Abstract
In HIV-infected individuals on antiretroviral treatment with viral suppression, structured treatment interruptions are designed to allow exposure to endogenous HIV antigens and to thereby boost HIV-specific immunity. AIDS Clinical Trials Group A5132 was an exploratory 2-arm randomized trial that evaluated two 4-week treatment interruptions in combination with 2 strategies for administering interleukin-2 (IL-2): 2.0 million international units of IL-2 subcutaneously daily during the final 2 weeks of treatment interruption and the first week of treatment reinitiation (arm A), or 4.5 million international units of IL-2 subcutaneously twice a day during the first 5 days of treatment reinitiation (arm B). Twenty-one subjects with HIV-1 RNA <50 copies/mL and CD4+ T cell counts ≥300 (median 615) cells/mm3 were randomized. The primary endpoint was the viral setpoint measured 11–12 weeks after a third treatment interruption (observed for 7 Arm A and 9 Arm B). The median HIV-1 RNA setpoints were 4.3 and 4.5 log10 copies/mL for Arm A and Arm B, respectively; there was no evidence of a difference between arms (P = 0.50, rank-sum test, worst rank for unobserved viral setpoint). The current study, the first to evaluate IL-2 during repeated short-term treatment interruptions, revealed no evidence for augmentation of HIV immunity. Viral setpoints were similar to historical controls, emphasizing the need for new strategies to enhance HIV-specific immunity.
Introduction
Structured treatment interruptions of antiretroviral therapy (ART) in patients with chronic HIV infection are designed to allow exposure to endogenous HIV antigens in ART-treated patients with maximal suppression of viral replication, and to thereby boost HIV-specific immunity (Oxenius and others 2002). The best available read-out of HIV-specific immune control induced by treatment interruptions, and through the administration of therapeutic vaccines, is an analytical treatment interruption to assess viral rebound kinetics and, in particular, the HIV RNA level (viral setpoint) 2–3 months after stopping ART (Kutzler and Jacobson 2008). While structured treatment interruptions have been seen to increase HIV-specific CD8 and CD4 responses (Oxenius and others 2002), viral setpoints were lowered only 0.3–0.4 log10 copies/mL by this strategy (Oxenius and others 2002; Jacobson and others 2006).
There is some evidence that interleukin-2 (IL-2), which increases CD4+ T cell counts, could enhance specific immune responses when administered with exposure to HIV antigens (Barouch and others 2000; Kuekrek and others 2005).
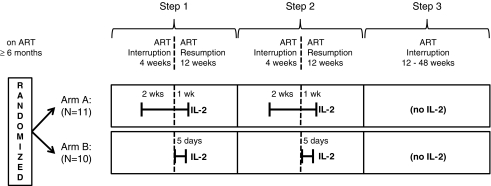
Since the ability of IL-2 to exert an adjuvant effect may vary with timing relative to antigen exposure and with IL-2 dose, 2 different regimens were compared in AIDS Clinical Trials Group (ACTG) study A5132. Arm A employed a longer (21-day) exposure with a lower dose of IL-2 [2.0 million international units (MIU)], beginning during a period of ART interruption, when antigen levels were expected to be high, and continuing into the resumption of ART, when both ART and the possibly enhanced immune response might be combined to suppress viral load (Fig. 1). Since IL-2 is known to drive HIV replication in a dose-dependent manner, Arm B employed a higher IL-2 dose (4.5 MIU twice daily) for a shorter period (5 days) (Kilby and others 2006; Bosch and others 2010) just when ART was resumed after treatment interruption, providing a margin of safety while testing the effect of the higher dose. The study was an exploratory, open-label, randomized trial of 2 antiretroviral treatment interruptions combined with these 2 timing strategies for administering IL-2, followed by an analytic treatment interruption to measure viral setpoint.
FIG. 1.
Study Schema for AIDS Clinical Trial Group A5132.
HIV-1-infected patients, with CD4 cell count ≥300 cells/mm3, HIV-1 RNA <50 copies/mL, laboratory measurements within acceptable ranges and on potent ART (defined as 3 or more antiretroviral agents in combination) for at least 6 months, were eligible for A5132. The study design involved 3 treatment interruptions (Fig. 1). At study entry (Step 1), subjects underwent the first 4-week interruption, after which they resumed ART for 12 weeks. Provided the Step 1 week 12 HIV-1 RNA was <10,000 copies/mL and the Step 1 week 14 CD4 cell count was ≥200 cells/mm3 and ≥50% of the baseline value (or CD4% ≥50% of the baseline value), they then underwent a second 4-week interruption (Step 2), after which they resumed ART for 12 weeks. The third treatment interruption (Step 3), for HIV-1 RNA primary endpoint readout, was at least 12 weeks and up to 48 weeks, depending on subjects' CD4 cell counts and HIV-1 RNA levels. Subjects, however, could restart treatment at any time during Step 3 at their or their clinicians' discretion. Subjects were randomized at study entry to Arm A: 2.0 MIU of IL-2 subcutaneously daily for 3 weeks (final 2 weeks of the Step 1 and Step 2 treatment interruptions and the first week of ART reinitiation), or Arm B: 4.5 MIU of IL-2 subcutaneously twice a day during the first 5 days of ART reinitiation in Steps 1 and 2. An additional criterion for treatment interruption in Steps 2 and 3 was compliance with IL-2 dosing (≥14 of 21 IL-2 injections for Arm A and ≥6 of 10 injections for Arm B). Subjects were thus to be followed for 44–104 weeks, depending on HIV-1 RNA and CD4 cell count measurements during the third treatment interruption. All subjects provided written informed consent and the study was approved by the institutional review boards of each participating site.
The primary endpoint was the viral setpoint at weeks 11 and 12 of the third (analytic) treatment interruption (average log10 HIV-1 RNA level at weeks 11 and 12), with worst-rank imputation for the rank-based treatment comparison for any subjects without an observed viral setpoint; comparison of the observed viral setpoints was a planned secondary analysis, together with other metrics of viral rebound. The study was to enroll at least 30 and up to 45 subjects, estimated to achieve a 95% confidence interval on the viral setpoint difference between arms of ± 0.4 log10 copies/mL. HIV-1 RNA testing (Roche Amplicor HIV-1 Monitor™) was performed at the ACTG central testing laboratory. Toxicities were graded using the 1992 Division of AIDS tables. Treatment arms were compared with the exact Wilcoxon rank-sum test for continuous endpoints and the log-rank test for time to event data.
Twenty-one subjects enrolled in A5132 in 2003–2004 (11 in Arm A and 10 in Arm B) and then the study closed to accrual due to the slow pace of enrollment. Eighteen proceeded to Step 2 (1 subject had CD4 cell count and CD4% <50% of the baseline value, 1 subject did not remain on ART, and 1 subject had HIV-1 RNA >10,000 copies/mL at weeks 12 and 14—all from Arm A); 16 entered Step 3 for primary endpoint readout (1 subject from Arm B had CD4 cell count and CD4% <70% of the baseline value, and 1 subject from Arm A received only 7 of the 21 required IL-2 doses), and 6 subjects restarted ART in Step 3.
The median age at entry was 45 years; 18 (86%) were male; 15 (71%) were white (non-Hispanic). The median CD4 cell count was 615 (25th–75th percentile: 485–990) cells/mm3.
Sixteen subjects (7 from Arm A and 9 from Arm B) had an observed viral setpoint. The median HIV-1 RNA setpoints were 4.3 and 4.5 log10 copies/mL for Arm A and Arm B, respectively (25th–75th percentile: 3.8–4.8, Arm A; 4.3–4.6, Arm B). There was no statistical difference between the 2 arms for the primary endpoint (P = 0.50 using worst rank for 5 subjects; P = 0.84 comparing observed setpoints); a 95% confidence interval on the difference between arms (Arm A–Arm B) based on observed setpoints was −0.9 to 0.6 log10 copies/mL. The time until HIV-1 RNA >5,000 copies/mL was similar between arms (median 7 versus 6 weeks, P = 0.88), as was the slope of the initial rise (median log10 week−1 0.39 and 0.55 for Arm A and Arm B, respectively, P = 0.47) and area under the log10 HIV-1 RNA curve during Step 3 weeks 0 to 12 (P = 0.84). CD4 cell count changes during 12 weeks of Step 3 treatment interruption were also similar between arms: median change −241 and −218 cells/mm3 for Arm A and Arm B, respectively (P = 0.61). Areas under the curve for CD4 cell counts in Step 3 also did not differ (P = 0.76).
Compliance with the IL-2 treatment and ART was very good. As noted above, 1 subject discontinued ART for more than 7 consecutive days during the ART treatment phase of Step 1, and 1 subject received only 7 out of the 21 required IL-2 doses while on Step 2. There were no deaths; 3 subjects had grade 3 or higher laboratory abnormalities [a grade 4 gamma-glutamyl transferase (Arm B) that returned to grade 3 three days later, a grade 3 bilirubin (Arm A) just after reinititation of an atazanavir-containing regimen, and a grade 3 lipase (Arm A)]. Five subjects (2 Arm A and 3 Arm B) reported 7 grade 3 or higher signs/symptoms (injection site vesication, chest pain, shortness of breath, perianal lesions, perianal pain, chest pain, and fever). No clinical AIDS events were seen in this study.
Although this study did not accrue the target sample size, most randomized subjects completed the multiple protocol steps and provided read-out for the primary endpoint, the viral setpoint during the analytic treatment interruption. There was no evidence of a difference between the 2 auto-vaccination strategies that combined IL-2 with two 4-week treatment interruptions. The median viral setpoint levels (4.3 and 4.5 log10 copies/mL) were similar to the placebo arms of treatment interruption and therapeutic vaccine studies that enrolled similar virally suppressed individuals (Jacobson and others 2006; Kilby and others 2006). A limitation of the present study is that there was not a concurrently randomized arm without either IL-2 or structured treatment interruptions. IL-2 has previously been evaluated when administered during viral suppression, but was not seen to alter viral setpoint after treatment interruption (Stellbrink and others 2002; Kilby and others 2006; Goujard and others 2007).
There were several adverse events related to IL-2 administration in this study, including a grade 3 injection-site reaction. In the much larger Evaluation of Subcutaneous Proleukin in a Randomized International Trial (ESPRIT) study, IL-2 plus ART showed significantly more serious toxicities than ART alone (Abrams and others 2009), while providing no clinical benefit. The current study, the first to evaluate strategies of administering IL-2 during repeated short-term treatment interruptions, provides additional information on the use of IL-2 in HIV infection, but revealed no evidence for augmentation of HIV immunity. Viral setpoints after the final treatment interruption were similar to historical controls identified from other studies, emphasizing the need for new strategies to enhance HIV-specific immunity.
Acknowledgments
The authors thank Anne Sevin (deceased), Peter Bohlin, the participating ACTG sites, and especially the study participants. This study was supported in part by the AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases (AI 38858, AI 68636, AI 38855, and AI 68634). The authors also thank and acknowledge the support of Chiron and Novartis, and the following institutions who participated in the study: Stanford University (AI 69556), University of Miami (AI 69477), University of California, Davis Medical Center, and University of Texas Medical Branch at Galveston (AI 32782).
References
- Abrams D. Lévy Y. Losso MH. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009;361:1548–1559. doi: 10.1056/NEJMoa0903175. ans others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH. Craiu A. Kuroda MJ. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc Natl Acad Sci USA. 2000;97:4192–4197. doi: 10.1073/pnas.050417697. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch RJ. Pollard RB. Landay A. Continuing or adding IL-2 in patients treated with antiretroviral therapy (ACTG Protocol A5051, a rollover trial of ACTG protocol A328) AIDS Res Ther. 2010;7:30. doi: 10.1186/1742-6405-7-30. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goujard C. Marcellin F. Hendel-Chavez H. Interruption of antiretroviral therapy initiated during primary HIV-1 infection: Impact of a therapeutic vaccination strategy combined with interleukin (IL)-2 compared with IL-2 alone in the ANRS 095 randomized study. AIDS Res Hum Retroviruses. 2007;23:1105–1113. doi: 10.1089/aid.2007.0047. and others. [DOI] [PubMed] [Google Scholar]
- Jacobson JM. Bucy RP. Spritzler J. Evidence that intermittent structured treatment interruption, but not immunization with ALVAC-HIV vCP1452, promotes host control of HIV replication: The results of AIDS Clinical Trials Group 5068. J Infect Dis. 2006;194:623–632. doi: 10.1086/506364. and others. [DOI] [PubMed] [Google Scholar]
- Kilby JM. Bucy RP. Mildvan D. A randomized, partially blinded phase 2 trial of antiretroviral therapy, HIV-specific immunizations, and interleukin-2 cycles to promote efficient control of viral replication (ACTG A5024) J Infect Dis. 2006;194:1672–1676. doi: 10.1086/509508. and others. [DOI] [PubMed] [Google Scholar]
- Kuekrek H. Schlingmann T. Valdez H. Differential effect of interleukin-2 treatment on primary and secondary immunizations in HIV infected individuals. AIDS. 2005;19:1967–1974. doi: 10.1097/01.aids.0000189859.59559.9b. and others. [DOI] [PubMed] [Google Scholar]
- Kutzler MA. Jacobson JM. Treatment interruption as a tool to measure changes in immunologic response to HIV-1. Curr Opin HIV AIDS. 2008;3:131–135. doi: 10.1097/COH.0b013e3282f54cde. [DOI] [PubMed] [Google Scholar]
- Oxenius A. Price DA. Günthard HF. Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proc Natl Acad Sci. 2002;99:13747–13752. doi: 10.1073/pnas.202372199. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellbrink HJ. van Lunzen J. Westby M. Effects of interleukin-2 plus highly active antiretroviral therapy on HIV-1 replication and proviral DNA (COSMIC trial) AIDS. 2002;16:1479–1487. doi: 10.1097/00002030-200207260-00004. and others. [DOI] [PubMed] [Google Scholar]