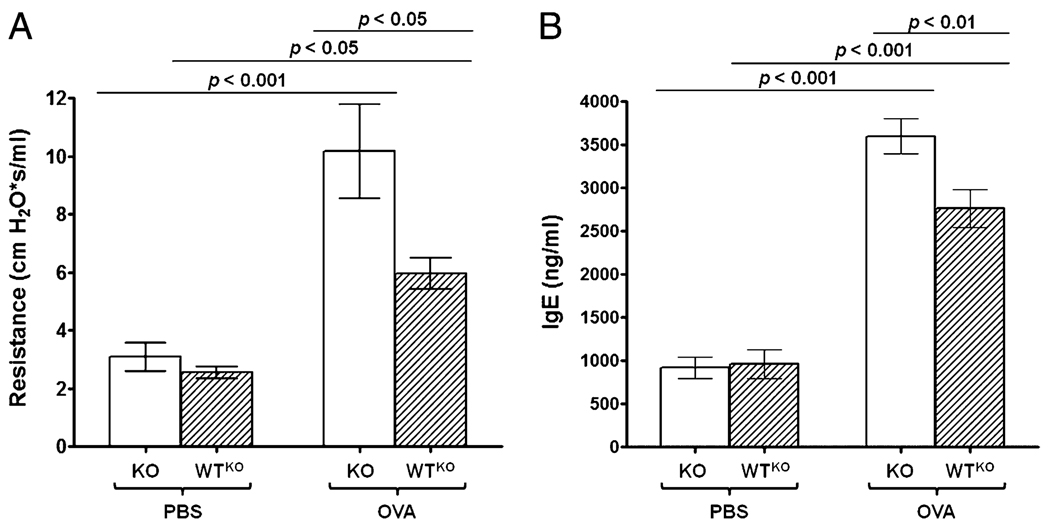
Allergen challenge led to a significant increase in airway responsiveness in SLPI KO and WTKO mice (Fig. 3A); levels in KO-OVA mice (10.2 ± 1.6 cm H2O × s/ml) were significantly higher (p < 0.001) compared with WTKO-OVA mice (6.6 ± 0.5 cm H2O × s/ml; p < 0.05) or KO-PBS mice (3.1 ± 0.5 cm H2O × s/ml). WTKO-PBS mice (2.6 ± 0.2 cm H2O × s/ml) displayed significantly lower (p < 0.05) lung resistance than WTKO-OVA mice. Similarly, in SLPI KO and WT mice, OVA challenge led to a marked and significant increase in plasma IgE titers (Fig. 3B). Again, ablation of the SLPI gene led to a more severe asthmatic phenotype in the form of higher plasma IgE levels. Plasma IgE levels in KO-OVA mice (3598 ± 204.7 ng/ml) were significantly higher compared with WTKO-OVA mice (2763 ± 220.3 ng/ml; p < 0.01) or the KO-PBS control group (920 ± 125.4 ng/ml; p < 0.001) control group, whereas WTKO-PBS mice (958 ± 166.8 ng/ml) showed lower (p < 0.001) IgE plasma levels than WTKO-OVA mice. We concluded that ablation of the SLPI gene enhanced AHR and IgE levels in OVA-sensitized and -challenged mice, whereas higher SLPI expression improved lung physiology and limited the atopic condition.
FIGURE 3.
Airway resistance and IgE levels in plasma from SLPI KO and WTKO mice. Bars represent the mean ± SEM from five independent experiments (n = 15 per group). A, Airway resistance. B, IgE levels in plasma. KO-OVA mice showed statistically significantly higher lung resistance and IgE levels in plasma compared with WTKO-OVA or KO-PBS mice.
Absence of SLPI promotes inflammation in an acute asthma model
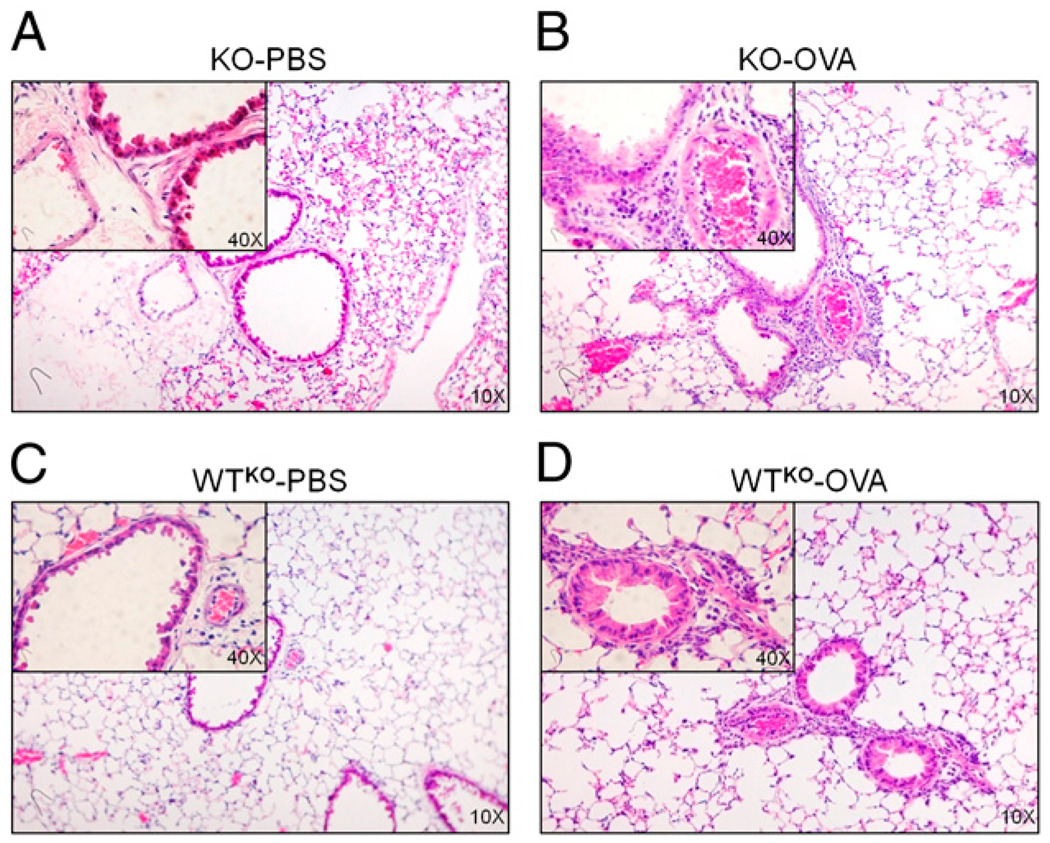
Using SLPI TG mice, we observed an anti-inflammatory effect in the lungs of OVA- challenged mice (Fig. 2B; TG-OVA). To demonstrate that SLPI is responsible for these results, we sensitized and challenged SLPI KO mice, expecting to observe higher inflammatory cell infiltration in their lungs compared with WT controls. As predicted, KO-OVA mice displayed a greater degree of infiltration than WTKO-OVA mice (Fig. 4). All PBS-challenged animals showed minimal inflammatory cell infiltration compared with the groups challenged with OVA. These results confirmed that, in the absence of SLPI, mice were unable to control the inflammatory process in response to OVA challenge, resulting in a much greater influx of inflammatory cells into peribronchial spaces of the lungs in OVA-sensitized and -challenged mice.
FIGURE 4.
H&E-stained lung sections from SLPI KO and WTKO mice. Lungs from KO-PBS (A), KO-OVA (B), WTKO-PBS (C), and WTKO-OVA (D) mice. Lungs from KO-OVA mice show an increase in cellular infiltration after OVA challenge compared with lungs of similarly treated WTKO-OVA mice.
SLPI ablation increases eosinophilia and goblet cell hyperplasia in SLPI KO mice
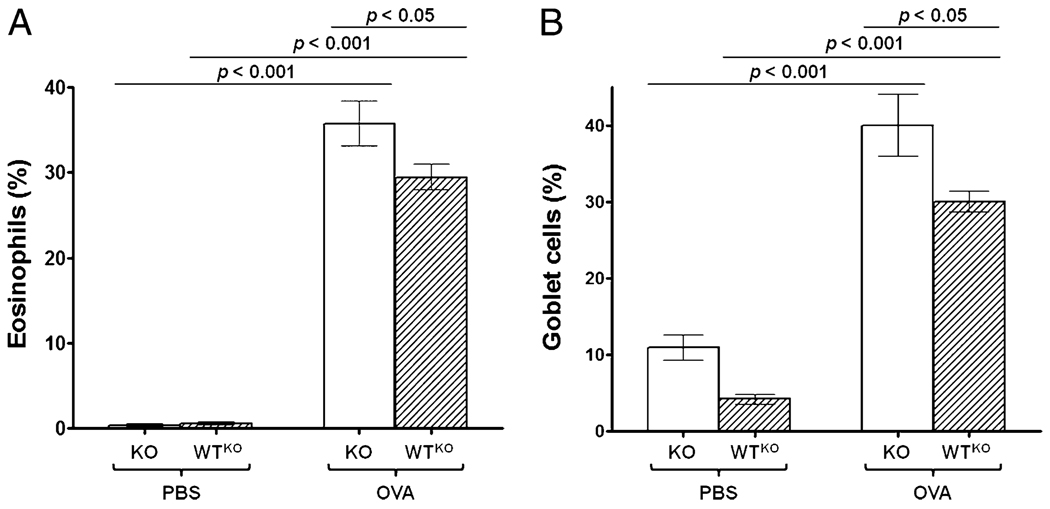
To further corroborate the effect of SLPI gene ablation on lung inflammation, we evaluated the percentage of eosinophils in the inflammatory infiltrate of the lungs and the degree of goblet cell hyperplasia. SLPI KO-OVA mice (36 ± 2.7%) exhibited a significantly higher percentage of eosinophils compared with WTKO-OVA mice (29 ± 1.5%; p < 0.05) or KO-PBS mice (1 ± 0.4%; p < 0.001) (Fig. 5A). Allergen challenge significantly induced goblet cell hyperplasia in KO and WT mice (Fig. 5B). Interestingly, ablation of the SLPI gene caused a significant (p < 0.05) increase in the percentage of goblet cells in KO-OVA mice (40 ± 4.1%) compared with WTKO-OVA mice (30 ± 1.4%), and this correlated (r = 0.75) with the eosinophil percentage, indicating that the antiproliferative effect that SLPI exerts on goblet cells is active in the presence of allergic inflammation.
FIGURE 5.
Eosinophils and goblet cells in lungs from SLPI KO and WTKO mice. Bars represent the mean ± SEM from five independent experiments (n = 15 per group). A, Percentage of eosinophils. B, Percentage of goblet cell hyperplasia. KO-OVA mice showed a statistically significantly higher percentage of eosinophils and goblet cell hyperplasia compared with WTKO-OVA and KO-PBS mice.
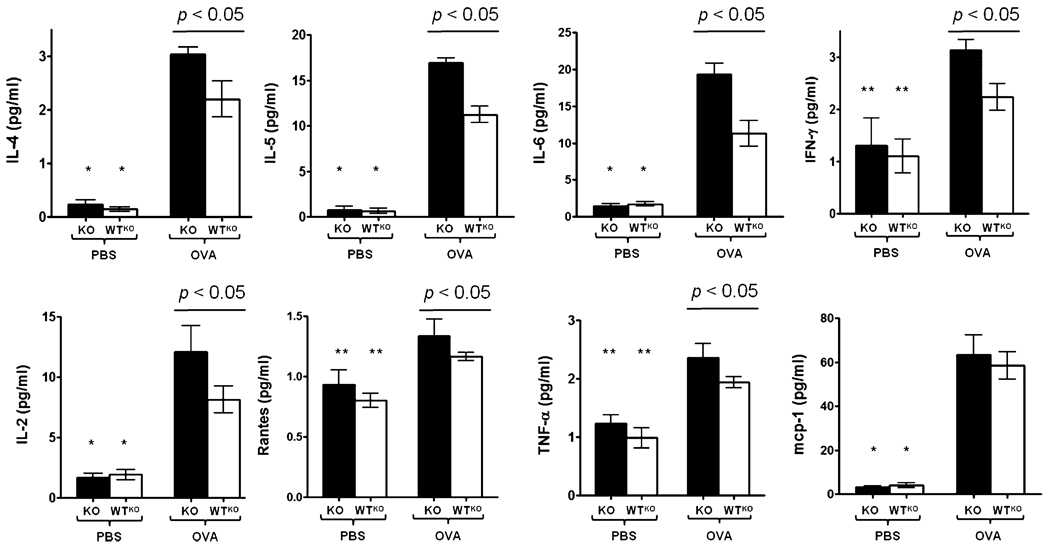
SLPI affects cytokine levels in an acute asthma model
In the assessment of the cytokine profiles of OVA-sensitized and -challenged mice, we found significantly higher (p < 0.001) levels of IL-4, IL-5, and IL-6 in OVA-challenged KO and WTKO mice compared with their respective PBS-challenged controls (Fig. 6). Consistent with their higher level of inflammation and plasma IgE levels, SLPI KO mice expressed cytokines at higher levels. The levels of IFN-γ and IL-2 followed similar patterns of expression, indicating that allergen challenge did not uniquely induce Th2 cytokines and that SLPI demonstrates a broad anti-inflammatory effect in the context of allergic asthma. RANTES and TNF-α were also significantly higher (p < 0.05) in KO-OVA mice compared with KO-PBS mice, but no significant difference was found in the assessment of MCP-1.
FIGURE 6.
Cytokine levels in lungs of SLPI KO and WTKO mice. The bars represent the mean ± SEM (n = 6 per group). *p < 0.001, for IL-4, IL-5, IL-6, IL-2, and MCP-1 in KO-PBS mice versus KO-OVA mice and for WTKO-PBS mice versus WTKO-OVA; **p < 0.05, for IFN-γ, RANTES, and TNF-γ in KO-PBS mice versus KO-OVA mice and WTKO-PBS mice versus WTKO-OVA mice.
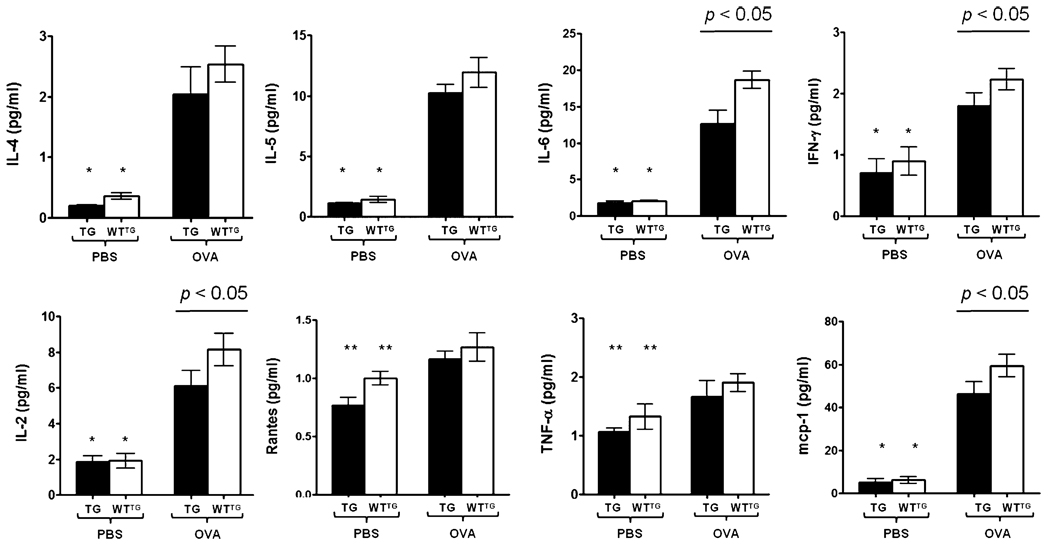
Using SLPI TG and WTTG mice, our results showed significantly higher (p < 0.001) levels for the entire panel of cytokines assessed in mice challenged with OVA compared with their PBS-challenged controls (Fig. 7). The levels of IL-6, IFN-γ, IL-2, and MCP-1 were significantly higher (p < 0.05) in WTTG mice challenged with OVA compared with TG mice under the same conditions. IL-4, IL-5, RANTES, TNF-α, and MCP-1 were also higher in WTTG-OVA mice, but there was no significant difference compared with TG-OVA mice.
FIGURE 7.
Cytokine levels in lungs of SLPI TG and WTTG mice. The bars represent the mean ± SEM (n = 3–5 per group). *p < 0.001 for IL-4, IL-5, IL-6, IFN-γ, IL-2, and MCP-1 in TG-PBS mice versus TG-OVA mice and WTTG-PBS mice versus WTTG-OVA mice; **p < 0.05 for RANTES and TNF-γ in TG-PBS mice versus TG-OVA mice and WTTG-PBS mice versus WTTG-OVA mice.
Our data showed that Th1 and Th2 cytokines were expressed at higher levels in KO and TG mice challenged with OVA compared with their respective WT controls, as assessed 3 h following the last allergen challenge. Additionally, TG-OVA mice showed lower cytokine levels compared with WTTG-OVA mice. These results can explain, in part, the degree of inflammation in the lungs and the magnitude of lung resistance observed in each group of animals.
Effect of RES treatment on SLPI mRNA and protein expression and IκB-β level
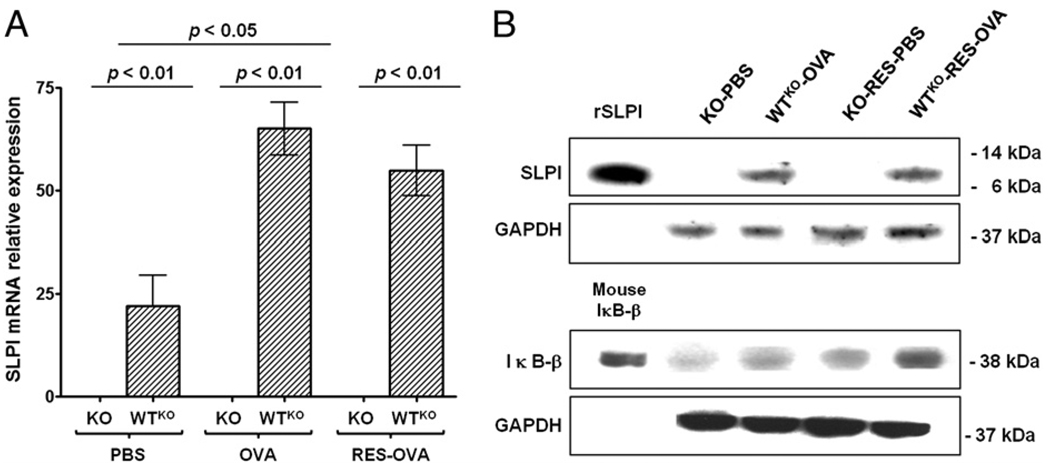
Previous studies by our laboratory demonstrated that RES treatment prevented the development of AHR in allergic A/J and C57BL/6 mice, as well as Brown Norway rats (28, 29). We also found higher SLPI expression in macrophages derived from OVA-sensitized and -challenged mice compared with control animals (data not shown). Those results led us to investigate the mRNA expression of SLPI after RES treatment in naive C57BL/6 mice. We found significantly higher (p < 0.05) SLPI mRNA expression 3 and 6 h after RES injection compared with nontreated mice. As expected, SLPI mRNA was absent from the lungs of SLPI KO mice (Fig. 8A), regardless of PBS or OVA challenge or RES treatment. In the analysis of SLPI protein expression (Fig. 8B), there was no difference between WTKO-OVA mice compared with the WTKO-RES-OVA group. WTKO-PBS and WTKO-RES-PBS mice displayed no SLPI protein expression in the Western blots.
FIGURE 8.
SLPI and IκB-β expression in lung homogenates from SLPI KO and WTKO mice after PBS or OVA challenge, including RES treatment. A, SLPI mRNA relative expression (mean ± SEM) (n = 10 per group). B, SLPI, IκB-β, and GAPDH protein expression. SLPI KO mice showed no SLPI mRNA and protein expression under any condition. SLPI WTKO mice displayed no variation in protein levels, and IκB-β level was higher after treatment with RES. rSLPI, recombinant SLPI.
SLPI regulates the activation of NF-κB through the protection of IκB-β (35). We evaluated whether RES was able to modify the protection effect of SLPI on IκB-β. Treatment with RES in SLPI KO and WTKO mice challenged with PBS or OVA (KO-RES-PBS and WTKO-RES-OVA) resulted in higher IκB-β levels compared with their respective untreated group (Fig. 8B). WTKO mice displayed higher levels of IκB-β compared with KO mice. These data showed the protective effect of constitutive SLPI expression over IκB-β and suggested a potential effect of RES in protecting the activation of NF-κB, which is independent of SLPI expression.
RES treatment prevents OVA-induced AHR independently of SLPI
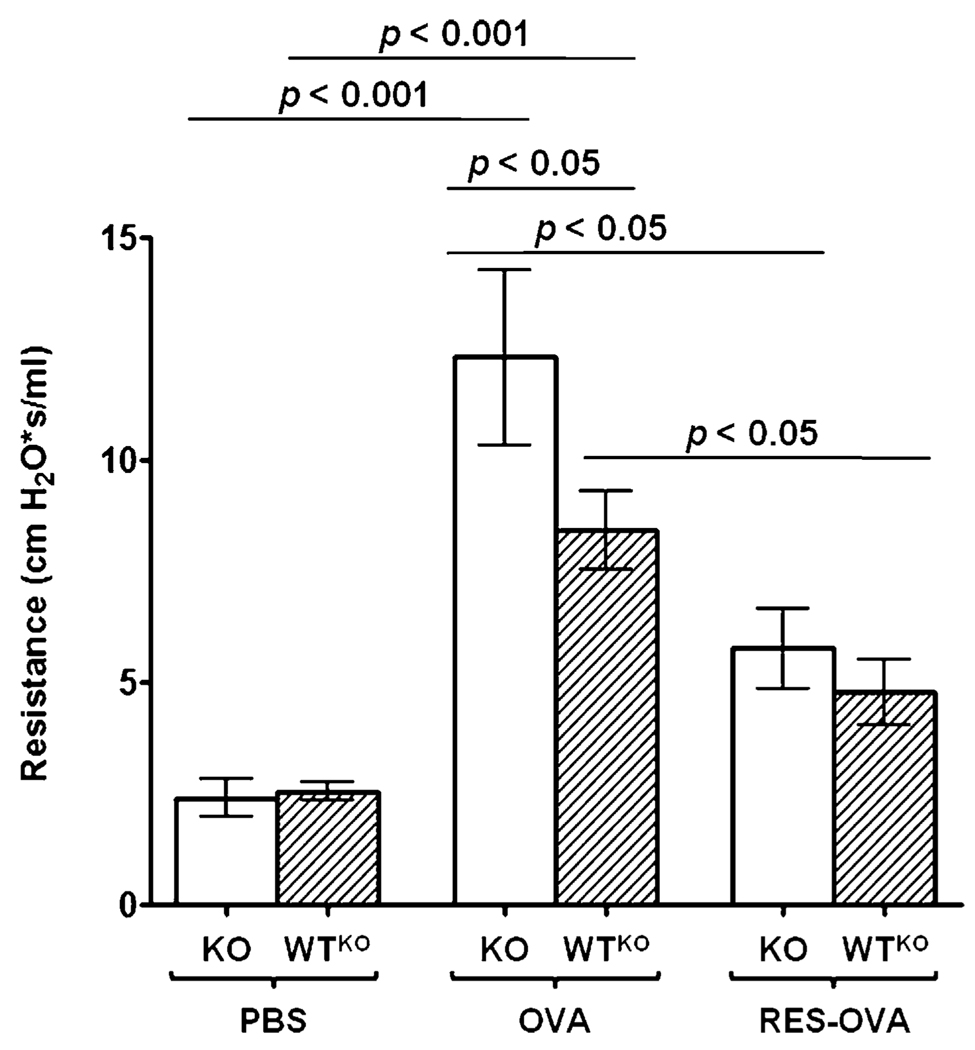
To evaluate the role of SLPI expression in RES treatment of allergic asthma, we treated SLPI KO mice with RES prior to OVA challenge. SLPI gene ablation in KO-OVA mice (12.3 ± 1.6 cm H2O × s/ml) increased the severity of AHR following allergen challenge compared with WTKO-OVA mice (8.4 ± 0.8 cm H2O × s/ml; p < 0.05) (Fig. 9). However, treatment with RES significantly reduced (p < 0.05) the lung responsiveness in KO-RES-OVA mice (5.8 ± 0.9 cm H2O × s/ml) and WTKO-RES-OVA mice (4.7 ± 1.2 cm H2O × s/ml) compared with untreated animals. Overall, these results showed that treatment with RES prevented AHR, and the effectiveness of the drug does not depend on the expression of SLPI.
FIGURE 9.
Airway resistance in OVA-sensitized and -challenged SLPI KO and WTKO mice after RES treatment. Bars represent the mean ± SEM from three independent experiments (n = 6 per group). RES treatment significantly reduces airway resistance in SLPI KO (KO-RES-OVA) and WT (WTKO-RES-OVA) mice compared with untreated mice under the same conditions.
RES treatment prevents inflammation in allergic asthma
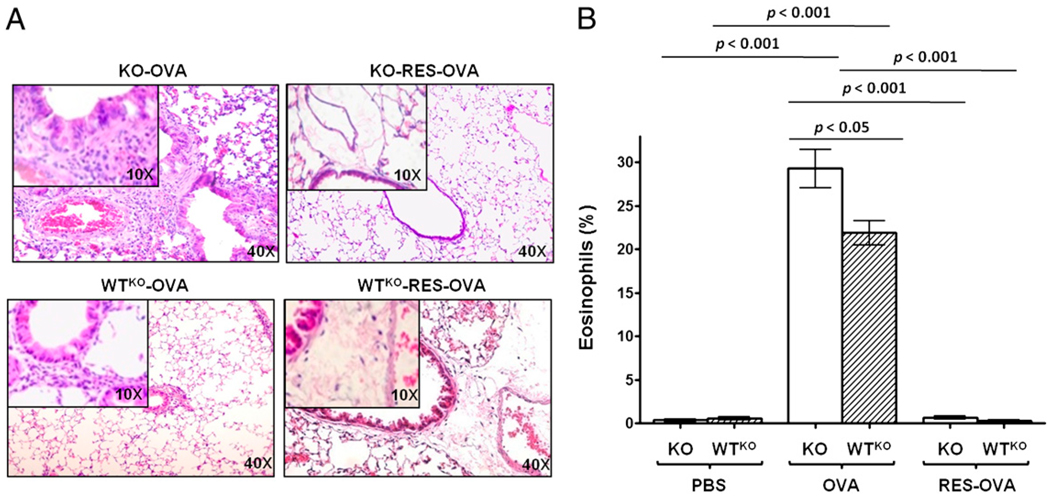
To determine whether the inhibition of allergic inflammation mediated by RES is dependent on SLPI expression, we assessed inflammatory cell infiltration and the percentage of eosinophils in RES-treated OVA-sensitized and -challenged SLPI KO and WTKO mice. We found that OVA-challenged SLPI KO mice and the group treated with RES (KO-RES-OVA) showed increased numbers of inflammatory cells compared with their respective controls (Fig. 10A). Also, the percentage of eosinophils was significantly higher (p < 0.001) in KO-OVA mice (29.0 ± 2.2%) (Fig. 10B) compared with the control mice. After treatment with RES, KO-RES-OVA mice (1.0 ± 0.2%) and WTKO-RES-OVA mice (0.5 ± 0.2%) showed a significantly lower (p < 0.001) fraction of eosinophils compared with KO-OVA or WTKO-OVA groups. These results confirmed that treatment with RES prevents AHR and diminishes inflammatory cell infiltration into allergen-sensitized and -challenged lungs and that the effect of the drug seems to be independent on the induction or even basal expression of SLPI.
FIGURE 10.
Inflammatory cell infiltration and eosinophils in lungs from SLPI KO and WTKO mice after RES treatment. A, H&E-stained lung sections. B, Percentage of eosinophils (mean ± SEM) (n = 6 per group). KO-RES-OVA and WTKO-RES-OVA mice show lower cellular infiltration and a significant decrease in eosinophil percentage after RES treatment compared with untreated mice. p < 0.001.
Discussion
The objective of this study was to evaluate the role of SLPI in allergic asthma and to assess whether the protective effect of RES treatment depends on the expression of SLPI. To test this, we evaluated lung physiology and inflammatory responses in a murine model of acute allergic asthma, using SLPI TG and KO mice. Our results demonstrated that overexpression of SLPI prevented the development of AHR and decreased the influx of inflammatory cells into the lungs following airway allergen challenge. Furthermore, ablation of SLPI expression resulted in more severe AHR and inflammation under similar conditions. Finally, treatment of allergic asthma with RES modulated the activation of NF-κB through the protection of IκB, but its effect is independent of SLPI expression.
AHR is one of the characteristics of allergic asthma in humans (36–38) that can be reproduced in animal models (28, 39, 40). Allergic C57BL/6 mice are hyporesponsive to Mch compared with BALB/c and A/J mice (41, 42). In preliminary experiments, we found that SLPI KO mice on a C57BL/6 genetic background were relatively hyperresponsive to Mch compared with WTKO C57BL/6 mice. To confirm whether this effect was generated by the ablation of expression of SLPI, we assessed airway resistance in KO and TG mice constitutively expressing different levels of SLPI. Our results showed that allergic SLPI TG mice were hyporesponsive to Mch compared with WTTG mice, whereas SLPI KO mice were hyperresponsive compared with WTKO mice. Hence, we were able to demonstrate that lung physiology is directly influenced by the differential expression of SLPI.
It was shown that acute OVA sensitization and challenge increase airway reactivity to the allergen, which causes inflammation, and increase resistance to expiratory airflow by narrowing of the airways (5, 42, 43). SLPI expressed in TG and WT mice may decrease the airway responsiveness to Mch by exerting its anti-inflammatory effect (21). Therefore, it is likely that ablation of the SLPI gene contributes to the hyperresponsiveness to allergen challenge observed in SLPI KO mice.
Allergen sensitization triggers the immune response characterized for the presence of eosinophils, neutrophils, and macrophages. These cells secrete proteases, which degrade the tissue matrix, increasing chemotaxis, improving the remodeling of damaged tissue, and clearing opsonized particles, including allergens (44). Considering that asthma is characterized by an important inflammatory component, we evaluated the inflammatory response and the role of SLPI in this process. Previous studies performed in our laboratory, using acute and chronic allergic asthma models, showed a significant inflammatory process in the lungs characterized by infiltration of the peribronchial space in the lungs (28, 29).
In the current study, we found that OVA-challenged SLPI TG mice showed significantly lower inflammatory cell influx, including eosinophils, surrounding the airways compared with WT controls; the opposite phenomenon was observed in KO mice under the same conditions. The presence of eosinophils could be explained by the triggering of an inflammatory response whereby epithelial cells secrete chemotactic factors that recruit granulocytes, including eosinophils (45). The milder inflammatory response found in mice in which SLPI expression was not ablated confirmed the effect of SLPI in controlling inflammation in this acute asthma model. These results support the work of Wright et al. (2), who reported that leukocyte influx into the airway could be inhibited by administration of intratracheal SLPI in guinea pigs after allergen sensitization and challenge.
To further evaluate the degree of inflammation, goblet cell hyperplasia was also assessed. The higher goblet cell hyperplasia found in KO-OVA mice is related to the inflammatory cell influx, and it correlated (r = 0.72) with the percentage of eosinophils in the peribronchial region. The increase in goblet cell activity could be explained by the fact that, in asthma, there is an inflammatory process in the lungs, which includes increased mucus production due to goblet cell hyperplasia. The excess of mucus, along with edema and smooth muscle contraction, results in the closure of distal airways and increased resistance in the airway (6).
Allergic asthma is an atopic disease characterized by an elevation of blood IgE levels (28, 46). Westin et al. (47) showed that SLPI regulates allergic reactions by inhibiting IgE. It is tempting to speculate that early inflammatory events in acute allergic asthma, characterized by cell infiltration in the lungs and IgE, are directly modulated by SLPI. We found higher levels of IgE in plasma of OVA-challenged SLPI KO mice, whereas animals constitutively expressing SLPI showed the opposite phenomenon. These findings are consistent with the observation by Nakamura et al. (48) of increased B cell proliferation and Ig production in SLPI KO mice. Our results could be explained by the theories that SLPI plays a role as a negative feedback protein in the production of Abs by interfering with transcription factor activation during the immune response (49) or the lack of control in the production of IgE in SLPI KO mice compared with those who express normal levels of SLPI (41, 46).
During the inflammatory process in the airway, epithelial cells interact with dendritic cells that activate CD4+ Th cells to initiate a Th2 response to produce IL-4 and IL-10; finally, the Th2 cytokines stimulate B cells to produce IgE. We assessed the cytokine profile of OVA-sensitized and -challenged mice in an attempt to further clarify the mechanism of action of SLPI in the immune response of allergic asthma, and we found a significant increase in the entire cytokine panel using KO-OVA mice compared with KO-PBS or WTKO-OVA mice. Additionally, IL-2, IL-6, IFN-γ, and MCP-1 showed significant increases using TG-OVA mice compared with WTTG-PBS or WTTG-OVA mice; interestingly, TG-OVA mice showed lower cytokine levels compared with WTTG mice under the same conditions. No specific Th1 or Th2 pattern of cytokine/chemokine expression seems to distinguish SLPI KO and TG mice from their respective WT controls. These results demonstrated that constitutive expression of SLPI is a determinant in the production of differential cytokines levels, which are associated with IgE in plasma and the severity of the inflammatory process.
RES is a TLR7/8 ligand in humans (50), and previous studies demonstrated that its protective effect is dependent on MyD88 but independent of MAPKAP-2 (28). The anti-inflammatory role of TLR7 signaling in allergic asthma is reflected by the complete inhibition of Th1 and Th2 cytokines following RES treatment in OVA-challenged animals (29). Additionally, several studies using RES treatment in mice performed in our laboratory showed that RES induced the expression of SLPI in macrophages derived from OVA-sensitized and -challenged mice compared with untreated controls. This observation led us to assess the effect of RES on SLPI expression and its role in modulating the development of inflammation in a murine model of allergic asthma. In this study, we found that RES increased mRNA expression in naive C57BL/6 mice and inhibited the inflammatory process in SLPI KO and TG OVA-sensitized and -challenged mice, which is reflected by the lower degree of inflammatory cell infiltration in the lungs, the decreased percentage of eosinophils, and the reduction in goblet cell hyperplasia compared with untreated animals.
The immunoregulatory role of SLPI occurs through the NF-κB–signaling pathway. Taggart et al. (51) demonstrated that SLPI is located in the cytoplasm and nucleus of U937 cells and peripheral blood monocytes. These results were confirmed by Xu et al. (49), who also found SLPI in the nucleus and cytoplasm of tonsillar epithelial cells. SLPI protects IκB-β and binds NF-κB in a specific manner, affecting its activation (51). These results were confirmed by Xu et al. (49), who also found SLPI in the nucleus and cytoplasm of tonsillar epithelial cells. Using SLPI KO mice, we studied the effect of RES on the activation of NF-κB and its effect on SLPI. We found higher levels of IκB-β in SLPI KO and WTKO mice treated with RES compared with untreated mice, showing a protective effect on the activation of NF-κB in those animals under RES treatment.
These results demonstrated how the expression of SLPI exerts a protective effect in the activation of NF-κB using SLPI KO and WT mice and showed that treatment with RES is effective against allergic asthma through the protection of IκB-β, but it is not directly associated with the expression of SLPI. An additional mechanism related to activation of NF-κB should be involved in the treatment with RES, and further studies must be performed to elucidate the participation of this NF in the treatment of allergic asthma with this drug.
Using an acute model of allergic asthma in SLPI TG and KO mice, we demonstrated that constitutive expression of SLPI directly reduces the inflammatory response in the respiratory tract and improves lung function. Because of the anti-inflammatory and antimicrobial functions attributed to SLPI, the lack of genetic polymorphism, and its early expression after tissue injury, this protein should be considered a target for novel therapies in allergic asthma.
Supplementary Material
Acknowledgments
This work was supported by grants from the Sandler Program for Asthma Research and the Canadian Institutes of Health Research (to D.R.). R.M. and P.C. are supported by a Canadian Institutes of Health Research Doctoral Award. C.K. is supported by the Fonds de la recherche en santé du Québec Master’s Training Award.
We thank Dr. Marie-Christine Guiot for expertise with histological analyses.
Abbreviations used in this article
- AHR
airway hyperresponsiveness
- CR
Congo red
- CT
threshold cycle
- KO
knockout
- Mch
methacholine
- PAS
periodic acid-Schiff
- RES
resiquimod
- SLPI
secretory leukocyte protease inhibitor
- TG
transgenic
- WT
wild-type
- WTKO
SLPI KO wild-type
- WTTG
SLPI TG wild-type
Footnotes
Supplementary Data http://www.jimmunol.org/content/suppl/2011/02/18/jimmunol.1001539.DC1.html
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Myers TR. Use of heliox in children. Respir. Care. 2006;51:619–631. [PubMed] [Google Scholar]
- 2.Wright CD, Havill AM, Middleton SC, Kashem MA, Lee PA, Dripps DJ, O’Riordan TG, Bevilacqua MP, Abraham WM. Secretory leukocyte protease inhibitor prevents allergen-induced pulmonary responses in animal models of asthma. J. Pharmacol. Exp. Ther. 1999;289:1007–1014. [PubMed] [Google Scholar]
- 3.Blais L, Beauchesne MF, Lévesque S. Socioeconomic status and medication prescription patterns in pediatric asthma in Canada. J. Adolesc. Health. 2006;38:607, e9–e16. doi: 10.1016/j.jadohealth.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Brown R, Turk F, Dale P, Bousquet J. Cost-effectiveness of omalizumab in patients with severe persistent allergic asthma. Allergy. 2007;62:149–153. doi: 10.1111/j.1398-9995.2006.01310.x. [DOI] [PubMed] [Google Scholar]
- 5.Burburan SM, Xisto DG, Rocco PR. Anaesthetic management in asthma. Minerva Anestesiol. 2007;73:357–365. [PubMed] [Google Scholar]
- 6.Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, Curran-Everett D, Erzurum SC, Gaston BM, Israel E, et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J. Appl. Physiol. 2008;104:394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 7.Shome GP, Starnes JD, III, Shearer M, Kennedy R, Way A, Arif A, Prabhakar S. Exhaled nitric oxide in asthma: variability, relation to asthma severity, and peripheral blood lymphocyte cytokine expression. J. Asthma. 2006;43:95–99. doi: 10.1080/02770900500497925. [DOI] [PubMed] [Google Scholar]
- 8.Bandi V, Velamuri S, Sirgi C, Wendt J, Wendt R, Guntupalli K. Deposition pattern of heliox-driven bronchodilator aerosol in the airways of stable asthmatics. J. Asthma. 2005;42:583–586. doi: 10.1080/02770900500216135. [DOI] [PubMed] [Google Scholar]
- 9.Eisenberg SP, Hale KK, Heimdal P, Thompson RC. Location of the protease-inhibitory region of secretory leukocyte protease inhibitor. J. Biol. Chem. 1990;265:7976–7981. [PubMed] [Google Scholar]
- 10.Odaka C, Mizuochi T, Yang J, Ding A. Murine macrophages produce secretory leukocyte protease inhibitor during clearance of apoptotic cells: implications for resolution of the inflammatory response. J. Immunol. 2003;171:1507–1514. doi: 10.4049/jimmunol.171.3.1507. [DOI] [PubMed] [Google Scholar]
- 11.Fakioglu E, Wilson SS, Mesquita PM, Hazrati E, Cheshenko N, Blaho JA, Herold BC. Herpes simplex virus downregulates secretory leukocyte protease inhibitor: a novel immune evasion mechanism. J. Virol. 2008;82:9337–9344. doi: 10.1128/JVI.00603-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomee JF, Hiemstra PS, Heinzel-Wieland R, Kauffman HF. Antileukoprotease: an endogenous protein in the innate mucosal defense against fungi. J. Infect. Dis. 1997;176:740–747. doi: 10.1086/514098. [DOI] [PubMed] [Google Scholar]
- 13.Sagel SD, Sontag MK, Accurso FJ. Relationship between antimicrobial proteins and airway inflammation and infection in cystic fibrosis. Pediatr. Pulmonol. 2009;44:402–409. doi: 10.1002/ppul.21028. [DOI] [PubMed] [Google Scholar]
- 14.Ramadas RA, Wu L, LeVine AM. Surfactant protein A enhances production of secretory leukoprotease inhibitor and protects it from cleavage by matrix metalloproteinases. J. Immunol. 2009;182:1560–1567. doi: 10.4049/jimmunol.182.3.1560. [DOI] [PubMed] [Google Scholar]
- 15.Ashcroft GS, Lei K, Jin W, Longenecker G, Kulkarni AB, Greenwell-Wild T, Hale-Donze H, McGrady G, Song XY, Wahl SM. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat. Med. 2000;6:1147–1153. doi: 10.1038/80489. [DOI] [PubMed] [Google Scholar]
- 16.Thuraisingam T, Sam H, Moisan J, Zhang Y, Ding A, Radzioch D. Delayed cutaneous wound healing in mice lacking solute carrier 11a1 (formerly Nramp1): correlation with decreased expression of secretory leukocyte protease inhibitor. J. Invest. Dermatol. 2006;126:890–901. doi: 10.1038/sj.jid.5700182. [DOI] [PubMed] [Google Scholar]
- 17.Jana NK, Gray LR, Shugars DC. Human immunodeficiency virus type 1 stimulates the expression and production of secretory leukocyte protease inhibitor (SLPI) in oral epithelial cells: a role for SLPI in innate mucosal immunity. J. Virol. 2005;79:6432–6440. doi: 10.1128/JVI.79.10.6432-6440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeely TB, Shugars DC, Rosendahl M, Tucker C, Eisenberg SP, Wahl SM. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood. 1997;90:1141–1149. [PubMed] [Google Scholar]
- 19.Py B, Basmaciogullari S, Bouchet J, Zarka M, Moura IC, Benhamou M, Monteiro RC, Hocini H, Madrid R, Benichou S. The phospholipid scramblases 1 and 4 are cellular receptors for the secretory leukocyte protease inhibitor and interact with CD4 at the plasma membrane. PLoS ONE. 2009;4:e5006. doi: 10.1371/journal.pone.0005006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Zheng T, Zhu Z, Homer RJ, Riese RJ, Chapman HA, Jr, Shapiro SD, Elias JA. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J. Exp. Med. 2000;192:1587–1600. doi: 10.1084/jem.192.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weldon S, McGarry N, Taggart CC, McElvaney NG. The role of secretory leucoprotease inhibitor in the resolution of inflammatory responses. Biochem. Soc. Trans. 2007;35:273–276. doi: 10.1042/BST0350273. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 23.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 24.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 25.Greene CM, McElvaney NG, O’Neill SJ, Taggart CC. Secretory leucoprotease inhibitor impairs Toll-like receptor 2- and 4-mediated responses in monocytic cells. Infect. Immun. 2004;72:3684–3687. doi: 10.1128/IAI.72.6.3684-3687.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 27.Gorden KK, Qiu XX, Binsfeld CC, Vasilakos JP, Alkan SS. Cutting edge: activation of murine TLR8 by a combination of imidazoquinoline immune response modifiers and polyT oligodeoxynucleotides. J. Immunol. 2006;177:6584–6587. doi: 10.4049/jimmunol.177.10.6584. [DOI] [PubMed] [Google Scholar]
- 28.Moisan J, Camateros P, Thuraisingam T, Marion D, Koohsari H, Martin P, Boghdady ML, Ding A, Gaestel M, Guiot MC, et al. TLR7 ligand prevents allergen-induced airway hyperresponsiveness and eosinophilia in allergic asthma by a MYD88-dependent and MK2-independent pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L987–L995. doi: 10.1152/ajplung.00440.2005. [DOI] [PubMed] [Google Scholar]
- 29.Camateros P, Tamaoka M, Hassan M, Marino R, Moisan J, Marion D, Guiot MC, Martin JG, Radzioch D. Chronic asthma-induced airway remodeling is prevented by toll-like receptor-7/8 ligand S28463. Am. J. Respir. Crit. Care Med. 2007;175:1241–1249. doi: 10.1164/rccm.200701-054OC. [DOI] [PubMed] [Google Scholar]
- 30.Belkowski SM, Boot JD, Mascelli MA, Diamant Z, de Garavilla L, Hertzog B, Polkovitch D, Towers M, Batheja A, D’Andrea MR. Cleaved secretory leucocyte protease inhibitor as a biomarker of chymase activity in allergic airway disease. Clin. Exp. Allergy. 2009;39:1179–1186. doi: 10.1111/j.1365-2222.2009.03247.x. [DOI] [PubMed] [Google Scholar]
- 31.Ghasemlou N, Bouhy D, Yang J, López-Vales R, Haber M, Thuraisingam T, He G, Radzioch D, Ding A, David S. Beneficial effects of secretory leukocyte protease inhibitor after spinal cord injury. Brain. 2010;133:126–138. doi: 10.1093/brain/awp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Jin FY, Nathan C, Radzioch D, Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88:417–426. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 34.Saglani S, Mathie SA, Gregory LG, Bell MJ, Bush A, Lloyd CM. Pathophysiological features of asthma develop in parallel in house dust mite exposed neonatal mice. Am. J. Respir. Cell Mol. Biol. 2009;41:281–289. doi: 10.1165/rcmb.2008-0396OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lentsch AB, Jordan JA, Czermak BJ, Diehl KM, Younkin EM, Sarma V, Ward PA. Inhibition of NF-kappaB activation and augmentation of IkappaBbeta by secretory leukocyte protease inhibitor during lung inflammation. Am. J. Pathol. 1999;154:239–247. doi: 10.1016/s0002-9440(10)65270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodman JE, Chandalia JK, Thakali S, Seeley M. Meta-analysis of nitrogen dioxide exposure and airway hyper-responsiveness in asthmatics. Crit. Rev. Toxicol. 2009;39:719–742. doi: 10.3109/10408440903283641. [DOI] [PubMed] [Google Scholar]
- 37.Motomura C, Odajima H, Tezuka J, Murakami Y, Moriyasu Y, Kando N, Taba N, Hayashi D, Okada K, Nishima S. Effect of age on relationship between exhaled nitric oxide and airway hyperresponsiveness in asthmatic children. Chest. 2009;136:519–525. doi: 10.1378/chest.08-2741. [DOI] [PubMed] [Google Scholar]
- 38.Komatsu Y, Fujimoto K, Yasuo M, Urushihata K, Hanaoka M, Koizumi T, Kubo K. Airway hyper-responsiveness in young adults with asthma that remitted either during or before adolescence. Respirology. 2009;14:217–223. doi: 10.1111/j.1440-1843.2008.01413.x. [DOI] [PubMed] [Google Scholar]
- 39.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 40.Hoymann HG. Invasive and noninvasive lung function measurements in rodents. J. Pharmacol. Toxicol. Methods. 2007;55:16–26. doi: 10.1016/j.vascn.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Fukunaga J, Abe M, Murai A, Akitake Y, Hosokawa M, Takahashi M. Comparative study to elucidate the mechanism underlying the difference in airway hyperresponsiveness between two mouse strains. Int. Immunopharmacol. 2007;7:1852–1861. doi: 10.1016/j.intimp.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Jonasson S, Hedenstierna G, Hedenström H, Hjoberg J. Comparisons of effects of intravenous and inhaled methacholine on airway physiology in a murine asthma model. Respir. Physiol. Neurobiol. 2009;165:229–236. doi: 10.1016/j.resp.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Lougheed MD, Fisher T, O’Donnell DE. Dynamic hyperinflation during bronchoconstriction in asthma: implications for symptom perception. Chest. 2006;130:1072–1081. doi: 10.1378/chest.130.4.1072. [DOI] [PubMed] [Google Scholar]
- 44.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front. Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 45.Dragon S, Takhar MK, Shan L, Hayglass KT, Simons FE, Gounni AS. T(H)2 cytokines modulate the IL-9R expression on human neutrophils. Biochem. Biophys. Res. Commun. 2009;384:167–172. doi: 10.1016/j.bbrc.2009.04.104. [DOI] [PubMed] [Google Scholar]
- 46.Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J. Exp. Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westin U, Lundberg E, Ohlsson K. IgE-mediated histamine release from nasal mucosa is inhibited by SLPI (secretory leukocyte protease inhibitor) to the level of spontaneous release. Mediators Inflamm. 1998;7:217–220. doi: 10.1080/09629359891162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura A, Mori Y, Hagiwara K, Suzuki T, Sakakibara T, Kikuchi T, Igarashi T, Ebina M, Abe T, Miyazaki J, et al. Increased susceptibility to LPS-induced endotoxin shock in secretory leukoprotease inhibitor (SLPI)-deficient mice. J. Exp. Med. 2003;197:669–674. doi: 10.1084/jem.20021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, Ding A, Knowles DM, Santini PA, Cerutti A. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat. Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- 50.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 51.Taggart CC, Cryan SA, Weldon S, Gibbons A, Greene CM, Kelly E, Low TB, O’Neill SJ, McElvaney NG. Secretory leucoprotease inhibitor binds to NF-kappaB binding sites in monocytes and inhibits p65 binding. J. Exp. Med. 2005;202:1659–1668. doi: 10.1084/jem.20050768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.