Abstract
Objective:
Our objective was to establish the feasibility of combining 2 minimally invasive procedures in patients with failed primary treatment (male sling) in post-prostatectomy incontinence (PPI) patients.
Methods:
From January 2007 to July 2008, 40 men with PPI were implanted with a suburethral tape (2 patients with Seratim, 3 with I-Stop and 35 with Advance). The median preoperative pad count was 4 (range 2–10). Prior to sling placement, 6 patients had undergone ProACT implantation. Of these, 4 patients required explantation due to balloon migration and 2 patients had their balloons kept in situ, with the balloons deflated.
Results:
Twenty-five patients were socially continent at this time. Fifteen patients (37.5%) did not improve or their improvement was not significant. These patients had a preoperative pad count between 7 and 10. Two of these patients had prostate adjustable continence therapy (ProACT) systems still in place. By gradually filling the balloons to 3 mL, both of these patients achieved complete continence, which was maintained at a mean follow-up of 8.5 months. Three patients with prior pelvic irradiation received an artificial urinary sphincter and achieved continence at mean follow-up of 8.3 months. The remaining 10 patients received a ProACT system in addition to the already implanted sling. After appropriate healing and filling of the balloons (average balloon volume 5 mL), all 10 patients reached complete continence; they were pad-free at a mean follow-up of 6 months (range 3–9).
Conclusions:
The combination of ProACT and a suburethral tape was demonstrated to be a possible treatment option in recurrent or persistent PPI.
Résumé
Objectif :
Notre objectif était d’établir la faisabilité d’une association de 2 interventions minimalement invasives chez des patients ayant subi un échec thérapeutique primaire (bandelettes sous-urétrales) chez des patients atteints d’incontinence post-prostatectomie.
Méthodologie :
De janvier 2007 à juillet 2008, on a placé une bandelette sous-urétrale chez 40 hommes atteints d’incontinence post-prostatectomie (2 patients ont reçu le dispositif de marque Seratim, 3 patients, de marque I-Stop et 35, de marque Advance). Le nombre médian de protections absorbantes avant l’opération était de 4 (2 à 10). Avant la mise en place de la bandelette, 6 patients avaient subi une implantation d’un système ProACT. De ce nombre, 4 patients ont dû se faire retirer les ballonnets en raison de leur déplacement; chez 2 patients, les ballonnets sont restés en place mais se sont dégonflés.
Résultats :
Vingt-cinq patients présentaient une continence sociale à ce moment. Quinze patients (37,5 %) n’ont présenté aucune amélioration, ou une amélioration non significative. Ces patients utilisaient de 7 à 10 protections absorbantes avant l’opération. Deux de ces patients étaient toujours porteurs d’un système ProACT. En remplissant graduellement les ballonnets de 3 mL, ces deux patients ont atteint une continence totale, maintenue après un suivi moyen de 8,5 mois. Trois patients ayant reçu antérieurement un traitement pelvien par rayonnement ont reçu un sphincter urinaire artificiel et ont atteint la continence après un suivi moyen de 8,3 mois. Chez les 10 derniers patients, on a implanté un système ProACT en plus de la bandelette déjà en place. Après un temps suffisant de guérison et le remplissage des ballonnets (volume moyen : 5 mL), les 10 patients ont atteint une continence complète. Ils n’avaient plus besoin de protection absorbante après un suivi moyen de 6 mois (entre 3 et 9 mois).
Conclusions :
L’association d’un système ProACT et d’une bandelette sous-urétrale s’est révélée une option thérapeutique possible en présence d’incontinence post-prostatectomie récurrente ou persistante.
Introduction
The increasing number of radical prostatectomies entails an increasing number of patients suffering from postoperative stress incontinence. Depending on the study and the definition of “incontinence,” the incidence of early stress incontinence varies between 0.8% and 87.0%.1–4 Incontinence that persists for more than 1 year postoperatively may decrease to <5%,5 and may even reach 1% to 2%.6
Non-invasive therapy is the first-line treatment for early incontinence experienced within the first 6 to 12 months after prostatectomy. In particular, pelvic floor muscle training (PFMT) is the most widely recommended non-invasive treatment. Surgical intervention is indicated only for patients who exhibit a persistent incontinence for more than 1 year postoperatively, despite conservative therapy.
Despite the new surgical treatment options, the artificial urinary sphincter (AUS) is still the gold standard for the surgical treatment of male incontinence. The technique is well-developed. Since the introduction of the AMS-721 (American Medical Systems, Minnetonka, MN) in 1972, the artificial sphincter has been modified several times to the current AMS-800. Nevertheless, the intervention is expensive and requires invasive surgery, and experienced surgeons. Furthermore, the rate of infection and erosion (4.5%–67%),7 as well as the high rate of revision rates (up to 20%),8 plays an important role in patients’ choice and reticence. In recent years, numerous minimally invasive treatment options with different success rates have been investigated. Adjustable, nonadjustable slings and prostate adjustable continence therapy (ProACT; Uromedica, Plymouth, MN) have been recommended for patients with persistent incontinence. Depending on the severity of incontinence, continence rates between 40% and 63% for the male sling have been reported.9,10 Dry rates using ProACT have even been reported between 67% and 75%.11,12 The patient’s demand for these minimally invasive treatments is high and often poorer results are accepted by the patients to avoid an artificial sphincter. On the other hand, failure of these treatment modalities would result in taking the decision to implant sphincter prosthesis.
Our aim was to study the possibility of combining 2 minimally invasive procedures before considering a sphincter prosthesis implantation.
Methods
From January 2007 to July 2008, 40 patients with post-prostatectomy incontinence were identified for this survey. Patient age ranged from 56 to 74 years (mean 68). All patients were reviewed preoperatively. A detailed history, physical examination, multichannel video urodynamic studies, 24-hour pad count, the International Consultation on Incontinence Questionnaire-Short Form (ICI-Q-SF)13 and cystoscopy were performed preoperatively in all patients. All patients had residual sphincteric function confirmed on cystoscopy, otherwise they were excluded from the study. All patients underwent PFMT and no continence was achieved within 12 months from the prostactectomy. Urodynamics were used to exclude the presence of detrusor overactivity. The preoperative 24-hour pad count ranged between 2 and 10 pads per day (median 4). The preoperative ICI-Q-SF questionnaire was completed in the office and scores ranged between 8 and 20 (mean 13.3). Fifteen patients had adjuvant radiotherapy because of biochemical relapse. Included in the total patient population were 6 patients with a previous ProACT implantation, who reached the maximum balloon inflation (8 mL) on each side and were still incontinent.
As the primary treatment, all options, including conservative methods other than PFMT, medical therapy, AUS placement, male slings, adjustable continence therapy (ProACT) and injection therapy were offered to all patients. Both the patient and the surgeon agreed on the choice of surgical intervention.
All patients in the study received retrourethral male slings according to Rehder and Gozzi.14 Thirty-five patients received an Advance (American Medical Systems, Minnetonka, MN), 3 I-Stop TOMS (CL Medical, Lyon, France) and 2 Seratim slings (Serag Wiessner KG, Naila, Germany). Four patients had a previously placed ProACT system removed because of distal migration of the balloons below the pelvic floor. The 2 remaining patients with a prior ProACT system received a retrourethral sling and the ProACT systems (with correct location) were left in place after deflation of both balloons.
All patients were followed for 8 months. The 24-hour pad count and the ICI-Q questionnaire were evaluated at the end of the follow-up. We defined those patients as cured who required a security pad/day. An improvement was defined as >50% reduction in the pad count.
Results
All patients underwent uncomplicated retrourethral sling placement by a single surgeon (CvdH) during a single procedure. Blood loss was minimal in all cases. No intraoperative complications were noted. At the end of the procedure, an indwelling Foley catheter was placed overnight. All patients were discharged within 48 hours after surgery. No cases of perineal pain or extended catheterization were noticed in this group.
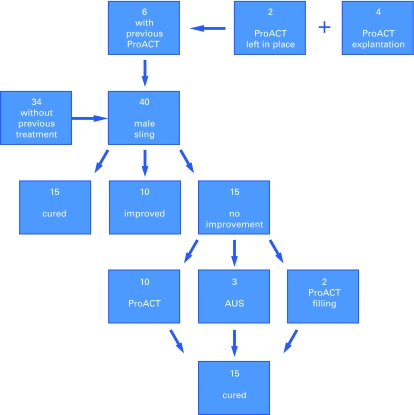
From the 40 patients, 15 patients were continent and 10 showed improvement and were satisfied with the treatment modality. The remaining 15 patients with moderate to severe incontinence failed the treatment. The mean postoperative ICI-Q-SF of the remaining 25 successful patients ranged between 3 and 10 (mean 6). All of the 25 patients were using only 1 pad postoperatively (ranging between a security pad and a wet pad). The other 15 patients had no change in their preoperative and postoperative ICI-Q-SF and pad count (Fig. 1).
Fig. 1.
This flow chart illustrates the patients’ distribution and results, with a total number of 40 patients (34 without any previous treatment and 6 with persistent incontinence after ProACT implantation). Cured = Security pad, Improved ≥50% reduction in pad count/day. AUS = artifical urinary sphincter.
From these 15 patients with failed primary treatment, 3 patients were previously irradiated and were therefore offered an AUS (2 Flow Secure, 1 AMS 800; (American Medical Systems, Minnetonka, MN). Two patients who still had their ProACT device in place were offered balloon filling. All 5 patients were continent at the end of the follow-up (8.5 months). The remaining 10 patients who failed the primary treatment and who were not previously irradiated were still unwilling to be treated with an AUS. They asked for another minimal invasive procedures and were offered a ProACT implantation (Fig. 1). No difficulties were noted during the introduction of the ProACT balloons or the implantation of the AUS. After a follow-up period of 6 months, all the patients were continent and satisfied with their treatment (according to a subjective assessment). Postoperatively, all patients were using a single pad as a safety pad. The mean postoperative ICI-Q-SF was 7.2 (range 4 to 10). No patient presented with significant postvoid residual urine volumes during the follow-up period. The average number of ProACT adjustments was 4 (range 3 to 6); all adjustments were done in an outpatient setting. The final balloon volumes ranged from 4 to 7 ml (mean 5.5). The ProACT placement was controlled using fluoroscopy before each adjustment and was done in line with the procedure described by Hübner and Schlarp.12 All adjustments were made within the first 6 months after the procedure. No cases of urethral erosion or other major complications were observed in this series. No ProACT balloon migrations were observed during the follow-up period.
Discussion
Implantation of an AUS is associated with continence and patient satisfaction rates of about 90% of refractory post-radical prostatectomy stress urinary incontinence cases and currently remains the standard.15 Although the AUS is an effective and durable treatment, many patients are hesitant or refuse implantation.16 They are not interested in (or capable of) manipulating the scrotal pump. Furthermore, the AUS is expensive and requires a complex surgical procedure that is associated with significant complication and revision rates. In fact, surgical revision or substitution of the device may be required in up to 40% of cases due to mechanical failures, infections or early and late erosions.17–20 Other treatment options should be pursued to encourage patients to treat a problem that will affect more (and younger) patients as the number of radical prostatectomies increases.
A number of minimal invasive procedures have been introduced. ProACT was first developed in 2000 and has been reported on extensively since that time.21 The original technique of implantation using fluoroscopic guidance has been well described over the last 6 years. Continence rates of 65% to 70% have demonstrated reproducible results in centres where fluoroscopy is readily available and by surgeons who are familiar with the implantation procedure.22 Similar results have also been demonstrated using transrectal ultrasound guidance. Gergori and colleagues described an overall dry rate of 75% in nonirradiated patients.11 Other minimally invasive procedures included the use of male slings. In 2007, Rehder and Gozzi published their pilot study analyzing the anatomic effects of placing a transobturator tape in cadavers, as well as its clinical efficacy in a small cohort of men with post-prostatectomy incontinence.14 Continence rates ranged between 40% and 63%.9,10 Other male slings, like bone-anchored slings, showed similar results.23
According to our knowledge, no data are available considering the secondary treatment of failed minimal invasive procedures in patients with post-prostatectomy incontinence using a combination of the 2 treatment modalities (following male retrourethral slings and/or ProACT).
In the present study, 40 patients with post-prostatectomy incontinence were treated with retrourethral slings. Six of these patients were initially treated with ProACT and did not improve. In 4 patients, the ProACT balloons were explanted at the time of sling placement. In the remaining 2 patients, the ProACT system was deflated and left in place. After a median follow-up of 6 months, 25 patients were socially continent with a postoperative 24-hour pad count of 1/day. Fifteen patients with failed slings were offered a secondary treatment. As previous radiation is considered as a relative contraindication for other minimal invasive procedures, like ProACT,12 3 patients who were previously irradiated were offered AUS. These were shown to be cured during the follow-up (ICI-Q-SF between 3 and 6 and 0 pads/day). The implantation of an AUS after failed retrourethal sling was shown to be technically feasible and did not affect the short-term outcome of the AUS. These findings, although observed in only 3 patients, mirror those published on AUS implantation after a bone-anchored male sling.24 Two patients who were initial ProACT failures were offered a system activation (balloons inflation) after the sling was implanted. At a follow up of 8.5 months, these patients had a postoperative 24-hour pad count of 1/day. Accordingly, the remaining 10 patients with Advance sling failures were offered a ProACT implantation. After a follow-up period of 6 months, all patients were continent and satisfied with their treatment (according to a subjective assessment). All patients were postoperatively using a single pad as a safety pad. The average number of ProACT adjustments was 4 (range 3 to 6), all of which were done in an outpatient setting. The final balloon volume ranged from 4 to 7 mL (mean 5.5). The ProACT position was controlled using fluoroscopy before each adjustment. No complications were noted. We showed that 10 patients who initially failed a retrourethral sling treatment were rendered cured through implanting an additional ProACT system. Furthermore, 2 patients who initially failed ProACT were also rendered cured through combining that system with a retrourethral sling (Fig. 1).
We defined those patients as continent when they required 0 to 1 pad daily. Regarding the quality of life assessment, a significant improvement was observed (as evaluated by the questionnaires). Similar to other reports, we observed an association between the recovery of continence and improvement in the quality of life.25 In terms of surgical technique, the ProACT and retrourethral sling procedures are relatively simple and require basic equipment, which is available in most hospitals. Placement of the retrourethral male sling (35 Advance, 3 I-Stop TOMS and 2 Seratim) showed fair results, with 37.5% (15 out of 40) of patients rendered pad-free and 25% (10 out of 40) of patients improved after more than 1 year. These data concur with the data already obtained from patients after a short follow-up period; they presented with a cure rate of 40% to 63% and an improvement rate of 17% to 30% after 3 months.9,10
Our results show that the previous implantation of a retrourethral sling, such as the Advance tape, does not negatively affect further surgical interventions, such as an artificial sphincter or the ProACT system. These results correlate with previous data published on the implantation of AUS after a failure with the bone-anchored male sling.24 However, there is no data on the use of ProACT, as a secondary option after a failure with the male sling, in curing post-prostatectomy incontinence.
Conclusion
We conclude that the combination of ProACT and suburethral male sling is technically feasible, and could be offered to patients with persistent incontinence without previous irradiation. However, the results of this work are limited by the small number of patients (n = 12) who received a combination therapy after failed primary treatment. Further studies are needed to compare these results before regarding this treatment combination as a standard. With judicious use of all available treatments, we were able to achieve meaningful improvement in continence in all 40 patients in this cohort.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Augustin H, Pummer K, Daghofer F, et al. Patient self-reporting questionnaire on urological morbidity and bother after radical retropubic prostatectomy. Eur Urol. 2002;42:112–7. doi: 10.1016/s0302-2838(02)00259-2. [DOI] [PubMed] [Google Scholar]
- 2.Burkhard FC, Kessler TM, Fleischmann A, et al. Nerve-sparing open radical retropubic prostatectomy—does it have an impact on urinary continence? J Urol. 2006;176:189–95. doi: 10.1016/S0022-5347(06)00574-X. [DOI] [PubMed] [Google Scholar]
- 3.Penson DF, McLerran D, Feng Z, et al. Five-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol. 2005;173:1701–5. doi: 10.1097/01.ju.0000154637.38262.3a. [DOI] [PubMed] [Google Scholar]
- 4.Rudy DC, Woodside JR, Crawford ED. Urodynamic evaluation of incontinence in patients undergoing modified Campbell radical retropubic prostatectomy: a prospective study. J Urol. 1984;132:708–12. doi: 10.1016/s0022-5347(17)49836-3. [DOI] [PubMed] [Google Scholar]
- 5.Peyromaure M, Ravery V, Boccon-Gibod L. The management of stress urinary incontinence after radical prostatectomy. BJU Int. 2002;90:155–61. doi: 10.1046/j.1464-410x.2002.02824.x. [DOI] [PubMed] [Google Scholar]
- 6.Bauer RM, Bastian PJ, Gozzi C, et al. Postprostatectomy incontinence: all about diagnosis and management. Eur Urol. 2009;55:322–33. doi: 10.1016/j.eururo.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Hussain M, Greenwell TJ, Venn SN, et al. The current role of the artificial urinary sphincter for the treatment of urinary incontinence. J Urol. 2005;174:418–24. doi: 10.1097/01.ju.0000165345.11199.98. [DOI] [PubMed] [Google Scholar]
- 8.Trigo Rocha F, Gomes CM, Mitre AI, et al. A prospective study evaluating the efficacy of the artificial sphincter AMS 800 for the treatment of postradical prostatectomy urinary incontinence and the correlation between preoperative urodynamic and surgical outcomes. Urology. 2008;71:85–9. doi: 10.1016/j.urology.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Gozzi C, Becker AJ, Bauer R, et al. Early results of transobturator sling suspension for male urinary incontinence following radical prostatectomy. Eur Urol. 2008;54:960–1. doi: 10.1016/j.eururo.2008.04.096. [DOI] [PubMed] [Google Scholar]
- 10.Cornu JN, Sèbe P, Ciofu C, et al. The AdVance Transobturator Male Sling for Postprostatectomy Incontinence: Clinical Results of a Prospective Evaluation after a Minimum Follow-up of 6 Months. Eur Urol. 2009;56:923–7. doi: 10.1016/j.eururo.2009.09.015. Epub 2009 Sep 8. [DOI] [PubMed] [Google Scholar]
- 11.Gregori A, Romano AL, Sceiri F, et al. Transrectal ultrasound-guided implantation of adjustable continence therapy (ProACT): surgical technique and clinical results after a mean follow-up of 2 years. Eur Urol. 2010;57:430–6. doi: 10.1016/j.eururo.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 12.Hübner WA, Schlarp OM. Treatment of incontinence after prostatectomy using a new minimally invasive device: adjustable continence therapy. BJU Int. 2005;96:587–94. doi: 10.1111/j.1464-410X.2005.05689.x. [DOI] [PubMed] [Google Scholar]
- 13.Avery K, Donovan J, Peters TJ, et al. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23:322–30. doi: 10.1002/nau.20041. [DOI] [PubMed] [Google Scholar]
- 14.Rehder P, Gozzi C. Transobturator sling suspension for male urinary incontinence including post-radical prostatectomy. Eur Urol. 2007;52:860–6. doi: 10.1016/j.eururo.2007.01.110. [DOI] [PubMed] [Google Scholar]
- 15.Venn SN, Greenwell TJ, Mundy AR. The long-term outcome of artificial urinary sphincters. J Urol. 2000;164(3 Pt 1):702–6. doi: 10.1097/00005392-200009010-00020. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Litt ER, Ballert KN, et al. Artificial urinary sphincter versus male sling for post-prostatectomy incontinence--what do patients choose? J Urol. 2009;181:1231–5. doi: 10.1016/j.juro.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Fleshner N, Herschorn S. The artificial urinary sphincter for post-radical prostatectomy incontinence: impact on urinary symptoms and quality of life. J Urol. 1996;155:1260–4. [PubMed] [Google Scholar]
- 18.Haab F, Trockman BA, Zimmern PE, et al. Quality of life and continence assessment of the artificial urinary sphincter in men with minimum 3.5 years of followup. J Urol. 1997;158:435–9. [PubMed] [Google Scholar]
- 19.Litwiller SE, Kim KB, Fone PD, et al. Postprostatectomy incontinence and the artificial urinary sphincter: a long-term study of patient satisfaction and criteria for success. J Urol. 1996;156:1975–80. doi: 10.1016/s0022-5347(01)65408-9. [DOI] [PubMed] [Google Scholar]
- 20.Carlson KV, Nitti VW. Prevention and management of incontinence following radical prostatectomy. Urol Clin North Am. 2001;28:595–612. doi: 10.1016/s0094-0143(05)70165-8. [DOI] [PubMed] [Google Scholar]
- 21.Hübner WA, Schlarp OM. Adjustable Continence Therapy (ProACTTM): evolution of the surgical technique and comparison of the original 50 patients with the most recent 50 patients at a single centre. Eur Urol. 2007;52:680–6. doi: 10.1016/j.eururo.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 22.Lebret T, Cour F, Benchetrit J, et al. Treatment of postprostatectomy stress urinary incontinence using a minimally invasive adjustable continence balloon device, ProACT: results of a preliminary, multicenter, pilot study. Urology. 2008;71:256–60. doi: 10.1016/j.urology.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 23.Rajpurkar AD, Onur R, Singla A. Patient satisfaction and clinical efficacy of the new perineal bone-anchored male sling. Eur Urol. 2005;47:237–42. doi: 10.1016/j.eururo.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Fisher MB, Aggarwal N, Vuruskan H, et al. Efficacy of artificial urinary sphincter implantation after failed bone-anchored male sling for postprostatectomy incontinence. Urology. 2007;70:942–4. doi: 10.1016/j.urology.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Gousse AE, Madjar S, Lambert MM, et al. Artificial urinary sphincter for post-radical prostatectomy urinary incontinence: long-term subjective results. J Urol. 2001;166:1755–8. [PubMed] [Google Scholar]