Abstract
Purpose
We conducted a multi-center randomized double-blind study to determine the effects of 6-month therapy with sulodexide on urinary protein excretion in patients with idiopathic Immunoglobulin A (IgA) nephropathy.
Materials and Methods
A total of seventy-seven patients participated in the study. They were randomly allocated to one of three groups: sulodexide 75 mg or 150 mg daily or the placebo for 6 months. The primary end point was the achievement, at 6 months, of at least 50% reduction in urine protein/creatinine ratio (UPCR) from the baseline value.
Results
At 6 months, the primary end point was achieved by 12.5% of the patients assigned to the placebo, 4.0% of the patients assigned to sulodexide 75 mg daily and 21.4% of those assigned to 150 mg (p=0.308). Treatment with sulodexide 150 mg daily for 6 months significantly reduced log UPCR from 6.38±0.77 at baseline to 5.98±0.94 at 6 months (p=0.045), while treatment with sulodexide 75 mg daily and placebo did not.
Conclusion
A 6-month treatment with sulodexide did not achieve 50% reduction of urinary protein excretion in IgA nephropathy patients, but showed a tendency to increase the time-dependent anti-proteinuric effect. Therefore, long-term clinical trials on a larger scale are warranted to elucidate the hypothesis that sulodexide affords renal protection in IgA nephropathy patients.
Keywords: IgA nephropathy, sulodexide, proteinuria
INTRODUCTION
Until recently, there was no effective treatment available for patients with IgA nephropathy. Although there remains no cure regimen, treatment options that slow disease progression are becoming available. Since IgA nephropathy may affect up to 1.3% of the population,1-4 and in large cohort studies of patients with IgA nephropathy, as many as 30-50% develop end-stage renal disease (ESRD) over a 20-year period after diagnosis,5-7 there is a need for novel therapeutic agents capable of preserving renal function.
Sulodexide, a mixture of heparan and dermatan sulfate (80/20%), targets metabolic defects in the synthesis of the matrix and basement membrane as well as in endothelial cell dysfunction. It has been shown to reduce proteinuria in animal models of diabetic nephropathy.8-11 Several small scale clinical studies in humans with diabetic nephropathy have demonstrated a consistent trend for the reduction of urinary albumin excretion in diabetes mellitus (DM).12-21 Gambaro, et al.22 reported that the agent achieved its renoprotective effects primarily through activity on the synthesis of the matrix/glomerular basement membrane (GBM) molecules by glomerular cells. Although the complete mechanism of sulodexide effects in the kidney has not been fully identified, the spectrum of reported effects include the prevention and correction of thickening of the GBM;22,23 restoration of the ionic charge barrier of the GBM;24,25 suppression of mesangial cell proliferation;26 reduction of the transforming growth factor-β1 expression and overproduction of collagen; inhibition of heparanase-1;27-29 suppression of proteinuria-induced endothelial cell endothelin production; and anti-thrombotic effects.
The most common alteration associated with IgA nephropathy identified by light microscopy is focal or diffuse proliferation of mesangial regions and extracellular matrix expansion. Taking into consideration the above alterations in IgA nephropathy and the mechanism of sulodexide effects, we investigated whether sulodexide reduces protein excretion rate in patients with idiopathic IgA nephropathy who have persistent proteinuria. We have conducted a multi-center randomized double-blind pilot study in order to determine the effects of a 6-month therapy with sulodexide on urinary protein excretion in patients with idiopathic IgA nephropathy who have persistent proteinuria.
MATERIALS AND METHODS
This is a randomized, double-blind, placebo controlled, multi-center dose-range finding trial for sulodexide which is composed of 80% fast-moving heparan and 20% dermatan sulfate (Vessel 2F, Alfa Wassermann SpA, Bologna, Italy). All patients gave written informed consent prior to enrollment in the study. The study was approved by the appropriate local research ethics committee at both Seoul National University Hospital and Seoul National University Bundang Hospital, and was performed in accordance with the Declaration of Helsinki.
Participants
Patients of either gender, aged between 18 and 70 years with a baseline estimated glomerular filtration rate (eGFR) of more than 30 mL/min/1.73 m2 were eligible for the study if they had biopsy-proven IgA nephropathy. Patients were required to have proteinuria, defined as a urinary protein/creatinine ratio (UPCR) between 0.1 g/g and 3.5 g/g, and to have been receiving treatment with an ACEI or an ARB. The diagnosis of IgA nephropathy was based on the histologic assessment of a renal biopsy performed by one investigator and was confirmed by immunofluorescence studies showing predominant or co-dominant mesangial deposition of IgA.7,30 The patients were also required to have stable metabolic control: PCR variation ≤30% from baseline for over 3 months and blood pressure (BP) ≤160/90 mmHg for at least 6 months with or without antihypertensive therapy.
Exclusion criteria were as follows: neoplasms; severe liver, cardiac, or systemic disease; known hypersensitivity to any glycosaminoglycans (GAGs); chronic treatment with corticosteroids, immunosuppressants or alkylating agents; urinary protein excretion rate ≥3.5 g/24 hours; symptomatic urinary tract infections; gross hematuria; and pregnancy or lactation.
Sample size
The sample size was estimated according to two independent hypotheses; 1) rejection of the hypothesis that P1=P2 (where P is the number of patients with a 50% reduction in UPCR after 6 month of therapy); 2) rejection of the null hypothesis that µ1=µ2=µ3 after 6 months of therapy, only if each independent variable accounts for at least 20% of the variation, α=0.05 and 1-β is 90%. A 15% withdraw and drop-out rate was also added to the calculation. Altogether, 102 patients were necessary (34 per treatment group).
Study design
At the study entry (time point 0, T0), a screening assessment was performed for all patients. It included a complete medical history, laboratory assessments (blood chemistry, blood hematology and urinalysis) and a pregnancy test for women with childbearing potential. Patients who met the inclusion criteria proceeded to randomization and were randomly assigned, based on a computer-generated randomization schedule, to treatment with the placebo, sulodexide 75 mg or sulodexide 150 mg daily at a 1:1:1 ratio. Patients were instructed to take their medication orally with water 30 minutes prior to morning and evening meals. A pharmaceutist from the 'Clinical Trials Center' without any of the patients' information distributed the drugs. Patients visited the clinic at 2, 4 and 6 months (T2, T4, and T6, respectively) after randomization. At each follow-up visit, efficacy and safety parameters were evaluated and a complete blood count, activated partial thromboplastin time (aPTT), fibrinogen, glycosylated hemoglobin (HbA1C), blood chemistry (glucose, lipids, urea, creatinine, total protein, albumin, and liver functions) were measured. In addition, the first morning voided urine was collected prior to the day of each follow-up visit for urine protein/creatinine ratio (UPCR).
Study end points
The primary end point was the achievement, at 6 months (T6), of at least a 50% reduction in UPCR from the baseline value. The secondary end point of the study was within-group change in log UPCR for 6 months (T6). As the primary efficacy parameter, we analyzed the proportion of patients who achieved the primary end point at 6 months (T6).
Statistical analyses
Analyses were performed according to the per protocol model on all randomized patients, regardless of adherence to treatment. Analysis was also performed from the evaluable patients without inclusion of missing values. A log-transformed value of UPCR was analyzed instead of UPCR due to the skewed distribution of the latter value. To evaluate the dose-dependent effect of sulodexide, we compared log-transformed UPCR at T0 and T6 in the two sulodexide groups with log-transformed UPCR at T0 and T6 in the placebo group using Pearson's chi-square test. Efficacy was also evaluated by analyzing within-group change in log-transformed UPCR for 6 months (T6) using the Wilcoxon signed rank test.
Baseline values were compared among the three groups using the Kruskal-Wallis test for continuous variables and the Pearson's chi-square test for categorical variables. Statistical analysis was performed using SPSS version 13.0 for Microsoft Windows.
RESULTS
Patients profiles and baseline characteristics
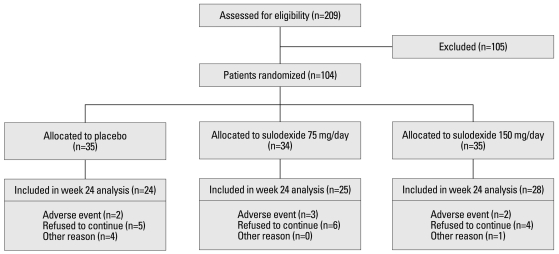
Between March 2007 and April 2009, 209 patients with IgA nephropathy were screened for study. One hundred and four patients were randomly assigned with equal allocation to treatment with the placebo, a single dose of sulodexide 75 mg daily, or sulodexide 150 mg daily in two divided doses. The profiles of these patients are summarized in Fig. 1. Of the 104 randomized patients, 77 completed the study. Twenty-seven patients discontinued participation due to adverse effects, were lost to follow-up, or spontaneously withdrew from the study.
Fig. 1.
Patient profiles; the number of patients who were screened for the study, underwent randomization, and completed the study. Some patients were excluded for more than one reason.
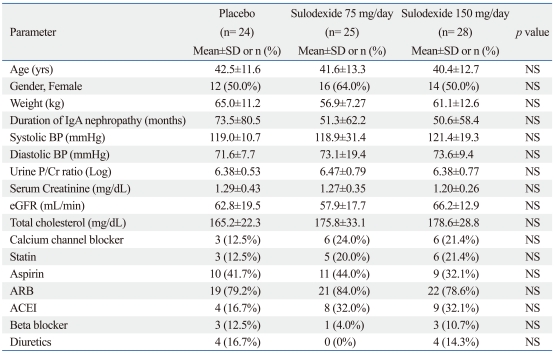
Demographic and clinical baseline characteristics were comparable among the three groups. No significant difference was found between the three groups in terms of age, body weight, gender ratio, duration of renal disease, systolic and diastolic blood pressure, dose and frequency of individual anti-hypertensive medications, metabolic control, serum creatinine and eGFR (Table 1).
Table 1.
Baseline Characteristics
NS, nonsignificant; IgA, Immunoglobulin A; BP, blood pressure; P/Cr, protein creatinine ratio; eGFR, estimated glomerular filtration ratio; ARB, angiotensin II receptor blocker; ACEI, angiotensin converting enzyme inhibitor.
Clinical parameters and adverse effects
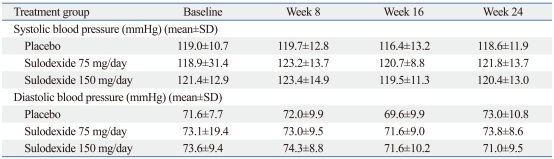
Systolic and diastolic blood pressure remained stable throughout the course of the study and no significant differences were observed among the three groups (Table 2). No serious adverse events, related or unrelated, to sulodexide was reported. Adverse events leading to withdrawal from the study were as follows: rash (two cases), gastrointestinal disturbance (four cases), and gross hematuria (one case). There was no substantial difference between the groups in the incidence and type of adverse events (Fig. 1). No significant changes in mean fibrinogen levels, aPTT, INR, prothrombin time and bleeding time were observed between the groups.
Table 2.
Blood Pressure throughout the Study
Student's t test: p>0.05 between three groups at baseline, week 8, 16 and 24, respectively.
Evaluation of sulodexide efficacy
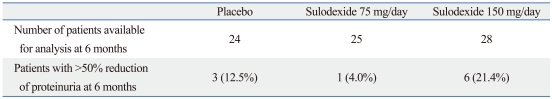
The proportion of patients reaching the primary endpoint is presented in Table 3. At T6, the primary endpoint was achieved in 12.5% of the patients assigned to the placebo, 4.0% of the patients assigned to sulodexide 75 mg daily and 21.4% of the patients assigned to sulodexide 150 mg daily. There was no significant difference among the three groups in the proportion of patients reaching the primary endpoint. The odds ratio for reaching the primary endpoint between the sulodexide 150 mg/day group versus the placebo group was 1.38 (95% CI 0.65 to 2.94, p=0.40). The odds ratio was 0.29 (95% CI 0.03 to 3.02, p=0.30) for the sulodexide 75 mg/day group versus placebo.
Table 3.
Patients Achieving >50% Reduction of Proteinuria at 6 months, n and (%)
p=0.308
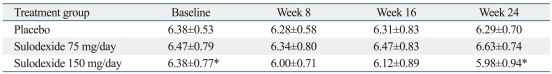
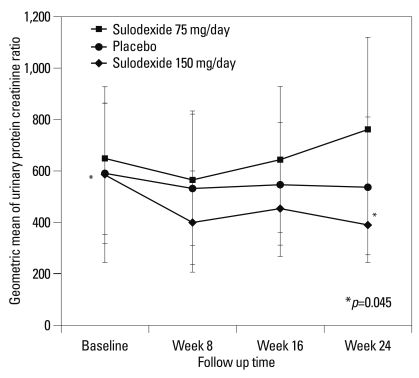
The secondary end point of the study was within-group change in log UPCR for 6 months. Treatment with sulodexide 150 mg daily for 6 months significantly reduced log UPCR from 6.38±0.77 at T0 to 5.98±0.94 at T6 (p=0.045). However, treatment with sulodexide 75 mg daily and the placebo did not show a significant reduction in logUPCR (75 mg group, logUPCR from 6.47±0.79 at T0 to 6.63±0.74 at T6, p=0.487; placebo group, logUPCR from 6.38±0.53 at T0 to 6.29±0.70 at T6, p=0.190) (Table 4). By the end of the study period, treatment with sulodexide 150 mg daily for 6 months had reduced the geometric mean in UPCR by 33%, as compared to the baseline (p=0.045) and showed a tendency to increase the time-dependent anti-proteinuric effect (Fig. 2). There were no significant differences between either of the sulodexide groups and the placebo group in the change in the logUPCR to any of the follow-up time points (Table 4, Fig. 2).
Table 4.
Effect of Sulodexide on Urine Protein/Creatinine Ratio in the Whole Study Group (Log scale)
*p=0.045 between baseline and week 24 in Sulodexide 150 mg/day group (p=0.190 at week 8 and p=0.487 at week 16 in Sulodexide 150 mg/day group)
Fig. 2.
Changes from baseline in urinary protein-to-creatinine ratio (UPCR) according to study group. The change from the baseline, in the geometric mean, with 95% confidence intervals, is shown for UPCR.
DISCUSSION
We investigated whether sulodexide is able to reduce the protein excretion rate in patients with idiopathic IgA nephropathy who have persistent proteinuria despite treatment with an ACEI or an ARB. This pilot study was the first to evaluate the efficacy of sulodexide in biopsy-proven IgA nephropathy patients. Many previous studies have shown the efficacy of sulodexide in diabetic nephropathy. However, a 6-month treatment with sulodexide did not achieve a 50% reduction of urinary protein excretion in IgA nephropathy patients. Yet the study results indicated a tendency to increase the time-dependent anti-proteinuric effect in the sulodexide 150 mg/day group. Although our findings were incongruent with those previously reported from small explorative investigations with diabetic nephropathy patients, we found that long-term treatment with a high dose of sulodexide might reduce urinary protein excretion in IgA nephropathy patients.
Sulodexide apparently alters glomerular permeability and effectively reduces proteinuria by a non-blood pressure, non-renin angiotensin aldosterone system (RAAS) related mechanism. As a result, sulodexide could add to the therapeutic options for IgA nephropathy patients who fail to respond to RAAS inhibition. Moreover, it may further reduce proteinuria in patients who show a partial response to RAAS inhibition. Such additive effects are very important.
Sulodexide is a preparation of low-molecular weight porcine GAG polysaccharides comprised of fast-moving heparin (80%), and dermatan sulfate (20%) with a mean molecular weight of 11,000-15,000 Da. The drug is absorbed orally8,31 and although it was first evaluated as an anti-thrombotic drug, it has no anticoagulant effect after oral administration.32 The drug has been shown to improve blood flow by lowering viscosity,33,34 to reduce the occurrence of cardiovascular events and to improve vascular disease-associated skin ulcers.35-37
Studies of proteoglycan sulfate incorporation have implied that diminished GAG content is, at least, the result of a diminished rate of biosynthesis of heparan sulfate proteoglycan.38,39 A simple explanation of the efficacy of sulodexide and related compounds is that they restore the anionic heparin sulfate charges on the glomerular basement membrane. Transforming growth factor-β1 (TGF-β1) has emerged as a predominant fibrogenic cytokine, which leads to glomerulosclerosis, interstitial fibrosis, and tubular atrophy in IgA nephropathy. Renal localization of TGF-β1 correlates with the severity of tubulointerstitial damage in patients with IgA nephropathy.40 Therefore, the beneficial effects of GAGs are partially related to downregulation of TGF-β expression.8 Inhibition of heparanase-1 has been proposed as a further nephroprotective mechanism of GAGs.27 Heparanase-1 is an endo-β(1-4)-D-glucuronidase that cleaves the glycosidic bond within the heparin sulfate chain. The GAG sulodexide has been shown to be an inhibitor of heparanse-1, whose activity is tightly regulated to ensure the structural integrity of the glomerular basement membrane (GBM) and to coordinate the release of growth factors.28 Heparanase-1 inhibition leads to the restoration of heparin sulfate levels in podocytes and to the complete blocking of albumin permeability through the GBM in an in vitro assay.27-29 On the other hand, our study was limited in the analysis by the small sample size. One hundred and four patients were enrolled in this study, but the number of patients enrolled (68 per group, making a total of over 204 patients) was considered adequate. Our study reflects the uncertainty of therapeutically successful results. However, the additive anti-proteinuric effect of sulodexide in patients with well-controlled BP and already receiving ACEI or ARB therapy was noteworthy.
Our preliminary study suggests that sulodexide had an additional anti-proteinuric effect for biopsy-proven IgA nephropathy patients who had already been treated with RAS inhibition. Larger-scale clinical trials including a total of over 204 patients are warranted to obtain greater certainty of the anti-proteinuric effects of sulodexide for IgA nephropathy.
ACKNOWLEDGEMENTS
This work was supported by a grant of Asia Pharmaceuticals. Institutional Review Board(IRB)/Ethics Committee approval was obtained.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.D'Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 1987;64:709–727. [PubMed] [Google Scholar]
- 2.Julian BA, Waldo FB, Rifai A, Mestecky J. IgA nephropathy, the most common glomerulonephritis worldwide. A neglected disease in the United States? Am J Med. 1988;84:129–132. doi: 10.1016/0002-9343(88)90019-8. [DOI] [PubMed] [Google Scholar]
- 3.Varis J, Rantala I, Pasternack A, Oksa H, Jantti M, Paunu ES, et al. Immunoglobulin and complement deposition in glomeruli of 756 subjects who had committed suicide or met with a violent death. J Clin Pathol. 1993;46:607–610. doi: 10.1136/jcp.46.7.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schena FP. A retrospective analysis of the natural history of primary IgA nephropathy worldwide. Am J Med. 1990;89:209–215. doi: 10.1016/0002-9343(90)90300-3. [DOI] [PubMed] [Google Scholar]
- 5.Velo M, Lozano L, Egido J, Gutierrez-Millet V, Hernando L. Natural history of IgA nephropathy in patients followed-up for more than ten years in Spain. Semin Nephrol. 1987;7:346–350. [PubMed] [Google Scholar]
- 6.Droz D. Natural history of primary glomerulonephritis with mesangial deposits of IgA. Contrib Nephrol. 1976;2:150–157. [Google Scholar]
- 7.Radford MG, Jr, Donadio JV, Jr, Bergstralh EJ, Grande JP. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. 1997;8:199–207. doi: 10.1681/ASN.V82199. [DOI] [PubMed] [Google Scholar]
- 8.Velussi M, Cernigoi AM, Dapas F, De Monte A. Glycosaminoglycans oral therapy reduces microalbuminuria, blood fibrinogen levels and limb arteriopathy clinical signs in patients with non-insulin dependent diabetes mellitus. Diab Nutr Metab. 1996;9:53–58. [Google Scholar]
- 9.Gambaro G, Venturini AP, Noonan DM, Fries W, Re G, Garbisa S, et al. Treatment with a glycosaminoglycan formulation ameliorates experimental diabetic nephropathy. Kidney Int. 1994;46:797–806. doi: 10.1038/ki.1994.335. [DOI] [PubMed] [Google Scholar]
- 10.Ceol M, Nerlich A, Baggio B, Anglani F, Sauer U, Schleicher E, et al. Increased glomerular alpha 1 (IV) collagen expression and deposition in long-term diabetic rats is prevented by chronic glycosaminoglycan treatment. Lab Invest. 1996;74:484–495. [PubMed] [Google Scholar]
- 11.Ceol M, Gambaro G, Sauer U, Baggio B, Anglani F, Forino M, et al. Glycosaminoglycan therapy prevents TGF-beta1 overexpression and pathologic changes in renal tissue of long-term diabetic rats. J Am Soc Nephrol. 2000;11:2324–2336. doi: 10.1681/ASN.V11122324. [DOI] [PubMed] [Google Scholar]
- 12.Skrha J, Perusicova J, Pont'uch P, Oksa A. Glycosaminoglycan sulodexide decreases albuminuria in diabetic patients. Diabetes Res Clin Pract. 1997;38:25–31. doi: 10.1016/s0168-8227(97)00076-4. [DOI] [PubMed] [Google Scholar]
- 13.Sorrenti G, Grimaldi M, Canova N, Palazzini E, Melchionda N. Glycosaminoglycans as a possible tool for micro- and macroalbuminuria in diabetic patients. A pilot study. J Int Med Res. 1997;25:81–86. doi: 10.1177/030006059702500204. [DOI] [PubMed] [Google Scholar]
- 14.Solini A, Vergnani L, Ricci F, Crepaldi G. Glycosaminoglycans delay the progression of nephropathy in NIDDM. Diabetes Care. 1997;20:819–823. doi: 10.2337/diacare.20.5.819. [DOI] [PubMed] [Google Scholar]
- 15.Gambaro G, Kinalska I, Oksa A, Pont'uch P, Hertlova M, Olsovsky J, et al. Oral sulodexide reduces albuminuria in microalbuminuric and macroalbuminuric type 1 and type 2 diabetic patients: the DiNAS randomized trial. J Am Soc Nephrol. 2002;13:1615–1625. doi: 10.1097/01.asn.0000014254.87188.e5. [DOI] [PubMed] [Google Scholar]
- 16.Szelachowska M, Poplawska A, Topolska J, Kinalska I, Grimaldi M. A pilot study of the effect of the glycosaminoglycan sulodexide on microalbuminuria in type I diabetic patients. Curr Med Res Opin. 1997;13:539–545. doi: 10.1185/03007999709113327. [DOI] [PubMed] [Google Scholar]
- 17.Velussi M. CADFdMA. A randomized, controlled study of sulodexide therapy for the treatment of diabetic nephropathy. Diabetes Nutr Metab. 1996;9:53–58. [Google Scholar]
- 18.Oksa A, Pontuch P, Kratochvilova H. [The effect of glycosaminoglycan sulodexide on albuminuria in patients with diabetes mellitus] Bratisl Lek Listy. 1999;100:486–489. [PubMed] [Google Scholar]
- 19.Dedov I, Shestakova M, Vorontzov A, Palazzini E. A randomized, controlled study of sulodexide therapy for the treatment of diabetic nephropathy. Nephrol Dial Transplant. 1997;12:2295–2300. doi: 10.1093/ndt/12.11.2295. [DOI] [PubMed] [Google Scholar]
- 20.Poplawska A, Szelachowska M, Topolska J, Wysocka-Solowie B, Kinalska I. Effect of glycosaminoglycans on urinary albumin excretion in insulin-dependent diabetic patients with micro- or macroalbuminuria. Diabetes Res Clin Pract. 1997;38:109–114. doi: 10.1016/s0168-8227(97)00096-x. [DOI] [PubMed] [Google Scholar]
- 21.Achour A, Kacem M, Dibej K, Skhiri H, Bouraoui S, El May M. One year course of oral sulodexide in the management of diabetic nephropathy. J Nephrol. 2005;18:568–574. [PubMed] [Google Scholar]
- 22.Gambaro G, Venturini AP, Noonan DM, Fries W, Re G, Garbisa S, et al. Treatment with a glycosaminoglycan formulation ameliorates experimental diabetic nephropathy. Kidney Int. 1994;46:797–806. doi: 10.1038/ki.1994.335. [DOI] [PubMed] [Google Scholar]
- 23.Gambaro G, Cavazzana AO, Luzi P, Piccoli A, Borsatti A, Crepaldi G, et al. Glycosaminoglycans prevent morphological renal alterations and albuminuria in diabetic rats. Kidney Int. 1992;42:285–291. doi: 10.1038/ki.1992.288. [DOI] [PubMed] [Google Scholar]
- 24.Ofosu FA. Pharmacological actions of sulodexide. Semin Thromb Hemost. 1998;24:127–138. doi: 10.1055/s-2007-995831. [DOI] [PubMed] [Google Scholar]
- 25.Nader HB, Buonassisi V, Colburn P, Dietrich CP. Heparin stimulates the synthesis and modifies the sulfation pattern of heparan sulfate proteoglycan from endothelial cells. J Cell Physiol. 1989;140:305–310. doi: 10.1002/jcp.1041400216. [DOI] [PubMed] [Google Scholar]
- 26.Caenazzo C, Garbisa S, Ceol M, Baggio B, Borsatti A, Marchi E, et al. Heparin modulates proliferation and proteoglycan biosynthesis in murine mesangial cells: molecular clues for its activity in nephropathy. Nephrol Dial Transplant. 1995;10:175–184. [PubMed] [Google Scholar]
- 27.Lewis EJ, Xu X. Abnormal glomerular permeability characteristics in diabetic nephropathy: implications for the therapeutic use of low-molecular weight heparin. Diabetes Care. 2008;31(Suppl 2):S202–S207. doi: 10.2337/dc08-s251. [DOI] [PubMed] [Google Scholar]
- 28.Goldshmidt O, Zcharia E, Cohen M, Aingorn H, Cohen I, Nadav L, et al. Heparanase mediates cell adhesion independent of its enzymatic activity. FASEB J. 2003;17:1015–1025. doi: 10.1096/fj.02-0773com. [DOI] [PubMed] [Google Scholar]
- 29.Gambaro G, Kong NC. Glycosaminoglycan treatment in glomerulonephritis? An interesting option to investigate. J Nephrol. 2010;23:244–252. [PubMed] [Google Scholar]
- 30.Donadio JV, Jr, Grande JP. Immunoglobulin A nephropathy: a clinical perspective. J Am Soc Nephrol. 1997;8:1324–1332. doi: 10.1681/ASN.V881324. [DOI] [PubMed] [Google Scholar]
- 31.Callas DD, Hoppensteadt DA, Jeske W, Iqbal O, Bacher P, Ahsan A, et al. Comparative pharmacologic profile of a glycosaminoglycan mixture, Sulodexide, and a chemically modified heparin derivative, Suleparoide. Semin Thromb Hemost. 1993;19(Suppl 1):49–57. [PubMed] [Google Scholar]
- 32.Andriuoli G, Mastacchi R, Barbanti M. Antithrombotic activity of a glycosaminoglycan (sulodexide) in rats. Thromb Res. 1984;34:81–86. doi: 10.1016/0049-3848(84)90108-7. [DOI] [PubMed] [Google Scholar]
- 33.Calabro A, Rossi A, Baiocchi MR, Coscetti G, Fellin R, Crepaldi G. [Effect of sulodexide on hemorheological parameters in a group of patients with peripheral arteriosclerotic vascular disease] Ric Clin Lab. 1985;15(Suppl 1):455–463. [PubMed] [Google Scholar]
- 34.Crepaldi G, Rossi A, Coscetti G, Abbruzzese E, Calveri U, Calabro A. Sulodexide oral administration influences blood viscosity and fibrinolysis. Drugs Exp Clin Res. 1992;18:189–195. [PubMed] [Google Scholar]
- 35.Coccheri S, Scondotto G, Agnelli G, Palazzini E, Zamboni V Arterial Arm of the Suavis (Sulodexide Arterial Venous Italian Study) group. Sulodexide in the treatment of intermittent claudication. Results of a randomized, double-blind, multicentre, placebo-controlled study. Eur Heart J. 2002;23:1057–1065. doi: 10.1053/euhj.2001.3033. [DOI] [PubMed] [Google Scholar]
- 36.Condorelli M, Chiariello M, Dagianti A, Penco M, Dalla Volta S, Pengo V, et al. IPO-V2: a prospective, multicenter, randomized, comparative clinical investigation of the effects of sulodexide in preventing cardiovascular accidents in the first year after acute myocardial infarction. J Am Coll Cardiol. 1994;23:27–34. doi: 10.1016/0735-1097(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 37.Koblik T, Sieradzki J, Sendur R, Biernat J, Czarnobilski K, Gryz E, et al. The effect of insulin and sulodexide (Vessel Due F) on diabetic foot syndrome: pilot study in elderly patients. J Diabetes Complications. 2001;15:69–74. doi: 10.1016/s1056-8727(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 38.Kanwar YS, Farquhar MG. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979;76:1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanwar YS, Rosenzweig LJ, Jakubowski ML. Distribution of de novo synthesized sulfated glycosaminoglycans in the glomerular basement membrane and mesangial matrix. Lab Invest. 1983;49:216–225. [PubMed] [Google Scholar]
- 40.Taniguchi Y, Yorioka N, Masaki T, Asakimori Y, Yamashita K, Yamakido M. Localization of transforming growth factors beta1 and beta2 and epidermal growth factor in IgA nephropathy. Scand J Urol Nephrol. 1999;33:243–247. doi: 10.1080/003655999750015853. [DOI] [PubMed] [Google Scholar]