Abstract
Objective To investigate whether vitamin D supplementation can decrease the mortality and morbidity of low birthweight infants in low income countries.
Design Randomised controlled trial.
Setting Large government hospital in New Delhi, India.
Participants 2079 low birthweight infants born at term (>37 weeks’ gestation).
Main outcome measures Primary outcome was admission to hospital or death during the first six months of life. Main secondary outcome was growth.
Interventions Weekly vitamin D supplements for six months at a dose of one recommended nutrient intake per day (35 µg/week). Infants were visited weekly at home for observed supplementation and were brought to the clinic monthly for clinical examination and anthropometric measurements.
Results Between group differences were not significant for death or hospital admissions (92 among 1039 infants in the vitamin D group v 99 among 1040 infants in the placebo group; adjusted rate ratio 0.93, 95% confidence interval 0.68 to 1.29; P=0.68), or referral to the outpatient clinic for moderate morbidity. Vitamin D supplementation resulted in better vitamin D status as assessed by plasma calcidiol levels at six months. In adjusted analyses, vitamin D treatment significantly increased standard deviation (z) scores at six months for weight, length, and arm circumference and decreased the proportion of children with stunted growth (length for age z score ≤2) or with arm circumference z scores of 2 or less.
Conclusion A weekly dose of vitamin D resulted in better vitamin D status and benefited the classic vitamin D function of bone growth but did not decrease the incidence of severe morbidity or death among young low birthweight infants.
Trial registration ClinicalTrials.gov NCT00415402.
Introduction
Vitamin D is well known for its role in calcium metabolism and bone health.1 In addition, vitamin D is active in the immune system.2 Low plasma levels of calcidiol, the accepted marker of vitamin D status, are associated with increased infectious disease, especially respiratory tract infections, in several populations,3 4 5 including young Indian children.6 Randomised controlled trials of vitamin D supplements to improve disease resistance have shown no therapeutic benefit of vitamin D for African patients with tuberculosis7 and no prophylactic benefit for infections among middle aged American adults8 or older British adults.9 Vitamin D supplements were associated with fewer seasonal influenza infections in Japanese schoolchildren.10 These trials were either small8 10 or carried out among groups where vitamin D status was not measured9 10 or where deficiency was mild.7 8
A low plasma calcidiol level is widespread, even among populations at low latitude. In Delhi, vitamin D deficiency is common among women and children, possibly as a result of limited exposure to sunlight and high smog levels.11 12 Low birth weight is also common in India and these infants are at high risk of respiratory tract infections and other morbidity.13 Low cost interventions such as improving vitamin D status are needed to improve the health and survival of these infants. We hypothesised that vitamin D supplements would decrease mortality and severe morbidity of low birthweight infants born at term in India. We studied this group in part because of their high mortality and in part because the study hospital routinely provides vitamin D supplements to preterm infants and we wanted to know whether to extend the practice to this other group of at risk infants.
Methods
We carried out an individually randomised, double blind, placebo controlled trial of weekly vitamin D supplementation of Indian infants for the first six months of life: the Delhi Infant Vitamin D Supplementation (DIVIDS) study. The study took place from March 2007 to July 2010.
The primary outcome was the rate of hospital admissions or death. Secondary outcomes were anthropometric measurements and vitamin D status at 6 months of age.
Recruitment
Infants born at Safdarjung Hospital, New Delhi were eligible if they were singleton; their gestation was 37 weeks or more, as defined by the last menstrual period; they had a birth weight between 1.8 and 2.5 kg; they were aged less than 48 hours; they lived within a 15 km radius of Safdarjung Hospital; and their parents gave written or thumb print informed consent. Exclusion criteria were severe congenital abnormalities, morbidity severe enough to result in death before age 7 days, intention to live outside the catchment area before the infant reached 6 months of age, or lack of consent. No families were included twice.
Safdarjung Hospital is a large government hospital in South Delhi giving subsidised medical treatment to a low to middle income population. The hospital has 20 000 deliveries per year, with about 20% resulting in low birthweight infants.
Randomisation and masking
A simple randomisation list without blocking was computer generated and held by the data safety and monitoring board only. At enrolment the infants were randomised to receive each week, starting at 7 days of age and continuing to 6 months (maximum of 25 doses), either 35 µg (1400 IU) granulated vitamin D3 (cholecalciferol), which is the Food and Agriculture Organization/World Health Organization recommended nutrient intake of 5 µg (200 IU) per day,14 or an identical appearing and tasting placebo (both prepared by Cadilla Pharmaceuticals, Gujarat, India). The ethics committee did not permit use of a larger vitamin D dose. The data safety and monitoring board individually labelled the sachets containing vitamin D or placebo crystals with the participant identification number. The study team remained blinded to treatment allocation until the primary and growth outcomes had been analysed.
Administering of supplement and compliance
The infants received the supplement or placebo dissolved in a small amount of expressed breast milk (or other milk for rare non-breastfed infants). Hospital nurses trained the mothers in how to express breast milk before discharge. Because breast milk was used for dosing, weekly supplementation began when the infant was 7 days old. Supplements were usually administered directly at the infants’ home by field workers, who recorded compliance. In cases of planned temporary absence from home, the parents received the sachets in advance and compliance was recorded as the number of empty sachets returned. In about 10% of cases, families planned permanent absence and were given the sachets to take with them. For these participants compliance and morbidity were recorded by weekly mobile phone calls; this is reflected in the different completion rates for morbidity and anthropometric measurements.
Participant follow-up
At recruitment at Safdarjung Hospital, we used standardised questionnaires to collect demographic and socioeconomic data of the families and anthropometric measurements of the infants. Fieldworkers recorded infant morbidity at weekly home visits using a pretested questionnaire. They were trained to identify conditions requiring clinical attention and to advise parents to take their infants to hospital.
Parents brought the infants to the hospital at ages 6, 10, 14, 18, 22, and 26 weeks for anthropometric measurements, clinical examination by a doctor, and standard vaccinations according to age. Standardised questionnaires were used to collect information on the overall health of the infants in the past month as well as specific symptoms in the past week. Information was also collected on the infants’ diet, including breast milk, other drinks, foods, or supplements. Exposure to sunlight was estimated as the average number of days outside multiplied by hours per day reported at each clinic visit, and recorded as “usual” exposure.
The parents were encouraged to bring their infants to Safdarjung Hospital whenever ill. Doctors not connected with the study decided whether or not to admit the infants to hospital. Illnesses detected at both scheduled and unscheduled visits were recorded along with information on where the infant was seen (inpatient or outpatient), diagnosis, treatment, duration of hospital stay if applicable, and outcome.
Anthropometric measures
The infants’ weight was measured to within 10 g (Prestige Baby Weighing Scale, HM-008A; Hardik Medi Tech, Delhi, India), length to the nearest 0.1 cm using an infantometer (Sumit Surgicals, Delhi), and head circumference and mid-upper arm circumference to the nearest 0.1 cm using non-stretchable tapes (Chasmors, London). Maternal weight was measured to within 100 g and mid-upper arm circumference to within 0.1 cm. Maternal height was measured at the first scheduled postnatal clinic visit at six weeks. All anthropometric data were collected in duplicate and the mean infant values were converted to z scores using the WHO growth reference.15 As flagged values, indicating low scores, were common in this low birthweight population we corroborated these against paper records and infant growth trajectories. We calculated the technical error of measurement using standard methods about every six months, which was within acceptable ranges.16
Vitamin D status
For logistical reasons blood sampling for vitamin D status at six months began in November 2008. Venous blood (5 mL) was collected in lithium heparin vacutainers and processed within two hours of collection. Plasma was frozen at −80°C until analysis for calcidiol by radioimmunoassay using 25OH Vitamin D Total Assay kits (Diasorin; Stillwater, MN). We used the mean of duplicate assays. The interassay coefficients of variation for both high and low control sera were 7% and values for external standards were within acceptable ranges. We classified vitamin D status as adequate (>50 nmol/L), mildly deficient (25-50 nmol/L), or severely deficient (<25 nmol/L).17
Statistical analyses
Data were double entered in Microsoft Access and cross checked before importing into Stata 11.1 for analysis. The primary outcome—incidence of death or hospital admission—in all children (intention to treat) was analysed by treatment group using Weibull regression with gamma shared frailty to account for multiple admissions within child. To avoid repeat counting of the same illness we considered infants to be at risk again three days after discharge from a previous admission for severe morbidity.
Secondary outcomes of the study were differences in vitamin D status and in weight for age, length for age, mid-upper arm circumference, and head circumference for age z scores, all measured at 6 months of age. We also analysed as a binary variable the occurrence of any admission to hospital or death. Linear regression was used to analyse these outcomes.
We defined any severe morbidity as death, hospital admission, or outpatient visits with diagnoses selected based on clinical judgment that represented severe illness: pneumonia, persistent diarrhoea, dysentery, severe fever, severe protein energy malnutrition, ear infections, meningitis, and septicaemia. We analysed the incidence of severe morbidities as for the primary outcome using Weibull regression with gamma shared frailty, again requiring a gap of at least three days between events.
Initial analyses compared the crude effect of vitamin D supplementation with placebo. Baseline characteristics were then assessed for their association with loss to follow-up. We then carried out analyses adjusting for all characteristics associated with missing data (assuming data were missing at random) and additionally adjusted for infants’ sex, quintiles of socioeconomic status, and quintiles of exposure to sunlight. We also assessed the effect of season—summer (April to June), monsoon (July to September), and winter (October to March)—for its relation with incidence rates. Socioeconomic status was generated using principal component analysis18 of household demographics, housing materials, and material possessions. As the true dates for stopping breast feeding were not known, we used Kaplan-Meier estimates to obtain the median durations of both exclusive (breast milk and nothing else) and predominant (breast milk plus non-nutritive liquids only) breast feeding, by using the midpoint between the dates the infant was known to have still been breast feeding exclusively or predominantly and was known to have stopped this feeding pattern.
Sample size
The sample size of 1000 per treatment arm was based on estimates of mortality from two studies in Delhi: a hospital audit showing a neonatal mortality rate of 4.5% of live births, with low birthweight infants contributing 84.5% of the deaths,19 and a controlled trial showing a mortality rate of 2.6% between 1 and 9 months of age in the control group.20 A hospital admission rate of 20% within the first six months of life was estimated from a study of low birthweight term infants in Brazil.21 We estimated the combined rate of mortality (about 6% based on the Delhi data) or admission to hospital (20% based on the Brazilian data) before 6 months of age in the placebo group of the study population as 26%. At a 5% significance level, we needed 903 infants per treatment arm to detect a 25% reduction in the combined rate of mortality and admission to hospital with 90% power. We allowed for 10% loss to follow-up and aimed to recruit 1000 infants per treatment group.
Results
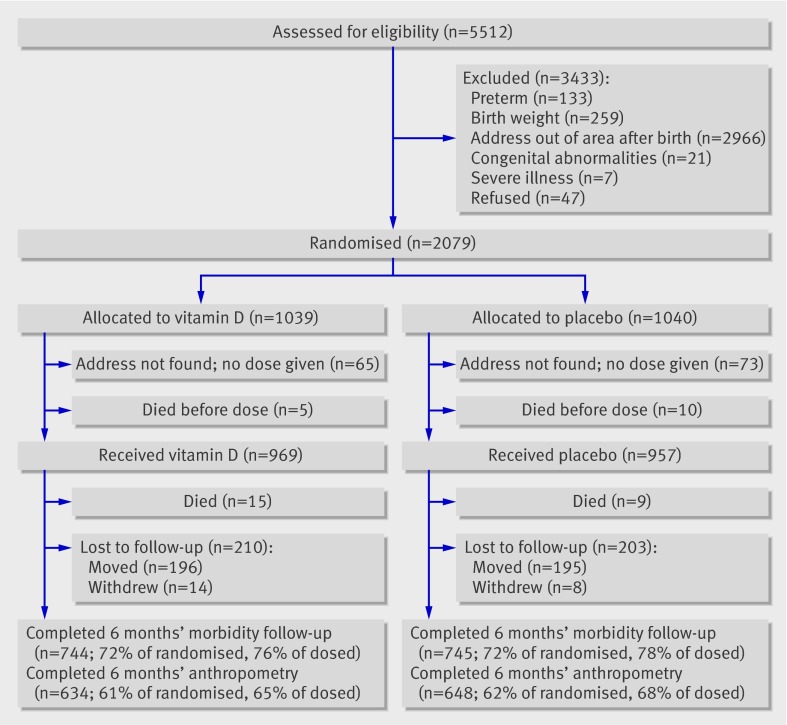
Figure 1 shows the flow of participants through the study. The addresses of 138 of 2079 (6.6%) infants allocated to treatment were not found and the parents could not be contacted as the poorest families lacked mobile phones. A further 413 (19.9%) infants were lost to follow-up, primarily because many from outlying rural areas returned to their homes after obtaining healthcare despite saying at recruitment that they intended to stay in the area for at least six months. Two hundred and seven (10%) infants were additionally lost to follow-up for anthropometric data, but morbidity and visits to clinics or hospital were collected by weekly mobile phone calls so that data were available for the primary outcome for 72% of those initially randomised but anthropometric data for only 62%. Losses to follow-up were biased to poorer families; those lost to morbidity follow-up were more likely to be from smaller and nuclear families, to have less well educated parents, and to be from the lowest quintiles for socioeconomic status (see web extra table 1). Similar results were found for those missing anthropometric data at follow-up. A larger number had missing results for plasma calcidiol levels owing to the late start of blood collection and refusals; factors associated with missing values were low quintile for socioeconomic status, low maternal education, low paternal education, and nuclear family type.
Fig 1 Flow of participants through study
Participants in the two treatment arms were similar at baseline (table 1). As inclusion criteria included an absolute birthweight cut-off point, the groups comprised more girls than boys. The population was fairly poor, with low education, low incomes, and crowded homes. Breast feeding was almost universal: the median duration of exclusive breast feeding was 15 weeks and of predominant breast feeding was 20 weeks and was similar in both trial arms (P values for log rank tests of differences were 0.58 for exclusive breast feeding and 0.50 for predominant breast feeding). The median reported exposure to sunlight in a four week period was 10.8 hours (interquartile range 3.8 to 25.9), and was similar in both trial arms. The proportion of infants receiving at least 23 doses was similar in both trial arms: 748/1039 (72%) in the vitamin D arm and 760/1040 (73%) in the placebo arm.
Table 1.
Characteristics of infants receiving vitamin D supplementation or placebo. Values are numbers (percentages) unless stated otherwise
| Characteristics | Vitamin D group (n=1039) | Placebo group (n=1040) |
|---|---|---|
| Girls | 551 (53) | 558 (54) |
| Mean (SD) birth weight (kg) | 2.2 (0.2) | 2.2 (0.2) |
| Mean (SD) maternal age (years) | 23.5 (3.3) | 23.5 (3.5) |
| Mean (SD) maternal body mass index* | 21.2 (3.0) | 21.0 (2.9) |
| Religion: | ||
| Hindu | 913 (88) | 915 (88) |
| Muslim | 108 (10) | 103 (10) |
| Other | 18 (2) | 22 (2) |
| Family type: | ||
| Nuclear | 462 (44.5) | 466 (45) |
| Joint | 379 (36.5) | 376 (36) |
| Extended | 198 (19) | 198 (19) |
| Family size: | ||
| 3-5 | 576 (55.5) | 564 (54) |
| 6-9 | 354 (34) | 361 (35) |
| >10 | 109 (10.5) | 115 (11) |
| Mean (SD) average No of family members | 5.9 (0.9) | 5.9 (0.9) |
| Maternal education: | ||
| None | 224 (22) | 221 (21) |
| Primary | 460 (44) | 478 (46) |
| Secondary | 283 (27) | 281 (27) |
| College or university | 72 (7) | 60 (6) |
| Paternal education: | ||
| None | 105 (10) | 103 (10) |
| Primary | 403 (39) | 450 (43) |
| Secondary | 419 (40) | 394 (38) |
| College or university | 112 (11) | 93 (9) |
| Paternal occupation: | ||
| Unemployed or student | 28 (3) | 26 (2.5) |
| Employed | 917 (88) | 910 (87.5) |
| Self employed | 94 (9) | 104 (10) |
| Maternal occupation: | ||
| Employed | 27 (3) | 28 (3) |
| Unemployed or housewife | 1012 (97) | 1012 (97) |
| Asset index score (quintiles): | ||
| Lowest | 213 (20.5) | 203 (20) |
| Low | 214 (21) | 202 (19) |
| Middle | 208 (20) | 208 (20) |
| High | 191 (18) | 225 (22) |
| Highest | 213 (20.5) | 202 (19) |
*Available for only 479 women in vitamin D arm and 469 women in placebo arm.
Infants in the vitamin D treatment group had significantly higher plasma calcidiol levels at six months (table 2); crude mean difference 19.00 nmol/L (95% confidence interval 14.7 to 23.5; P<0.001). After adjustment for sunlight exposure and for factors associated with not having a result for calcidiol, the adjusted mean difference was 18.7 nmol/L (14.2 to 23.5; P<0.001). In the vitamin D group fewer infants had severe vitamin D deficiency (<25 nmol/L); nevertheless, almost half the infants in this group did not have adequate vitamin D status (>50 nmol/L) despite 210/219 (93%) receiving 23 or more doses of vitamin D. As rickets becomes more apparent once children start to walk, only one child, in the placebo group, was found to have bone pain but no other symptoms of rickets on examination at six months.
Table 2.
Effect of vitamin D supplementation on plasma calcidiol* levels at six months. Values are numbers (percentages) unless stated otherwise
| Variables | Vitamin D group (n=216) | Placebo group (n=237) | P value |
|---|---|---|---|
| Mean (SD) calcidiol level (nmol/L) | 55.0 (22.5) | 36.0 (25.5) | <0.001 |
| Type of deficiency: | |||
| Severe (<25 nmol/L) | 18 (8) | 92 (39) | <0.001 |
| Mild (10-20 nmol/L) | 76 (35) | 82 (35) | |
| Adequate (>50 nmol/L) | 122 (57) | 63 (27) |
*25-hydroxyvitamin D.
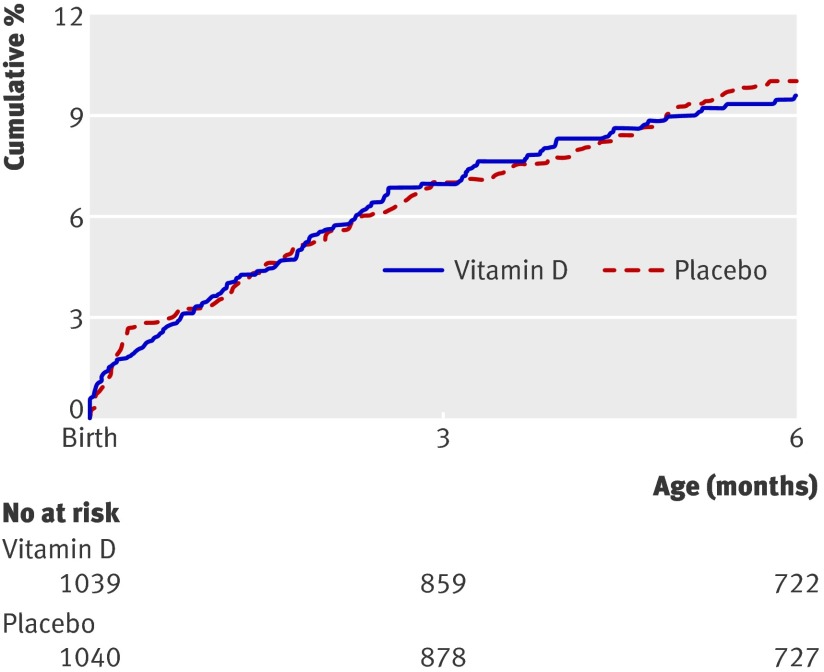
Figure 2 shows the cumulative incidence of hospital admission or death. Twenty deaths occurred in the vitamin D arm and 19 in the placebo arm but 15 were before any supplementation (fig 1). Causes of death for those dying after receiving supplements were based on hospital records (n=8), or on verbal autopsy for those dying at home (n=16): pneumonia (n=7), septicaemia (n=6), diarrhoea (n=4), fever (n=2), not feeding (n=1), and unknown (n=4). Causes of death were known for only two (one septicaemia and one pneumonia) of the 15 infants who died before supplementation. Rates of death and admission to hospital did not differ between groups in either unadjusted or adjusted analyses (table 3). Vitamin D had no significant effects on mortality alone or on any severe morbidity in either unadjusted or adjusted analyses. Similar effect estimates were obtained when the analyses were repeated excluding the 153 children who never received a dose of vitamin D or placebo (see web extra table 2). For the binary analysis of any hospital admission or death, the unadjusted odds ratio was 0.92 (95% confidence interval 0.67 to 1.26; P=0.59) and the odds ratio adjusted for sex, quintile of socioeconomic status, family type, and maternal education was 0.91 (95% confidence interval 0.66 to 1.26; P=0.57).
Fig 2 Kaplan-Meier plot of time to admission to hospital or death of infants receiving vitamin D supplementation or placebo
Table 3.
Incidence of admissions to hospital and death among infants up to 6 months of age and rate ratio of effect of vitamin D supplementation
| Variables | Incidence rate* (95% CI) | Unadjusted rate ratio (95% CI) | P value | Adjusted† rate ratio (95% CI) | P value | Adjusted‡ rate ratio (95% CI) | P value | |
|---|---|---|---|---|---|---|---|---|
| Vitamin D group (n=1039) | Placebo group (n=1040) | |||||||
| Hospital admission or death: | ||||||||
| No of events/No of children | 92/85 | 99/88 | — | — | — | — | — | — |
| Rate/child year | 0.22 (0.18 to 0.27) | 0.23 (0.19 to 0.29) | 0.93 (0.68 to 1.27) | 0.64 | 0.93 (0.68 to 1.27) | 0.63 | 0.98 (0.70 to 1.38) | 0.92 |
| Death: | ||||||||
| No of children | 20 | 19 | — | — | — | — | — | — |
| Rate/child year | 0.048 (0.031 to 0.077) | 0.045 (0.029 to 0.074) | 1.05 (0.56 to 1.98) | 0.87 | 1.05 (0.56 to 1.97) | 0.88 | 1.97 (0.74 to 5.28) | 0.18 |
| Any severe morbidity: | ||||||||
| No of events/No of children | 174/147 | 207/177 | — | — | — | — | — | — |
| Rate/child year | 0.41 (0.35 to 0.49) | 0.49 (0.42 to 0.57) | 0.84 (0.67 to 1.06) | 0.14 | 0.84 (0.67 to 1.05) | 0.12 | 0.87 (0.69 to 1.09) | 0.23 |
*Total person time in vitamin D group was 419 child years and in placebo group it was 422 child years.
†Adjusted for sex, quintile of socioeconomic status, family type, and maternal education.
‡Adjusted for sex, quintile of socioeconomic status, family type, maternal education, exclusive breastfeeding status, exposure to sunlight, and season: n=930 in vitamin D arm and n=881 in placebo arm.
Vitamin D supplementation was associated with borderline increases in z scores for length, weight, and arm circumference and a significant decrease in head circumference (table 4). After adjustment for infant sex, socioeconomic status, and factors associated with missing data, the strength of the benefits of vitamin D for length, weight, and arm circumference was increased, whereas the negative effect on head circumference was decreased. Similar results were obtained when the anthropometric measures were analysed as proportion with z scores of 2 or less.
Table 4.
Effect of vitamin D supplementation on anthropometric z scores at age 6 months in infants receiving vitamin D supplementation or placebo. Values are means (standard deviations), number in group, unless stated otherwise
| Variables | Vitamin D group | Placebo group | Unadjusted difference (95% CI) | P value | Adjusted difference* (95% CI) | P value |
| Weight | −1.51 (0.98), n=627 | −1.60 (0.98), n=646 | 0.09 (−0.02 to 0.20) | 0.096 | 0.12 (0.01 to 0.22) | 0.026 |
| Length | −1.84 (0.98), n=620 | −1.95 (0.99), n=638 | 0.11 (−0.001 to 0.022) | 0.053 | 0.12 (0.02 to 0.21) | 0.014 |
| Weight/length | −0.34 (1.17), n=620 | −0.36 (1.16), n=637 | 0.02 (−0.11 to 0.14) | 0.811 | 0.03 (−0.09 to 0.16) | 0.615 |
| Head circumference | −0.91 (0.94), n=617 | −0.77 (0.95), n=642 | −0.14 (−0.25 to −0.04) | 0.008 | −0.08 (−0.17 to 0.01) | 0.080 |
| Arm circumference | −0.49 (0.91), n=622 | −0.59 (0.95), n=641 | 0.10 (−0.002 to 0.20) | 0.055 | 0.11 (0.01 to 0.21) | 0.033 |
| No (%) with z scores ≤2: | ||||||
| Weight | 190 (30) | 206 (32) | 0.93 (0.73 to 1.18)† | 0.541 | 0.87 (0.68 to 1.12)‡ | 0.270 |
| Length | 270 (44) | 310 (49) | 0.82 (0.65 to 1.02)† | 0.073 | 0.73 (0.57 to 0.95)‡ | 0.018 |
| Weight/length | 44 (7) | 36 (6) | 1.28 (0.81 to 2.01)† | 0.294 | 1.16 (0.72 to 1.86)‡ | 0.546 |
| Head circumference | 66 (11) | 49 (8) | 1.45 (0.98 to 2.14)† | 0.059 | 1.29 (0.85 to 1.97)‡ | 0.231 |
| Arm circumference | 25 (4) | 43 (7) | 0.58 (0.35 to 0.97)† | 0.034 | 0.55 (0.33 to 0.93)‡ | 0.026 |
*Adjusted for baseline anthropometric z score (except for arm circumference where birth arm circumference was used instead), sex, quintile of socioeconomic status, family type, maternal education, exposure to sunlight, and breast feeding for more than six months.
†Unadjusted odds ratio (95% CI).
‡Adjusted odds ratio (95% CI).
Discussion
In this severely vitamin D deficient population, weekly supplementation with the recommended daily allowance of vitamin D3 resulted in higher mean plasma calcidiol levels and a lower proportion of infants severely deficient at 6 months of age compared with infants given placebo. Vitamin D treatment benefited the classic vitamin D function of bone growth, as evidenced by greater length. However, supplementation did not significantly benefit the disease resistance functions of vitamin D as shown by the lack of difference between groups in death or severe or moderate morbidity.
Strengths and limitations of the study
The study has several strengths, including its large sample size, its successful blinding, its high and well documented compliance, its well balanced treatment groups, and that it was carried out in a population with documented poor vitamin D status, which improved at the end of the trial in the vitamin D group compared with the placebo group. The study’s main limitation was the large loss to follow-up, but controlling for factors associated with missing data did not alter the results. In addition, although the death rate was similar to that used in the calculations of sample size, inpatient admissions were much lower so power was reduced and the confidence interval was wide and included the original estimate of a 25% reduction in the primary outcome. We estimate that, with the observed rate for the primary outcome in the placebo group and the observed loss to follow-up, we would have needed to recruit 1500 infants per group to detect the planned 25% reduction in mortality plus admission to hospital. We increased the number of events in the secondary analysis which included outpatient visits for severe illness; many ill children were not admitted overnight in part for logistical reasons such as lack of beds in this crowded hospital. Even with this larger number of events, vitamin D supplementation was associated with no significant benefit. This, together with the near superimposition of the Kaplan-Meier curves for rates of hospital admission or death in the two groups, suggests that it would take a large sample size in this population to detect a statistically significant benefit of vitamin D supplementation that is unlikely to be of biological importance.
Effects on anthropometric measures
The significant benefit of vitamin D supplements for these low birthweight infants on stunting, underweight, and low arm circumference is an important finding. Stunting is common in many low income populations, starts early in life, and is largely irreversible.22 Stunting has also proved difficult to prevent, possibly because of the large number of related nutritional, infectious, social, and environmental factors.
The borderline negative effect of vitamin D on head circumference is difficult to explain, especially when other anthropometric measures were increased. Although it is possible that vitamin D promoted long bone growth at the expense of head and brain development, this seems unlikely given that maternal vitamin D status was positively associated with head circumference in a prospective study23 and that the limited evidence on vitamin D and brain functions indicates that it is deficient, not supplemental, vitamin D that may be a problem.24 Therefore, we conclude that the slightly lower head circumference was a chance finding and that we have no evidence of adverse effects of vitamin D.
Comparisons with other studies
In spite of the many benefits of vitamin D for immune functions measured in vitro,2 and the benefits for all cause mortality in mainly elderly participants recruited to trials of vitamin D for other outcomes,25 clinical trials of vitamin D for the prevention or treatment of infectious disease have shown little benefit. A recently published trial among young Afghan children26 found a benefit for prevention of recurrent pneumonia of a single large dose of vitamin D. Vitamin D status was not measured, although previous work in the area showed it was likely to be poor, and the improvement in vitamin D status after supplementation was not measured. Trials showing no effect of vitamin D supplementation on infections were done in mildly vitamin D deficient populations.7 8 9 Our study extends current knowledge by showing a lack of benefit among a population where severe vitamin D deficiency was documented as highly prevalent and where supplements resulted in higher vitamin D status.
Potential reasons for different effects on primary and secondary outcomes
It is possible that different amounts of vitamin D are required for optimisation of different vitamin D dependent functions. Evidence shows that higher doses of vitamin D are needed to improve responses to tuberculosis than are needed for prevention of bone problems, the basis for current recommended daily allowances.27 This could explain why the one recommended daily allowance given to the infants in our study improved growth but did not significantly affect morbidity and mortality. It is also possible that a larger dose of vitamin D, such as used in the Afghan trial,26 would have had even greater benefits on both growth and morbidity. It was notable that the one recommended nutrient intake dose left almost half the supplemented infants with inadequate status at six months. We were not permitted by the ethics committee to use a larger dose.
It is also possible that breast feeding, almost universal in our cohort, protected the infants against most severe infections, which is why the benefits of vitamin D were not seen. This is one difference between our study and the Afghan study26 since the children in that study were older and recruited at about the same age as median breastfeeding cessation; furthermore, about 10% of their children had never breast fed.
Conclusions
Vitamin D supplements of one recommended nutrient intake improved growth in this vitamin D deficient cohort of low birthweight term infants but did not decrease mortality and severe morbidity, although the large loss to follow-up reduced power for this outcome. Infant vitamin D status depends on maternal status, particularly during pregnancy.28 The low status we found in the infants suggests that mothers were also deficient and it would be worth investigating the benefits for both maternal and infant health of providing vitamin D supplements during pregnancy.29 Improving the vitamin D status of such infants, through supplementation or increased exposure of mothers or infants to sunlight, might help reduce the prevalence of stunting and its adverse short and long term consequences. Trials should also investigate higher doses of vitamin D to determine whether its benefits can be further increased.
What is already known on this topic
Poor vitamin D status has been associated with increased morbidity and mortality among several populations
Animal model and cell culture data provide potential mechanisms whereby improving vitamin D status could improve immune function
What this study adds
Providing low birthweight term Indian infants with supplementation with the recommended daily intake of vitamin D had no significant effect on morbidity or mortality despite improving vitamin D status and length growth
We thank the infants and their families for their participation; the field staff (Praveen Kumar, Aparna Srivastava, Bunty, Sanjeev, and Dinesh) for their work; R N Salhan (Safdarjung Hospital) and the data safety and monitoring board (Siddharth Ramji, Shinjini Bhatnagar, and R M Pandey) for their help with the trial; Ann Prentice and her team for helping set up the vitamin D assay in our laboratory; the Department of Biotechnology, Ministry of Science and Technology, Government of India; Nutrition Third World; and Sight and Life for funding the study; and Cadilla Pharmaceuticals, Gujarat, India, for preparing the vitamin D and placebo sachets.
Contributors: GTK and SF were coprincipal investigators of the study, together supervised all aspects of the work, and drafted the manuscript; they are guarantors of the study. HSS and HC were medical supervisors and contributed to data interpretation and writing. AMR was project statistician. VS and HA carried out the field work and contributed to the writing.
Funding: This study was funded by the Department of Biotechnology, Ministry of Science and Technology, Government of India; Nutrition Third World; and Sight and Life. None of the funding sources was involved in any aspect of the study design, conduct, analysis, or interpretation.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by the Institute of Home Economics (University of Delhi), Safdarjung Hospital, Sitaram Bhartia Institute of Science and Research, and the London School of Hygiene and Tropical Medicine. The data were reviewed annually by the data safety and monitoring board.
Data sharing: No additional data available.
Cite this as: BMJ 2011;342:d2975
Web Extra. Extra material supplied by the author
Characteristics of study population completing morbidity follow-up and those lost to follow-up; per protocol analysis of admissions to hospital and death
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. [DOI] [PubMed] [Google Scholar]
- 2.Van Etten E, Stoffels K, Gysemans C, Mathieu C, Overbergh L. Regulation of vitamin D homeostasis: implications for the immune system. Nutr Rev 2008;66(10 suppl 2):S125-34. [DOI] [PubMed] [Google Scholar]
- 3.Roth DE, Shah R, Black RE, Baqui AH. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr 2010;99:389-93. [DOI] [PubMed] [Google Scholar]
- 4.Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamaki H, et al. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr 2007;86:714-7. [DOI] [PubMed] [Google Scholar]
- 5.Gibney KB, MacGregor L, Leder K, Torresi J, Marshall C, Ebeling PR, et al. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis 2008;46:443-6. [DOI] [PubMed] [Google Scholar]
- 6.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr 2004;58:563-7. [DOI] [PubMed] [Google Scholar]
- 7.Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2009;179:843-50. [DOI] [PubMed] [Google Scholar]
- 8.Li-Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect 2009;137:1396-404. [DOI] [PubMed] [Google Scholar]
- 9.Avenell A, Cook JA, Maclennan GS, Macpherson GC. Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing 2007;36:574-7. [DOI] [PubMed] [Google Scholar]
- 10.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 2010;91:1255-60. [DOI] [PubMed] [Google Scholar]
- 11.Goswami R, Gupta N, Goswami D, Marwaha RK, Tandon N, Kochupillai N. Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in Delhi. Am J Clin Nutr 2000;72:472-5. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Mawer EB, Puliyel JM. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Arch Dis Child 2002;87:111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neonatal Mortality Formative Research Working Group. Developing community-based intervention strategies to save newborn lives: lessons learned from formative research in five countries. J Perinatol 2008;28(suppl 2):S2-8. [DOI] [PubMed] [Google Scholar]
- 14.Food and Agriculture Organization, World Health Organization. Expert consultation on human vitamin and mineral requirements. FAO/WHO, 2002.
- 15.World Health Organization. Child growth standards. 2006. www.who.int/childgrowth/software/en/.
- 16.Ulijaszek S, Kerr D. Anthropometric measurement error and the assessment of nutritional status. Br J Nutr 1999;82:165-77. [DOI] [PubMed] [Google Scholar]
- 17.Lucas RM, Ponsonby AL, Pasco JA, Morley R. Future health implications of prenatal and early-life vitamin D status. Nutr Rev 2008;66:710-20. [DOI] [PubMed] [Google Scholar]
- 18.Filmer D, Pritchett L. Estimating wealth effects without expenditure data—or tears: an application to educational enrolments in states of India. Demography 2001;38:115-32. [DOI] [PubMed] [Google Scholar]
- 19.Sarna MS, Saili A, Dutta AK, Kumari S. Neonatal mortality patterns in an urban hospital. Indian Pediatr 1991;28:719-24. [PubMed] [Google Scholar]
- 20.Sazawal S, Black RE, Menon VP, Dinghra P, Caulfield LE, Dhingra U, et al. Zinc supplementation in infants born small for gestational age reduces mortality: a prospective, randomized, controlled trial. Pediatrics 2001;108:1280-6. [DOI] [PubMed] [Google Scholar]
- 21.Lira PI, Ashworth A, Morris SS. Effect of zinc supplementation on the morbidity, immune function, and growth of low-birth-weight, full-term infants in northeast Brazil. Am J Clin Nutr 1998;68(suppl 2):418-24S. [DOI] [PubMed] [Google Scholar]
- 22.Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 2010;125:e473-80. [DOI] [PubMed] [Google Scholar]
- 23.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr 2008;62:68-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J 2008;22:982-1001. [DOI] [PubMed] [Google Scholar]
- 25.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 2007;167:1730-7. [DOI] [PubMed] [Google Scholar]
- 26.Manaseki-Holland S, Qader G, Isaq Masher M, Bruce J, Zulf Mughal M, Chandramohan D, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health 2010;15:1148-55. [DOI] [PubMed] [Google Scholar]
- 27.Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet 2011;377:242-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacs CS. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. Am J Clin Nutr 2008;88:520-8S. [DOI] [PubMed] [Google Scholar]
- 29.Sahu M, Das V, Aggarwal A, Rawat V, Saxena P, Bhatia V. Vitamin D replacement in pregnant women in rural north India: a pilot study. Eur J Clin Nutr 2009;63:1157-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of study population completing morbidity follow-up and those lost to follow-up; per protocol analysis of admissions to hospital and death