ABSTRACT
Streptococcal surface dehydrogenase (SDH) (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) is an anchorless major multifunctional surface protein in group A Streptococcus (GAS) with the ability to bind important mammalian proteins, including plasmin(ogen). Although several biological properties of SDH are suggestive of its possible role in GAS virulence, its direct role in GAS pathogenesis has not been ascertained because it is essential for GAS survival. Thus, it has remained enigmatic as to “how and why” SDH/GAPDH is exported onto the bacterial surface. The present investigation highlights “why” SDH is exported onto the GAS surface. Differential microarray-based genome-wide transcript abundance analysis was carried out using a specific mutant, which was created by inserting a hydrophobic tail at the C-terminal end of SDH (M1-SDHHBtail) and thus preventing its exportation onto the GAS surface. This analysis revealed downregulation of the majority of genes involved in GAS virulence and genes belonging to carbohydrate and amino acid metabolism and upregulation of those related to lipid metabolism. The complete attenuation of this mutant for virulence in the mouse model and the decreased and increased virulence of the wild-type and mutant strains postcomplementation with SDHHBtail and SDH, respectively, indicated that the SDH surface export indeed regulates GAS virulence. M1-SDHHBtail also displayed unaltered growth patterns, increased intracellular ATP concentration and Hpr double phosphorylation, and significantly reduced pH tolerance, streptolysin S, and SpeB activities. These phenotypic and physiological changes observed in the mutant despite the unaltered expression levels of established transcriptional regulators further highlight the fact that SDH interfaces with many regulators and its surface exportation is essential for GAS virulence.
IMPORTANCE
Streptococcal surface dehydrogenase (SDH), a classical anchorless cytoplasmically localized glycolytic enzyme, is exported onto the group A Streptococcus (GAS) surface through a hitherto unknown mechanism(s). It has not been known why GAS or other prokaryotes should export this protein onto the surface. By genetic manipulations, we created a novel GAS mutant strain expressing SDH with a 12-amino-acid hydrophobic tail at its C-terminal end and thus were able to prevent its surface exportation without altering its enzymatic activity or growth pattern. Interestingly, the mutant was completely attenuated for virulence in a mouse peritonitis model. The global gene expression profiles of this mutant reveal that the surface exportation of SDH is mandatory to maintain GAS virulence. The ability of GAS as a successful pathogen to localize SDH in the cytoplasm as well as on the surface is physiologically relevant and dynamically obligatory to fine-tune the functions of many transcriptional regulators and also to exploit its virulence properties for infection.
Introduction
Streptococcus pyogenes (group A Streptococcus [GAS]) is the human pathogen that causes the widest variety of diseases, ranging from mild pharyngitis and impetigo to severe and often fatal toxic shock syndrome, and it also causes autoimmune heart and kidney diseases as poststreptococcal sequelae (1). Although GAS is known to cause primarily localized, noninvasive, and mild infections, the invasive and more-severe GAS infections are not uncommon as there are 10,000 cases of invasive GAS disease in the United States and over 500,000 GAS infection-related deaths per year worldwide (2). Despite the availability of sequence information for several GAS genomes and detailed characterization of their virulence factors, the pathogenic mechanisms of GAS still remain elusive (1). Therefore, elucidation of precise mechanisms underlying GAS pathogenesis is expected to facilitate development of effective therapeutics against S. pyogenes.
GAS has developed complex gene regulatory networks to sense and respond to subtle environmental changes (3–5) in order to survive in their intricate and continuously changing habitats within the human body and mucosal surfaces. Regulation of virulence genes and GAS pathogenesis are primarily dependent on these networks (4, 6–8). Many of the genes belonging to these networks also interface with GAS metabolism or nutrient transport and regulation of catabolic-controlled protein (CcpA) genes (5, 9–11).
The glycolytic pathway is a universal and conserved metabolic pathway through which two energy-rich ATPs are produced from one glucose molecule. GAS metabolizes glucose and derives energy from the glycolytic pathway for its growth and survival. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is the key glycolytic enzyme that catalyzes the oxidation and phosphorylation of glyceraldehyde-3-phosphate to an energy-rich intermediate, 1,3-bisphosphoglycerate, in the presence of NAD as a cofactor. Typically, GAPDH is a cytoplasmic protein lacking a signal sequence or a hydrophobic anchor. Since our first report on the identification of GAPDH as a major surface protein of S. pyogenes (and hence termed the streptococcal surface GAPDH or streptococcal surface dehydrogenase [SDH]/Plr/SPy0274) (12), several reports have demonstrated that GAPDH is either expressed on the cell surface or secreted in numerous Gram-positive and Gram-negative bacteria, fungi, and parasites, including a bioterror agent, Bacillus anthracis (13–15). SDH has been categorized as an anchorless bacterial surface protein (12, 14). Various nonglycolytic functions of SDH, such as auto-ADP-ribosylation (16), ability to bind to mammalian structural proteins (fibronectin, laminin, myosin, actin, lysozyme) (12) and to proteins belonging to the human fibrinolytic system (plasmin [12, 17, 18] and uPAR [19]), and ability to regulate host cell signaling (20) indicate that SDH plays an important role in GAS virulence. Similarly, the ability of Listeria monocytogenes GAPDH to ADP-ribosylate Rab5a and subsequently impair host phagolysosome (21) and the recently reported antiphagocytic activity of surface GAPDH/SDH (22) indicate that GAS SDH/GAPDH can indeed serve as a virulence factor. Moreover, the ability of anti-SDH/GAPDH antibody to mediate opsonophagocytosis of GAS (19) and to provide protection against GAS challenge (V. Pancholi, unpublished report), in conjunction with similar reports on other pathogens such as Brucella abortus (23), Edwardsiella tarda, (24), Streptococcus agalactiae (25), and B. anthracis (15), indicate that GAPDH in general can serve as a potential vaccine target.
However, it is still unclear (i) how and why SDH/GAPDH is exported onto the bacterial surface, and (ii) how SDH participates in GAS pathogenesis. SDH is encoded by a single gene and hence, being indispensable, our several attempts to create a GAS mutant lacking SDH were unsuccessful. Previously, by exploiting a novel strategy, we created a GAS mutant (M1-SDHHBtail) expressing SDH with a hydrophobic tail at its C terminus (18) that did not export SDH (SDHHBtail) onto the cell surface and was retained exclusively within the cytoplasm. The growth pattern and GAPDH activity of this mutant were comparable to those of the wild-type strain. Interestingly, the mutant demonstrated impaired antiphagocytic activity and reduced ability to adhere to human pharyngeal cells (18), which are considered the two major determinants of virulence in GAS. These findings thus strongly indicate that there is functional relevance for the exportation of anchorless surface SDH onto the GAS cell surface. The present study, therefore, was carried out to address why SDH/GAPDH is exported onto GAS and other bacterial surfaces and to elucidate its role in GAS pathogenesis.
RESULTS
Prevention of SDH export in the M1-SDHHBtail mutant leads to substantial changes in gene regulation.
To determine the effect of inhibition of surface exportation of SDH, microarray analysis was performed employing the wild-type M1-SF370 (M1-WT) and isogenic mutant (M1-SDHHBtail) GAS strains grown to late log phase. The global gene expression profile of M1-SDHHBtail in comparison to M1-WT revealed significant (≥2 fold; P < 0.05) changes in the transcript abundance of 192 (10.6%) of the total reported genes in the M1-SF370 genome (26). Among the differentially regulated genes in M1-SDHHBtail, 128 genes (66.7%) were downregulated, while the other 64 genes (33.3%) were upregulated (Table 1). Notably, about 46% of the differentially expressed genes (89 of 192 genes) were related to bacterial metabolism (Table 1), most of which belonged to carbohydrate metabolism (37 genes), followed by energy production and conversion (14 genes), lipid metabolism (12 genes), and amino acid transport and metabolism (11 genes). Except for the genes involved in lipid and nucleotide metabolism, a substantial decrease in the transcript abundance was observed for the genes belonging to general metabolism and nutrient transport. Additionally, 18 (9.37%) of the total 192 genes displaying differential regulation were found to be associated with the virulence. Transcript abundance of ~72.2% (13/18 genes) of these genes was found to be significantly decreased, indicating that the prevention of the SDH exportation or accumulation of SDH within the cytoplasm (18) adversely affects streptococcal metabolism and virulence. Apart from validating the data obtained from microarray analysis with real-time quantitative reverse transcription-PCR (qRT-PCR) (see Table S3 and Table S4 in the supplemental material), we also studied several corresponding biochemical functions to establish a functional correlation with the observed transcript abundance.
TABLE 1 .
Microarray-based global gene expression profiles for the M1-SDHHBtail mutant strain versus the wild-type M1-SF370 strain showing the number of significantly differentiated genes belonging to different functional categories
| Functional category of genes |
Total no. of significantly differentiated genesa |
% of significantly differentiated genes |
No. of upregulated genes |
No. of downregulated genes |
|---|---|---|---|---|
| Amino acid transport and metabolism |
11 | 5.7 | 3 | 8 |
| Carbohydrate transport and metabolism |
37 | 19.3 | 1 | 36 |
| Cell division and chromosome partition |
1 | 0.5 | 1 | 0 |
| Cell envelope and biogenesis |
2 | 1.04 | 0 | 2 |
| Cell motility and secretion |
1 | 0.5 | 0 | 1 |
| Coenzyme metabolism | 3 | 1.6 | 0 | 3 |
| DNA replication and recombination |
4 | 2.1 | 3 | 1 |
| Energy production and conversion |
14 | 7.3 | 3 | 11 |
| Function unknown | 36 | 18.8 | 10 | 26 |
| General function prediction only |
15 | 7.8 | 5 | 10 |
| Inorganic ion transport |
1 | 0.5 | 0 | 1 |
| Lipid metabolism | 12 | 6.3 | 11 | 1 |
| Nucleotide transport and metabolism |
4 | 2.1 | 4 | 0 |
| Phage | 15 | 7.8 | 10 | 5 |
| Posttranslational modification | 5 | 2.6 | 3 | 2 |
| Secondary metabolite biosynthesis |
5 | 2.6 | 2 | 3 |
| Transcription | 5 | 2.6 | 1 | 4 |
| Translation and ribosomal structure |
4 | 2.1 | 2 | 2 |
| Virulence genes | 18 | 9.4 | 5 | 13 |
| Total no. (%) | 192 | 100 | 64 (33.3) | 128 (66.7) |
The total number of significantly differentiated genes for the different functional categories of genes and the totals are shown in boldface type.
Prevention of SDH export adversely affects carbohydrate transport and metabolism.
Prevention of SDH exportation resulted in downregulation of 36 out of 37 differentially regulated genes in the carbohydrate transport and metabolism category in the M1-SDHHBtail mutant strain as revealed by the comparative microarray and qRT-PCR-based analyses (see Table S3 and Table S4 in the supplemental material). Notably, this included downregulation of 11 out of 18 predicted or identified genes specific to carbohydrate phosphotransferase (PTS) systems/ABC transporters (Table S5). In particular, multiple operons (SPy1057-1060, SPy1294-1296, SPy1299-1301, SPy1306, and SPy1986) containing genes for utilization/transport of nonglucose substrates, such as maltose/maltodextrin, mannose, mannose/fructose, N-acetylneuraminate, β-glucoside, fructose, cellobiose, galactose, lactose, and trehalose were downregulated (8- to 32-fold or 2 to 5 log2 units). We also observed a 2-fold decrease in the SPy1986 transcript (with 90% confidence limit), which was originally annotated as glucose-specific PTS system and recently has been annotated as MalT (maltose transporter) in a type M1 GAS strain, MGAS_5005 (27).
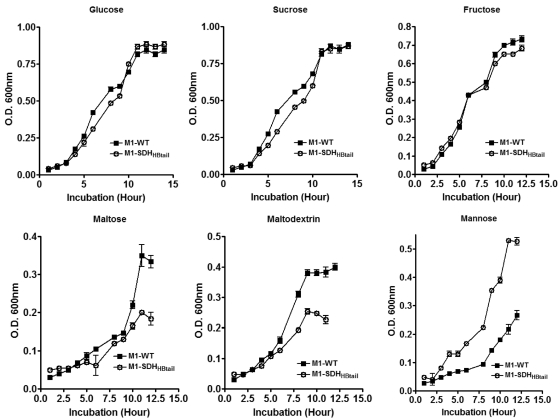
Since about half of the differentially regulated genes in the M1-SDHHBtail mutant were related to metabolism, most of which were downregulated and essentially belonged to carbohydrate transporters, we investigated the growth properties of the M1-SDHHBtail mutant and the wild-type GAS strains in chemically defined medium (CDM) supplemented with different sugars as the sole carbon source (Fig. 1). Our results revealed that the lack of surface exportation of SDH did not change the growth characteristics of the mutant in complex nutritionally enriched THY medium (Todd-Hewitt broth supplemented with 0.5% yeast extract). However, when the mutant was grown in CDM with different sugars as the sole carbon source, different growth patterns of M1-SDHHBtail were observed compared to M1-WT. More importantly, the growth rates of the M1-SDHHBtail mutant were not affected when grown in CDM with glucose, sucrose, fructose, and mannose (Fig. 1). On the other hand, when maltose or maltodextrin was used as the sole carbon source, the growth rate of the M1-SDHHBtail mutant was reduced (Fig. 1), indicating that the inhibition of SDH exportation or increased intracellular concentration of SDH directly influences the ability of GAS to metabolize and transport specific carbon sources.
FIG 1 .
Growth curves of the wild-type M1-SF370 (M1-WT) and its isogenic mutant (M1-SDHHBtail) GAS strains in chemically defined medium supplemented with glucose, sucrose, fructose, maltose, maltodextrin, and mannose. Error bars represent standard error of the mean OD600nm obtained from three independent experiments.
Cytoplasmic retention of SDH results in increased intracellular ATP concentrations and decreased acid tolerance.
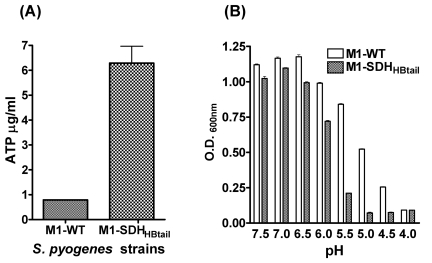
Microarray analysis of the M1-SDHHBtail mutant revealed downregulation of 11 genes (SPy0148 to SPy0151, SPy0154, SPy0155, SPy0157, SPy0414, SPy0739, SPy1128, and SPy1849) and upregulation of three genes (SPy0755, SPy0757, and SPy0759) related to energy production and conversion (see Table S3 in the supplemental material). Since most of the downregulated genes belonged to V-type Na+ ATPase and upregulated genes belonged to proton-translocating ATPase, we hypothesized that the downregulation of ATPase may result in a surplus intracellular concentration of ATP. To validate the microarray data, we measured the intracellular concentration of ATP in the M1-SDHHBtail mutant and wild-type strains by luciferin-luciferase assay. An 8-fold increase in the intracellular ATP concentration in the mutant (Fig. 2A) indicated that the prevention of SDH export and its subsequent increase in the cytosol positively regulate the levels of ATP in the M1-SDHHBtail mutant.
FIG 2 .
(A) Intracellular ATP concentrations in the wild-type M1-SF370 (M1-WT) and its isogenic mutant (M1-SDHHBtail) GAS strains. ATP concentrations in samples were determined using luciferin-luciferase bioluminescence assay based on the standard curve obtained with known concentrations of ATP. Error bars represent standard errors of the mean ATP concentrations obtained from three independent experiments (triplicate wells for each experiment). (B) Effect of pH on growth of the wild-type M1-SF370 (M1-WT) and mutant (M1-SDHHBtail) GAS strains. GAS strains were grown overnight in chemically defined medium (CDM), and culture density (600 nm) was determined as described in Materials and Methods. Error bars represent standard error of the mean OD600nm obtained from three independent experiments.
The results described above also indicated that increased intracellular concentration of SDH may impair the ability of GAS to expel H+ due to the significantly reduced expression of V1V0ATPases, resulting in reduced acid tolerance. Arginine deaminase system (ADS) has been previously reported to confer acid tolerance in GAS (28) and influence its ability to adhere and invade host epithelial cells (29). We observed a significant downregulation of SPy1541-SPy1549 genes (~32-fold) encoding enzymes in the arginine deaminase system (ADS) of S. pyogenes (Table 1; also see Table S3 in the supplemental material). To validate these results, we analyzed the ability of the wild-type and mutant GAS strains to survive and grow in the acidic environment by monitoring their growth patterns in THY broth at pHs ranging from 4.0 to 7.5 (Fig. 2B). The M1-SDHHBtail mutant showed significantly retarded growth (P < 0.001) at pHs ranging from 4.5 to 6.0 in comparison to the wild-type strain (Fig. 2B), indicating that inhibition of SDH export onto the cell surface significantly affected the intracellular proton homeostasis in GAS. Thus, prevention of SDH exportation and the concomitant increase in its intracellular concentration adversely affect the ability of the GAS mutant to survive in the acidic environment.
Inhibition of SDH exportation affects phosphorylation of HPr, a central regulatory protein of the carbohydrate phosphotransferase system.
As described above, the prevention of SDH exportation resulted in downregulation of several genes related to carbohydrate transport and metabolism. Many of these genes belong to the phosphotransferase (PTS) system (see Table S5 in the supplemental material). Bacterial systems have developed complex mechanisms to adapt to environmental conditions, such as carbon catabolite repression (CCR), which facilitates utilization of preferential substrates like glucose instead of other nonpreferred substrates when more than one carbohydrate exists in the environment (11). The phosphoenolpyruvate (PEP):carbohydrate phosphotransferase system is a well-described regulatory system for CCR through which bacteria transport and phosphorylate the carbohydrates (30). In this study, our microarray analysis revealed downregulation of several PTS-related genes without significantly altering the transcript abundance of the ccpA gene (SPy0514). This indicates that the observed differential expression of PTS-related genes is likely due to an altered interaction between Hpr and CcpA, which in turn is influenced by the phosphorylation status of Hpr. Since the ATP levels were significantly elevated in the SDH mutant (Fig. 2A), we speculated that the increased intracellular concentration of SDH somehow modulates the Hpr phosphorylation and/or function of CcpA.
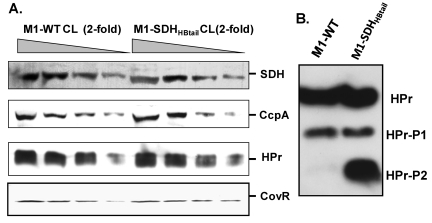
To examine the relative changes in the phosphorylation status of Hpr in the M1-SDHHBtail mutant strain in comparison to that in the wild-type GAS strain, we determined expression levels of differentially resolved phosphorylated forms of HPr by native PAGE analysis. This analysis allowed us to differentiate between fast-migrating Ser-phosphorylated HPr (HPr-Ser-P or HPr-P1) and/or His/Ser-phosphorylated HPr (HPr-His/Ser-P or HPr-P2). Parallel Western blot analysis of the whole-cell lysates of M1-SDHHBtail mutant and M1-WT strains was carried out to determine relative endogenous protein levels of HPr and CcpA. Although the protein expression profiles of CcpA and Hpr were comparable in both GAS strains (Fig. 3A), a significant increase in the amount of doubly phosphorylated Hpr (Hpr-Ser-P, -His-P [HPr-P2]) in the M1-SDHHBtail mutant was observed (Fig. 3B). This indicated that the retention of SDH in the cytoplasm results in increased phosphorylation of Hpr possibly due to increased ATP production (Fig. 2A) as also reported previously (31).
FIG 3 .
Western blot analysis of the whole-cell lysate (CL) of the overnight-grown wild-type M1-SF370 (M1-WT) and mutant (M1-SDHHBtail) GAS strains for the presence of SDH, CcpA, HPr, and CovR, using protein-specific antibodies. Equal amounts (50 µg total protein [25 ul] as the starting concentration) of cell lysates of M1-WT and M1-SDHHBtail were used for the assay. (A) Western blot analysis of equal volume (25ul) of the four serially 2-fold diluted samples of whole-cell lysates to determine the concentrations of different proteins as indicated in the wild-type and mutant GAS strains. (B) Reactivity of mono- and doubly phosphorylated forms of HPr (HPr-P1 and HPr-P2) in the whole-cell lysates of M1-WT and M1-SDHHBtail GAS strains.
Retention of SDH within the cytosol increases lipid/fatty acid biosynthesis.
Unlike the expression profiles of carbohydrate metabolism genes in the M1-SDHHBtail mutant as described above, microarray-based differential gene expression analysis for the mutant in comparison to the wild-type M1-WT GAS strains revealed 2- to 4-fold upregulation of 13 lipid biosynthesis genes located in a cluster (fabM/phaB/SPy1758, fabT/SPy1755, fabH/SPy1754, acpP/SPy1753, fabK/SPy1751, fadD/SPy1750, fabG/SPy1749, fabF/SPy1748, accB/SPy1747, fabZ/SPy1746, accC/SPy1745, accD/SPy1744, and accA/SPy1743) (see Table S3 in the supplemental material). These genes collectively encode enzymes that belong to the Gram-positive fatty acid synthesis type II (FASII) pathway involving a series of enzymatic steps (32). FASII of Gram-positive bacteria is well characterized in the human pathogen Streptococcus pneumoniae and is considered the universal fatty acid synthesis pathway, since similar gene clusters are also found in Enterococcus, Clostridium, and Lactococcus species (32). Comparison of the fatty acid synthesis cluster of S. pyogenes with that of S. pneumoniae revealed significant homology and similar genetic organization. We therefore predicted that the increased transcript abundance of fatty acid biosynthesis genes may result in the increased fatty acid content in the M1-SDHHBtail mutant.
To validate these data, we analyzed the total fatty acid content and compositional variation in the M1-SDHHBtail mutant and wild-type strains by gas chromatography (Table 2). The significant increase in the total fatty acid content in the M1-SDHHBtail mutant (1.64 fold; P = 0.013) was in accordance with the increased transcript abundance of lipid/fatty acid biosynthesis genes, SPy1743-SPy1758. The unsaturated fatty acid (UFA) and saturated fatty acid (SFA) profile in the mutant revealed significantly increased (P < 0.03) synthesis of individual saturated fatty acids (12:0, 14:0, 16:0, and 18:0) except those possessing carbon chain lengths of 10:0 (not shown for M1-WT and not detected in the M1-SDHHBtail mutant) and 13:0. Also, the reduction in the UFA/SFA (131.9:96.5) ratio from 1.37 (M1-WT) to 1.02 (166.43:163.1 in M1-SDHHBtail) indicated an upregulation of saturated fatty acid content in the mutant (Table 2). Collectively, these results revealed that the prevention of SDH exportation directly or indirectly affects lipid biosynthesis and fatty acid composition in the GAS.
TABLE 2 .
Fatty acid composition of the wild-type (M1-WT) and mutant (M1-SDHHBtail) GAS strainsa
| Fatty acid peak | Fatty acid compositionb
|
P value | |
|---|---|---|---|
| M1-WT | M1-SDHHBtail | ||
| 12:0 | 1.94 ± 0.41 | 5.04 ± 0.46 | 0.0016 |
| 13:0 | 1.10 ± 0.21 | 1.37 ± 0.17 | 0.0926 |
| 14:0 | 3.66 ± 0.79 | 8.41 ± 0.24 | 0.0050 |
| 16:1 w9cc | 13.53 ± 1.77 | 19.18 ± 4.53 | 0.0917 |
| 16:1 w7c | 21.21 ± 4.14 | 31.27 ± 7.04 | 0.0613 |
| 16:1 w5c | 3.18 ± 0.39 | 4.29 ± 0.92 | 0.0978 |
| 16:0 | 71.58 ± 9.37 | 109.79 ± 9.54 | 0.0078 |
| 18:1 w9c | 20.9 ± 1.07 | 29.19 ± 3.02 | 0.0233 |
| 18:1 w7c | 66.88 ± 1.16 | 74.76 ± 9.52 | 0.1455 |
| 18:1 w5c | 4.35 ± 0.12 | 4.82 ± 0.51 | 0.1300 |
| 18:0 | 18.23 ± 1.54 | 38.49 ± 7.37 | 0.0215 |
| 19:1 iso I | 1.82 ± 0.21 | 2.92 ± 0.24 | 0.0049 |
| Total fatty acid | 228.71 ± 19.5 | 330.69 ± 26.6 | 0.0064 |
| % fatty acid in cell mass | 0.47 + 0.06 | 0.77 ± 0.10 | 0.0129 |
The rows with significant differences as evaluated by one-tailed nonparametric t test with Welch’s correction are shaded.
Mean ± SD of three independent experiments. Shaded rows highlight significant increase in specific fatty acid contents.
16:1 w9c, fatty acid with 16-carbon chain with one double bond located at the ninth carbon from the ω (omega) end of the chain.
Prevention of SDH exportation downregulates major GAS virulence factors.
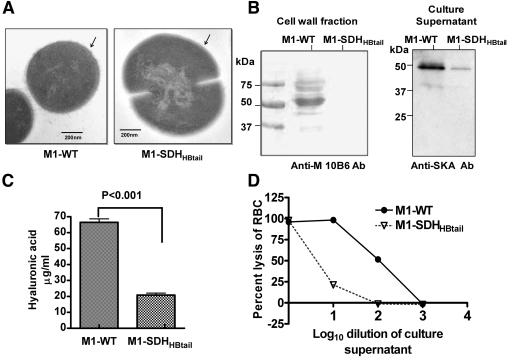
Microarray analysis of the M1-SDHHBtail mutant revealed differential expression profiles for 33 of the most important and well-characterized virulence genes (Table 3). The latter include adenine dinucleotide glycohydrolase (Nga/SPy0165), streptolysin O (Slo/SPy0167), C3 family ADP-ribosyltransferase (SpyA/SPy0428), exotoxins (SpeJ/SPy0436, SpeB/SPy2039), streptolysin S (SagA operon/SPy0738-SPy00746), pullulanase (PulA/SPy1972), streptokinase A (Ska/SPy1979), collagen-like surface protein (Scl/SPy1983), inhibitor of complement-mediated lysis (Sic/SPy2016), M protein (Emm1/SPy2018), mitogenic factor (mf/SPy2043) and capsule (Has operon/SPy2200-SPy2202). These gene products have been shown to play an important role in S. pyogenes colonization, persistence, dissemination, and proliferation (1, 27). Many of these virulence factors which are displayed as surface proteins contribute to the outer, electron-dense, fuzzy layer on the surface of GAS. The mutant (0.94 µm) was significantly larger (34%) than the wild-type strain (~0.72 µm). In the mutant, this fuzzy layer was found to be absent, which suggests the loss of expression of several surface proteins, including M protein, which concurs with the microarray and qRT-PCR data (Fig. 4A; see also Table S4 in the supplemental material).
TABLE 3 .
Differentially expressed genes encoding virulence factors in the M1-SDHHBtail mutant straina
| M1-SF370 ORF | Gene | Annotation | Microarray median log2 ratio | P value |
|---|---|---|---|---|
| SPy0165 | nga | Nicotine adenine dinucleotide glycohydrolase precursor | −2.35 | 0.001 |
| SPy0167 | slo | Streptolysin O precursor | −2.25 | 0.001 |
| SPy0428 | spyA | C3 family ADP-ribosyltransferase | −1.29 | 0.003 |
| SPy0436 | speJ | Putative exotoxin (superantigen) | −1.24 | 0.004 |
| SPy0738 to -746 | sagA-sagI | Streptolysin S-associated ORF | −2.25 to −4.18 | 0.0002 to 3.72E−-06 |
| SPy1972 | pulA | Putative pullulanase | −1.16 | 0.0238 |
| SPy1979 | ska | Streptokinase A precursor | −1.50 | 0.0041 |
| SPy1983 | scl | Collagen-like surface protein | −5.59 | 4.94E−09 |
| SPy1985 | rgfB | Exodeoxyribonuclease III | −1.96 | 0.0044 |
| SPy2016 | sic | Inhibitor of complement-mediated lysis | −2.13 | 4.76E−07 |
| SPy2018 | emm1 | M-protein type 1 | −1.13 | 0.0121 |
| SPy2039 | speB | Pyrogenic exotoxin B | −2.81 | 0.0007 |
| SPy2043 | mf | Mitogenic factor | −1.83 | 0.0016 |
| SPy2200 to -2202 | hasA-hasC | Hyaluronate synthase, UDP-G-6-D, UDP-GPPIaseb | −4.32 to−4.49 | 1.29 to 1.72 E−08 |
| SPy0711 | speC | Pyrogenic exotoxin C precursor, phage associated | 1.54 | 0.0012 |
| Spy0712 | mf2 | Putative DNase, phage associated | 1.69 | 0.0012 |
| Spy0737 | epf | Putative extracellular matrix-binding protein | 3.45 | 0.0005 |
| Spy1357 | grab | Protein GRAB (protein G-related α 2M-binding protein) | 2.42 | 5.45E−05 |
Shaded rows highlight significant up-regulation of specific genes in the mutant strain.
UDP-G-6-D, UDP-glucose-6-dehydrogenase; UDP-GPPlase, UDP-glucose pyrophosphorylase.
FIG 4 .
Effects of the cytoplasmic retention of SDH on the expression of virulence factors in GAS. (A) Transmission electron microscopy of the wild-type (M1-WT) and mutant (M1-SDHHBtail) GAS strains. (B) Relative expression levels of the M1 protein and streptokinase in the cell wall fractions and culture supernatants of the M1-WT and M1-SDHHBtail GAS strains as determined by type M1-reactive 10B6 monoclonal antibody (Anti-M 10B6 Ab) and antistreptokinase antibody (Anti-SKA Ab) in Western blot analysis. (C) Hyaluronic acid contents in M1-WT and M1-SDHHBtail GAS strains. (D) Relative percent hemolysin activity on sheep RBCs of the serially diluted (10-fold-diluted) culture supernatants of M1-WT and M1-SDHHBtail GAS strains. Lysis of RBCs in the undiluted culture supernatant of the M1-WT strain was set at 100%.
We further validated the above results by demonstrating the absence of the antiphagocytic M protein in the cell wall extracts and the secretory streptococcal plasminogen activator, Ska, in the culture supernatants of the M1-SDHHBtail mutant, using anti-M-protein specific and anti-SKA antibodies, respectively, in Western blot analysis (Fig. 4B). Additionally, a substantial decrease in the hyaluronic acid content in the M1-SDHHBtail mutant (20.82 ± 1.79 µg/ml) compared to the wild-type strain (66.46 ± 2.41 µg/ml) is in agreement with the observed downregulation of genes belonging to the has operon in the M1-SDHHBtail mutant (Fig. 4C). These results corroborated our earlier report showing significantly reduced survival of the M1-SDHHBtail mutant in whole blood (18).
Additionally, we also observed a 10- to 16-fold downregulation of the streptococcal exotoxin-encoding genes of the sag operon (sagA-sagI, SPy0738-SPy0746) (33), which correlated with the 25-fold decrease in streptolysin S (Sls) production in the mutant (100 hemolytic units in M1-WT versus 3.98 hemolytic units in M1-SDHHBtail) (Fig. 4D). To rule out the possibility that the observed hemolysis was not due to streptolysin O (Slo), we analyzed the contribution of Slo to hemolysis by the addition of trypan blue (an Sls inhibitor) to the reaction mixtures. The resulting hemolytic ratio, 0.0398 (M1-SDHHBtail/M1-WT) (not shown) indicated that the reduction in the hemolytic activity in the mutant was not caused by Slo secretion but is essentially mediated by Sls (Fig. 4D).
Prevention of SDH export results in downregulation of SpeB and possibly its secretion.
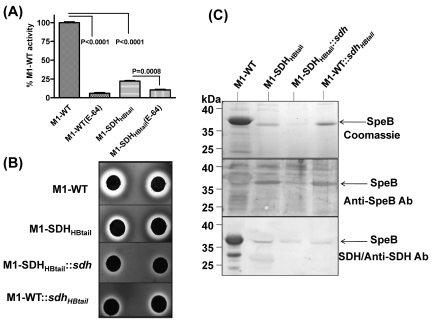
Streptococcal pyogenic exotoxin B (SpeB/SPy0274) is another virulence factor which degrades host serum proteins, such as human extracellular matrix, immunoglobulins, complement components, and even S. pyogenes surface and secreted proteins (34). Downregulation of speB (7.01-fold downregulation found by microarray and 3.22-fold downregulation by qRT-PCR [P < 0.0001]; see Table S4 in the supplemental material) in the M1-SDHHBtail mutant prompted us to compare the SpeB-specific cysteine protease activity in the culture supernatants of M1-SDHHBtail and M1-WT strains. This was accomplished by measuring the fluorescence released upon casein hydrolysis in the reaction mixture containing casein-fluorescein isothiocyanate (FITC) (Fig. 5A). About 94% of the cysteine protease activity observed in the culture supernatants of M1-WT was inhibited by cysteine protease inhibitor E-64, indicating that most of the detected hydrolytic activity in the supernatant was contributed by cysteine protease (Fig. 5A). Compared to the wild type, the M1-SDHHBtail mutant displayed significantly reduced cysteine protease activity (22% for M1-SDHHBtail and 100% for M1-WT) (Fig. 5A).
FIG 5 .
Relationship of the surface export of SDH and SpeB secretion. (A) Determination of the relative cysteine protease activities of SpeB present in the culture supernatants of M1-WT and M1-SDHHBtail GAS using FITC-labeled casein. The released fluorescence activity by the culture supernatant of the wild-type GAS strain was set at 100% activity, and its specificity was determined in the presence of cysteine protease inhibitor E-64. Values are means plus standard errors of the means (error bars) of three to six independent experiments. (B) Determination of SpeB-specific cysteine protease activity (hydrolysis of milk casein) present in the culture supernatants of M1-WT, M1-SDHHBtail, M1-SDHHBtail::sdh, and M1-WT::sdhHBtail GAS strains using milk agar. (C) Western blot analysis showing the effect on the secretion of SpeB in the culture supernatants of M1-WT, M1-SDHHBtail, M1-SDHHBtail::sdh, and M1-WT::sdhHBtail strains and the ability of SDH to bind secreted SpeB as determined by the blot overlay method.
Similarly, SpeB-specific protease activity was also determined by visualizing the zones of clearance due to casein digestion on milk agar plates. The reduced size of the clearance zones around the disk impregnated with the supernatant obtained from M1-SDHHBtail mutant strain on milk agar plates clearly demonstrated that the retention of SDH in the cytoplasm significantly reduced SpeB activity in the mutant (Fig. 5B). Further, to determine whether SpeB secretion is directly related to SDH surface exportation, we similarly measured SpeB-associated cysteine protease activity for the M1-WT strain complemented with the gene encoding SDHHBtail. The significantly reduced size of the clearance zone around the disk impregnated with supernatant obtained from the complemented strain in comparison to the wild-type strain confirmed that SpeB secretion is directly related to SDH export. Additionally, when the supernatants obtained from the M1-SDHHBtail mutant complemented with the gene encoding wild-type SDH was examined for SpeB activity, a further reduction in the size of the clearance zone around the test disks was observed (Fig. 5B). These results also concurred with the anti-SpeB reactivity in the same volume of trichloroacetic acid (TCA)-precipitated supernatants obtained from the wild-type (M1-WT), mutant (M1-SDHHBtail), and their corresponding complemented strains (Fig. 5C). These results indicate that the exportation of SpeB is directly related to the surface export of SDH and that SDH might bind to SpeB, facilitating its secretion. To determine the above contention, we performed a blot overlay assay using culture supernatants obtained from the M1-WT, M1-SDHHBtail, M1-WT::SDHHBtail, and M1-SDHHBtail::SDH strains. The direct binding of SDH to the pro-SpeB molecule (~35-kDa protein) as revealed by anti-SDH reactivity after the blot was overlaid with purified recombinant SDH indicated that SDH may potentially serve as a chaperone/carrier protein for SpeB, although SpeB itself is a secretory protein (Fig. 5C). These results thus emphasize the significance of the process of surface export of SDH in terms of facilitating transport of other proteins or virulence factors which may or may not possess classical surface export or secretion machinery.
Surface export of SDH is essential for the maintenance of GAS virulence.
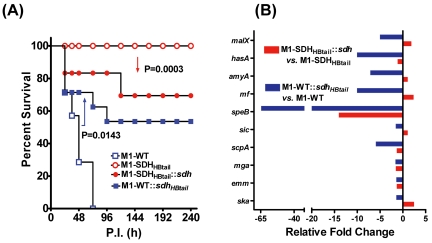
Microarray analysis in conjunction with other functional analyses collectively indicated that the prevention of SDH export onto the cell surface results in downregulation of several virulence factors (Table 3). We therefore hypothesized that the inhibition of SDH export might adversely affects GAS virulence in an experimental mouse intraperitoneal infection model. A group of 20 mice injected with the M1-SDHHBtail strain displayed 100% survival, while all 20 mice injected with the wild-type strain died by day 3 postinfection (P < 0.0001) (Fig. 6A). While live bacteria were recovered from the spleen (106 CFU/mg of tissue), lung, liver, and kidneys (5 × 104 to 8 × 104 CFU/mg of tissue) of mice infected with M1-WT, no bacteria were recovered from the mice infected with the M1-SDHHBtail mutant, indicating its attenuation for virulence. Together, these results indicate that the mere retention of SDH in the cytoplasm by preventing its exportation onto the GAS surface adversely affect both GAS metabolism and virulence. Hence, the accumulation of SDH/GAPDH in the cytoplasm beyond its physiological concentration results in the attenuation of GAS virulence.
FIG 6 .
Retention of SDH in the cytoplasm attenuates GAS virulence in an experimental mouse intraperitoneal infection model. (A) Survival/mortality curves for M1-WT and M1-SDHHBtail (20 mice per group) and their corresponding complemented strains (10 mice per group), M1-SDHHBtail::sdh and M1-WT::sdhHBTail (complemented strains created with pDC123 plasmid containing genes encoding SDHHBtail and SDH, respectively). Mice infected with GAS strains were monitored for 10 days postinfection (P.I.) and statistically evaluated by the log rank test, and the results were plotted using GraphPad Prism 4 software. All the mock-infected mice survived throughout the observation period (not shown). (B) qRT-PCR-based expression analysis of the indicated genes in the complemented strains. The relative fold change in the expression level was calculated with respect to the expression levels in the corresponding parent strains (M1-SDHHBtail::sdh versus M1-SDHHBtail and M1-WT::sdhHBTail versus M1-WT) after normalization with the housekeeping gene.
The above results raise an important question whether surface exportation of SDH per se is directly related to GAS virulence. To address this question, we included an additional two groups of mice (10 mice/group), one group infected with the M1-SDHHBtail mutant strain complemented with the gene encoding wild-type SDH and one group infected with the M1-WT strain complemented with the gene encoding SDHHBtail. In the first group (M1-SDHHBtail::sdh), 30% mice died, indicating that the attenuated M1-SDHHBtail regained significant virulence (P = 0.0003) upon expression of wild-type SDH from the plasmid in conjunction with the SDHHBtail from the genome (Fig. 6A). In the second group (M1-WT::sdhHBtail), 50% of the mice survived throughout a 10-day period in comparison to no mice survived (100% death observed within 3 days) in the group infected with the wild-type (M1-WT) strain. This indicated that the virulence of the wild-type strain was significantly reduced (P = 0143) as a result of the expression of SDHHBtail, which was not exported and was retained solely in the cytoplasm (Fig. 6A). Together, these results confirmed that the surface export of SDH is essential to maintain GAS virulence and overaccumulation of SDH by preventing its transport results in attenuation of virulence.
To further delineate the phenomenon of the surface export-related virulence regulation in GAS, we compared the expression levels of certain important genes, including those related to virulence in both of the aforementioned complemented strains. Although several genes were downregulated in the M1-SDHHBtail mutant upon complementation with the gene encoding SDH, 5 out of 10 genes showed relative upregulation, although they did not achieve the wild-type expression levels (Fig. 6B; also see Table S6 in the supplemental material) possibly because of the expression of cytoplasmically retained SDHHBtail in the background. The latter trend may be responsible for the increase in virulence (Fig. 6A). Similarly, downregulation of all 10 genes in M1-WT::sdhHBtail mimicking the gene expression profile of the M1-SDHHBtail concurred with its significantly decreased virulence, although it did not become avirulent because of the presence of SDH capable of being exported onto the surface.
Collectively, our data highlight the significance of the SDH export process per se in controlling various aspects of metabolism and virulence maintenance in GAS.
DISCUSSION
GAPDH is one of the key cytoplasmic enzymes involved in glycolysis. As much as the surface exportation of GAPDH in the absence of a classical protein exporting machinery in GAS and many Gram-positive pathogens is intriguing, it has also raised concerns regarding its physiological relevance. While it is still not clear how this anchorless surface protein is exported to the surface, we demonstrate in the present study for the first time the significance of the GAPDH export process on the GAS cell surface. This could not have been achieved without employing our previously described novel strategy of creating a GAS mutant expressing SDH (with a genetically introduced hydrophobic tail at its C terminus) solely in the cytoplasm or at the cytoplasmic side of the membrane (18). While we clearly demonstrated previously that the exportation of SDH to the cell surface is not a mere artifact, in the present study we were able to unravel the physiological relevance of the surface export of SDH by subjecting the M1-SDHHBtail mutant strain to microarray analysis in conjunction with various other in vitro and in vivo biochemical/biological assays.
The major highlights of the present study emerge from two important findings. (i) Simply by retaining SDH in the cytoplasm, carbohydrate metabolism- and many virulence-related genes in GAS were downregulated, and as a result, (ii) the M1-SDHHBtail mutant was completely attenuated. SDH being a key glycolytic enzyme, its intimate association with GAS carbohydrate metabolism is quite obvious. However, its role in the regulation of GAS carbohydrate metabolism could not be established until now owing to the fact that SDH is encoded by a single gene and is indispensable for GAS survival. The role of carbohydrate metabolism in GAS pathogenesis was also not evident until carbohydrate utilization proteins (CUPs) important in the pathogenesis of GAS were reported (10, 27). It has been demonstrated that many genes encoding these CUPs and especially those that can utilize maltodextrin and maltose are upregulated when GAS is grown in human saliva (10, 27). Moreover, the downregulation of these genes in the absence of the SptR/S (SPy0874/SPy0875) two-component system indicated that SPy0874 or SptR plays a crucial role in the regulation of complex carbohydrate metabolism in GAS (6). The poor ability of the M1-SDHHBtail mutant to grow in the presence of maltose and maltodextrin concurs with the downregulation of 37 out of 38 carbohydrate metabolism genes. However, no changes in the transcript abundance of the sptR or sptS gene in the M1-SDHHBtail mutant indicate that the cytoplasmically retained SDH may interfere with SptR/S-mediated functional regulation (e.g., binding to specific promoter) rather than at the sptR/S transcriptional level.
Carbohydrate metabolism in bacteria is also regulated by a catabolite control (CcpA) protein (11). Recent reports have elucidated the role of CcpA (SPy0514) as a repressor of carbohydrate metabolism and established its association with GAS virulence (5, 9, 10, 35, 36). CcpA represses multiple operons involved in utilization of nonglucose substrates, including mannose, cellobiose, mannose/fructose, beta-glucosidase PTSs, and also a maltose ABC transporter (11). Significant downregulation of CcpA-regulated genes in the M1-SDHHBtail mutant without altering the expression levels of ccpA and hpr indicates that intracellular SDH may potentially interact with these proteins to regulate their function. Hpr is the central regulatory protein of the PTS system that plays an important role in catabolite control repression (CCR) by its differential phosphorylation at His (HPr-His~P) and Ser (HPr-SerP) residues. In GAS, the latter binds strongly to catabolite control protein A (CcpA), and the resulting CcpA–HPr-SerP complex binds strongly (50- to 100-fold higher) to the catabolite response element (cre) in the promoter region of CcpA-regulated genes (9, 11, 27). It has also been observed that large amounts of doubly phosphorylated HPr [HPr (Ser-P/His~P)] are generated in streptococcal and lactococcal species during growth (37). While the significance of this dual phosphorylation remains unclear, we observed that retention of SDH in the cytoplasm resulted in an increase in the doubly phosphorylated Hpr species. This could possibly be attributed to the increased intracellular ATP production which in turn is a consequence of significant downregulation of 14 of 25 predicted V-type Na+-ATPase or V1V0ATPase genes (SPy0147-SPy0157 and SPy0754-0760) (38) and arginine deaminase system (28).
The role of lipid biosynthesis in virulence regulation has been studied for only a few Gram-positive pathogens, including S. pneumoniae and Staphylococcus aureus (39, 40), and there is no such information available for GAS. In contrast to the expression profile of genes regulating carbohydrate metabolism, upregulation of genes responsible for lipid biosynthesis (FAS-II genes SPy1743-SPy1755) prompted us to determine total lipid and fatty acid content. While the saturated fatty acid content increased significantly in the M1-SDHHBtail mutant, it displayed complete virulence attenuation unlike the phenotype of FAS-II knockout in S. mutans (41). To understand the role of lipid biosynthesis in GAS virulence, the present study invokes future studies on how SDH regulates fatty acid biosynthesis, whether the increased expression of FAS-II genes is the marker of attenuation, and whether the biosynthesis of fatty acid plays any role in virulence as in the case of S. aureus (40).
Although the role of SDH/GAPDH in many nonglycolytic functions is directly or indirectly associated with bacterial virulence (12, 19, 20, 42), the mechanism of SDH-mediated regulation of GAS/bacterial virulence has so far not been investigated. In the present investigation, besides downregulation of carbohydrate transport/metabolism-related genes, we also observed the downregulation of several virulence-associated genes (e.g., has and sag operon genes, ska, emm1, collagen-binding protein [Scl], speB, mf, and others). A similar profile for virulence genes was observed for the M1-GAS mutants (albeit in different type M1 strain MGAS_5005) that lack virulence and carbohydrate gene-regulating two-component systems (TCSs) such as CovR, Mga, and CcpA (5, 27, 43, 44). Interestingly, despite the unaltered expression profiles of the latter genes (covR, mga, or ccpA), the downregulation of virulence genes in M1-SDHHBtail mutant suggests a possible role of SDH in altering the interaction between Mga and CcpA at the cre site or CovR binding to its promoter sites when intracellular equilibrium of SDH is perturbed. The possibility of interaction of SDH with other stand-alone transcription factors regulating GAS pathogenesis and carbohydrate metabolism became evident when we observed downregulation of LacD.1 (SPy1704 encoding putative tagatose-1,6-bisphosphate aldolase, 3.13-fold) and speB gene (7.01-fold) in the SDHHBtail mutant despite the fact that SPy1704 is a negative regulator of SpeB. The expression of SpeB is also shown to be regulated by ropB/rgg/SPy2042 (45). Significantly reduced SpeB-associated cysteine protease activity with no change in the expression level of SpeB regulator ropB/rgg/SPy2042 in the M1-SDHHBtail mutant is additional evidence for the above-mentioned argument for the ability of SDH to interact with one or more stand-alone transcription regulator(s).
To determine whether the export process of SDH per se is responsible for the regulation of virulence, we complemented M1-WT with the gene encoding SDHHBtail and complemented M1-SDHHBtail mutant with the gene encoding SDH. The relative decrease in the virulence of the M1-WT::sdhHBtail and increase in the virulence of M1-SDHHBtail::sdh indicated that export of SDH is certainly essential for GAS to remain virulent, since the retention of SDH in the cytoplasm results in its attenuation. These results also concur with qRT-PCR-based expression profiles of certain important genes studied for these complemented strains (Fig. 6B). In conjunction with these results, the direct binding of SDH to SpeB and the way SpeB (microdomain/ExPortal) and SDH are exported and localized on the cell surface (14, 18, 46, 47) lead us to believe that SDH-mediated virulence regulation in GAS could happen in three specific ways. (i) SDH by itself can serve as a transcription factor. (ii) SDH can function as an important component of the transcription complex to regulate the expression of virulence-associated genes. (iii) The process of surface export of SDH by itself regulates the bacterial gene expression profile. SDH may serve as a chaperone facilitating the export of certain virulence factors destined for secretion or exportation.
Together, our findings indicate that the surface exportation of SDH is an essential phenomenon to maintain GAS virulence. This export is thought to circumvent the imbalance (increased concentration) in the intracellular concentration of SDH which otherwise may enhance its multifarious interactions with CovR, SptR, CcpA, Mga, LacD.1, and/or Rgg. While the nature and specificity of these potential interactions are presently unknown, our findings open a new avenue of novel regulatory pathways for fine-tuning gene regulation in GAS. Considering the conserved nature of SDH/GAPDH throughout biological evolution and the exportation of SDH/GAPDH onto the cell surface in almost all types of prokaryotes, fungi, and protozoans, we believe that microorganisms in general devised this simple export mechanism long before sophisticated secretion systems came into existence. Thus, exportation of SDH/GAPDH on the surface may be an essential function of certain bacteria to remain as successful pathogens. In this context, SDH, being an ancient key glycolytic enzyme, serves as a quintessential regulator fulfilling necessary regulatory functions of virulence maintenance as and when necessary in a constantly changing host environment. While the surface exportation of SDH is indeed an essential process for GAS persistence as a pathogen, the strategy of preventing this exportation can be exploited as a novel way of attenuation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. pyogenes wild-type strain M1-SF370 (M1-WT; ATCC 700294; American Type Culture Collection, Manassas, VA) and the isogenic mutant strain (M1-SDHHBtail) (18) were grown in Todd-Hewitt broth (Difco Laboratories) supplemented with 0.5% yeast extract (THY) or on proteose peptone no. 3 agar plates (Difco Laboratories) supplemented with 5% defibrinated sheep blood. Escherichia coli XL1-Blue was grown in Luria-Bertani (LB) medium (with or without agar) at 37°C. Antibiotics were added at the following concentrations when needed: ampicillin, 100 µg/ml; and spectinomycin, 500 µg/ml (for S. pyogenes and 50 µg/ml for E. coli). Chemically defined medium (CDM) was prepared as described previously (48), except that 1% glucose was replaced with other sugars in the same concentrations as carbohydrate sources, when needed. Unless otherwise mentioned, all the chemicals were procured from Sigma-Aldrich. Growth curves of the wild-type and mutant strains in the presence of various sugar sources were measured by recording the absorbance at 600 nm.
RNA isolation.
RNA was extracted from the wild-type (M1-SF370) and mutant (M1-SDHHBtail) strains grown till late exponential phase (OD600 = 0.8), using Qiagen RNeasy kit (Qiagen) as described previously (49). RNase-free DNase I was added to the RNA preparation for removal of residual DNA contamination according to the manufacturer’s protocol. RNA was quantified, and its integrity was checked by using an Agilent 2100 bioanalyzer as described previously (49).
Microarray analysis and real-time PCR (qRT-PCR).
Oligonucleotide-based microarray analysis was performed using polylysine-coated slides spotted with oligonucleotides designed to correspond to 1,769 open reading frames (ORFs) of the S. pyogenes M1-SF370 genome (49). Synthesis and labeling of the first-strand cDNA, hybridization of probes with microarrays, scanning the signals of bound reagents on the microarray spots, Lowess normalization, and R-based microarray analysis and t statistics using Bioconductor multitest (https://carmaweb.genome.tugraz.at/carma/) were performed as described previously (49). A difference of at least 2-fold (log2 ratio of ≥1 or ≤−1 with a P value of ≤0.05) in the transcript abundance ratio was considered a significant change in the gene expression. Results were deposited to GEO database and an approved accession number (GSE 15231) was obtained. Microarray analysis was validated by real-time quantitative reverse transcription-PCR (qRT-PCR) for randomly selected genes, using brilliant SYBR green QPCR master mix (Roche) employing gene-specific primers (see Table S1 in the supplemental material) and using a LightCycler480 (Roche) real-time PCR instrument as described previously (49). The copy numbers for all the genes were normalized with gyrA and proS using two biological replicates each with three technical replicates. The data were analyzed using the Exor-4 software (Roche).
Cell fractionation.
Cell wall, cell membrane, and cytoplasmic proteins from the wild-type and mutant GAS strains were fractionated by digesting intact GAS cells with recombinant phage lysin (Plys) in phosphate-buffered saline (PBS) buffer containing 30% (wt/vol) raffinose, 5 mM EDTA, and 0.5 mM dithiothreitol (DTT), followed by differential high-speed centrifugation as described previously (12, 50).
Production of recombinant proteins CovR and CcpA and specific polyclonal antibodies.
Recombinant 6×His-tagged CovR (SPy0336) and CcpA (SPy0514) were prepared essentially as described before using pET-14b His-tagged vector and employing nickel-nitrilotriacetic acid (Ni2+-NTA) affinity chromatography (50). The sequences of the specific primers used for cloning the genes in the expression vector are shown in Table S2 in the supplemental material. Recombinant proteins were used to raise polyclonal antibodies in rabbits. Polyclonal antisera were custom-made by Lampire Biological Laboratories, Inc., using the 50-day express-line protocol (Lampire Biological Laboratories, Inc., Pipersville, PA).
Blot overlay and Western blot analysis.
Proteins were resolved on SDS-polyacrylamide gels, followed by electroblotting on polyvinylidene difluoride (PVDF) membranes (Bio-Rad). The membranes were blocked and probed with specific antibody followed by anti-rabbit/mouse/goat (for polyclonal antibody) immunoglobulin G alkaline phosphatase-conjugated secondary antibodies. Immunoreactive bands were visualized using either chromogenic (nitroblue tetrazolium [50 µg/ml] and 5-bromo-4-chloro-3-indolylphosphate [25 µg/ml]) or chemiluminescent substrates (Lumi-Phos; Pierce) (19).
Monoclonal antibody 10B6 (51) was used to detect the expression of M protein. Sheep antistreptokinase antibody was purchased from AbD Serotec Inc. Rabbit anti-HPr was obtained from Joseph Deutscher. Anti-SpeB antibody was purchased from Santa Cruz Laboratory. Anti-SDH (12), anti-CcpA, and anti-CovR polyclonal antibodies were custom-made by Lampire Biological Laboratories, Inc., in rabbits injected with purified recombinant proteins. The custom-made antibodies were affinity purified using a protein G-Sepharose 4B column followed by corresponding purified protein-bound 3M phase activated beads as described previously (19).
To determine the binding ability of SDH to SpeB secreted in the culture supernatants of the wild-type, mutant, and complemented strains grown to late log phase (optical density [OD600] of 0.8), the supernatants were precipitated with trichloroacetic acid (TCA) (30% final concentration), neutralized, and washed with 100% ethanol containing 1% (vol/vol) M sodium acetate. The proteins present in the precipitates were resolved on 15% SDS-polyacrylamide gels, Western blotted, and subjected to the blot overlay method as described previously (19) using a purified SDH (12). Briefly, the Western blots were overlaid with purified SDH (10 µg/ml) for 2 h and washed, and the binding of SDH to specific protein (SpeB) was monitored using anti-SDH monoclonal antibody (MAb) 13D5 (52) as described above.
Total cell ATP assays.
Total ATP content in the wild-type and mutant GAS strains were tested by an ENLITEN ATP assay bioluminescence detection kit (Promega) according to the manufacturer’s instructions. Briefly, pellets obtained from 50-ml samples of the GAS cultures grown to late log phase were suspended in 100 µl of 2 mM EDTA, pH 7.75. The cells were lysed in the presence of glass beads using FastPrep-120, precipitated with 10% trichloroacetic acid in 2 mM EDTA, and quickly centrifuged at 16,000 × g for 2 min at 4°C. The supernatant diluted 1:10 (10 µl) was then subjected to luminescence measurement using FluoStart plate reader. The total ATP was estimated from the standard curve generated using known concentrations of ATP.
Total cell fatty acid assays.
Total fatty acid content and the composition of the individual fatty acids were custom analyzed by gas chromatography (Microbial ID Inc., Newark, DE). Briefly, fatty acid methyl esters were extracted from the GAS cultures grown until late logarithmic phase and separated by gas chromatography (MIDI). The resulting fatty acid profiles were analyzed using Sherlock pattern recognition software. Data were analyzed from three independently grown cultures of the wild-type and mutant strains. Data were analyzed by one-tailed nonparametric t test with Welch’s correction using GraphPad Prism 4 software.
TEM.
Late-log-phase-grown cultures of the wild-type and M1-SDHHBtail mutant strains were washed, resuspended in fixative (4% paraformaldehyde and 2.5% gluteraldehyde in 0.1 M cacodylate buffer [pH 7.4]), and subsequently processed for transmission electron microscopy (TEM) at the Ohio State University (OSU) Central Microscopy and Imaging Facility (CMIF) using Technai2 electron microscope as described previously (49, 50).
Measurement of the capsular hyaluronic acid.
The capsular hyaluronic acid from the late-log-phase-grown culture of GAS strains was extracted with chloroform, and the amount was measured spectrophotometrically (640 nm) after the extracts were treated with Sigma Stains-All solution as described previously (49, 50).
SLS hemolysis assays.
The ability of streptolysin S (SLS) present in the THY culture supernatants of the late-log-phase-grown (OD at 600 nm [OD600] of 0.8) wild-type and M1-SDHHBtail strains to lyse sheep red blood cells (shRBCs) was measured as described previously (53). The released hemoglobin from the lysed shRBCs was spectrophotometrically measured at 570 nm (Bio-Rad). The complete lysis (100% lysis) of shRBCs was achieved by the addition of 4 M NH4Cl and was used as the positive control. Samples containing only RBCs and PBS were used as blank or negative control. Percent hemolysis was calculated as follows: [(sample A − blank B)/(100% lysis Control C)] × 100. One hemolytic unit (HU) was defined as the reciprocal of the highest dilution of hemolysin that released 50% hemoglobin from 2.5% shRBC solution. To estimate the hemolytic activity contributed by streptolysin O, the SLS inhibitor, trypan blue (50 µg/ml), was added to the samples prior to incubation with shRBCs.
Measurement of SpeB activity.
SpeB-specific cysteine protease activity in the culture supernatant was estimated by visualizing casein hydrolysis in the form of a clearance zone around the 8-mm 3M paper discs impregnated with 10 µl of culture supernatants on 5% milk agar plates as described previously (54).
For the quantitative measurement of cysteine protease activity, casein hydrolysis was analyzed using the fluorescein isothiocyanate (FITC)-labeled casein method (55). For this, filter-sterilized supernatants from overnight GAS cultures were activated by mixing an equal volume of activation buffer-SAED (0.1 M sodium acetate buffer [pH 5.0], 1 mM EDTA, 20 mM DTT) and incubating for 30 min at 40°C. The activated supernatants were then mixed with an equal volume of FITC-conjugated casein (5 µg/ml; AnaSpec Inc.) prepared with 20 µM E-64 or without E-64, a cysteine-specific protease inhibitor (obtained from Sigma). The reaction mixtures were incubated for 1 h at 40°C, and the increase in the fluorescence intensities (excitation [Ex]/emission [Em] = 485/520 nm) was measured by a spectrofluorimeter (Bio-Rad).
In vivo GAS virulence in mice.
GAS strains (M1-WT, M1-SDHHBtail, M1-WT::sdhHBtail, and M1-SDHHBtail::sdh) grown until late exponential phase (OD600 of 0.8) were centrifuged, washed twice with sterile PBS, suspended in the same buffer, and adjusted to an OD600 of 1.0 (representing ~4 × 108 CFU/ml). Five-week-old female CD-1 mice (18 to 20 g, 10 to 20 mice/group, Charles River Laboratories) were infected intraperitoneally with 0.5 ml (~2 × 108 CFU) of the GAS strain and monitored for 10 days per the protocol approved by the Ohio State University Institutional Animal Care and Use Committee (OSU-IACUC) as described previously (49). Mortality/survival profiles for each infected group were plotted and statistically analyzed by the log rank test using GraphPad Prism 4 software. After 4 days of infection, the internal organs (spleen, lung, liver, and kidneys) from 4 to 6 sacrificed animals were removed and homogenized. GAS strains from the homogenized tissues were recovered on sheep blood agar plates.
Microarray data accession number.
Data from eight experiments were submitted to the Gene Expression Omnibus (GEO) database, assigned accession number GSE15231, and approved.
SUPPLEMENTAL MATERIAL
List of primers used in real-time PCR.
Primers used for cloning genes.
Microarray-based differential gene expression analysis for M1-SDHHBtail versus M1-SF370 (GSE 15231).
Comparison of microarray- and qRT-PCR-based gene-expression profiles.
Microarray-based differential gene expression profiles for PTS- and carbohydrate metabolism/transport-related genes.
qRT-PCR-based gene-expression profiles of selected genes for M1-SDHHBtail::sdh versus M1-SDHHBtail and M1-WT::sdhHBtail versus M1-WT.
ACKNOWLEDGMENTS
We thank Vincent Fischetti (Rockefeller University, New York, NY) for 10B6 monoclonal antibody, Joseph Deutscher (INRA, France) for anti-HPr antibody, Daniel Nelson (University of Maryland Biotechnology Institute [UMBI], University of Maryland, Bethesda) for recombinant PlyS (lysin), and Haritha Adhikarla for editorial help.
This study was supported in part by the Bill and Melinda Gates Foundation Grand Challenges Explorations Round-3 award (OPP1006841GCE) to V.P. We have no competing financial interests.
Footnotes
Citation Jin H, Agarwal S, Agarwal S, Pancholi V. 2011. Surface export of GAPDH/SDH, a glycolytic enzyme, is essential for Streptococcus pyogenes virulence. mBio 2(3):e00068-11. doi:10.1128/mBio.00068-11.
REFERENCES
- 1. Tart AH, Walker MJ, Musser JM. 2007. New understanding of the group A Streptococcus pathogenesis cycle. Trends Microbiol. 15:318–325 [DOI] [PubMed] [Google Scholar]
- 2. Carapetis JR, Steer AC, Mulholland EK, Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5:685–694 [DOI] [PubMed] [Google Scholar]
- 3. Kreikemeyer B, McIver KS, Podbielski A. 2003. Virulence factor regulation and regulatory networks in Streptococcus pyogenes and their impact on pathogen-host interactions. Trends Microbiol. 11:224–232 [DOI] [PubMed] [Google Scholar]
- 4. Churchward G. 2007. The two faces of Janus: virulence gene regulation by CovR/S in group A streptococci. Mol. Microbiol. 64:34–41 [DOI] [PubMed] [Google Scholar]
- 5. McIver KS. 2009. Stand-alone response regulators controlling global virulence networks in Streptococcus pyogenes. Contrib. Microbiol. 16:103–119 [DOI] [PubMed] [Google Scholar]
- 6. Shelburne SA, et al. 2005. Central role of a bacterial two-component gene regulatory system of previously unknown function in pathogen persistence in human saliva. Proc. Natl. Acad. Sci. U. S. A. 102:16037–16042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cho KH, Caparon MG. 2005. Patterns of virulence gene expression differ between biofilm and tissue communities of Streptococcus pyogenes. Mol. Microbiol. 57:1545–1556 [DOI] [PubMed] [Google Scholar]
- 8. Virtaneva K, et al. 2005. Longitudinal analysis of the group A Streptococcus transcriptome in experimental pharyngitis in cynomolgus macaques. Proc. Natl. Acad. Sci. U. S. A. 102:9014–9019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Almengor AC, Kinkel TL, Day SJ, McIver KS. 2007. The catabolite control protein CcpA binds to Pmga and influences expression of the virulence regulator Mga in the group A Streptococcus. J. Bacteriol. 189:8405–8416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shelburne SA, et al. 2008. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc. Natl. Acad. Sci. U. S. A. 105:1698–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deutscher J. 2008. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 11:87–93 [DOI] [PubMed] [Google Scholar]
- 12. Pancholi V, Fischetti VA. 1992. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate dehydrogenase with multiple binding activity. J. Exp. Med. 176:415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pancholi V. 2001. Multifunctional alpha-enolase: its role in diseases. Cell. Mol. Life Sci. 58:902–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pancholi V, Chhatwal GS. 2003. Housekeeping enzymes as virulence factors for pathogens. Int. J. Med. Microbiol. 293:1–11 [DOI] [PubMed] [Google Scholar]
- 15. Matta SK, Agarwal S, Bhatnagar R. 2010. Surface localized and extracellular glyceraldehyde-3-phosphate dehydrogenase of Bacillus anthracis is a plasminogen binding protein. Biochim. Biophys. Acta 1804:2111–2120 [DOI] [PubMed] [Google Scholar]
- 16. Pancholi V, Fischetti VA. 1993. Glyceraldehyde-3-phosphate dehydrogenase on the surface of group A streptococci is also an ADP-ribosylating enzyme. Proc. Natl. Acad. Sci. U. S. A. 90:8154–8158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lottenberg R, et al. 1992. Cloning, sequence analysis, and expression in Escherichia coli of a streptococcal plasmin receptor. J. Bacteriol. 174:5204–5210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boel G, Jin H, Pancholi V. 2005. Inhibition of cell surface export of group A streptococcal anchorless surface dehydrogenase affects bacterial adherence and antiphagocytic properties. Infect. Immun. 73:6237–6248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jin H, Song YP, Boel G, Kochar J, Pancholi V. 2005. Group A streptococcal surface GAPDH, SDH, recognizes uPAR/CD87 as its receptor on the human pharyngeal cell and mediates bacterial adherence to host cells. J. Mol. Biol. 350:27–41 [DOI] [PubMed] [Google Scholar]
- 20. Pancholi V, Fischetti VA. 1997. Regulation of the phosphorylation of human pharyngeal cell proteins by group A streptococcal surface dehydrogenase (SDH): signal transduction between streptococci and pharyngeal cells. J. Exp. Med. 186:1633–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alvarez-Dominguez C, et al. 2008. Characterization of a Listeria monocytogenes protein interfering with Rab5a. Traffic 9:325–337 [DOI] [PubMed] [Google Scholar]
- 22. Terao Y, Yamaguchi M, Hamada S, Kawabata S. 2006. Multifunctional glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pyogenes is essential for evasion from neutrophils. J. Biol. Chem. 281:14215–14223 [DOI] [PubMed] [Google Scholar]
- 23. Rosinha GM, Myioshi A, Azevedo V, Splitter GA, Oliveira SC. 2002. Molecular and immunological characterisation of recombinant Brucella abortus glyceraldehyde-3-phosphate-dehydrogenase, a T- and B-cell reactive protein that induces partial protection when co-administered with an interleukin-12-expressing plasmid in a DNA vaccine formulation. J. Med. Microbiol. 51:661–671 [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Oshima S, Kurohara K, Ohnishi K, Kawai K. 2005. Vaccine efficacy of recombinant GAPDH of Edwardsiella tarda against edwardsiellosis. Microbiol. Immunol. 49:605–612 [DOI] [PubMed] [Google Scholar]
- 25. Madureira P, et al. 2007. Streptococcus agalactiae GAPDH is a virulence-associated immunomodulatory protein. J. Immunol. 178:1379–1387 [DOI] [PubMed] [Google Scholar]
- 26. Ferretti JJ, et al. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. U. S. A. 98:4658–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shelburne SA, Davenport MT, Keith DB, Musser JM. 2008. The role of complex carbohydrate catabolism in the pathogenesis of invasive streptococci. Trends Microbiol. 16:318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Degnan BA, et al. 1998. Inhibition of human peripheral blood mononuclear cell proliferation by Streptococcus pyogenes cell extract is associated with arginine deiminase activity. Infect. Immun. 66:3050–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marouni MJ, Ziomek E, Sela S. 2003. Influence of group A streptococcal acid glycoprotein on expression of major virulence factors and internalization by epithelial cells. Microb. Pathog. 35:63–72 [DOI] [PubMed] [Google Scholar]
- 30. Postma PW, Lengeler JW. 1985. Phosphoenolpyruvate: carbohydrate phosphotransferase system of bacteria. Microbiol. Rev. 49:232–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thevenot T, Brochu D, Vadeboncoeur C, Hamilton IR. 1995. Regulation of ATP-dependent P-(Ser)-HPr formation in Streptococcus mutans and Streptococcus salivarius. J. Bacteriol. 177:2751–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schujman GE, de Mendoza D. 2008. Regulation of type II fatty acid synthase in gram-positive bacteria. Curr. Opin. Microbiol. 11:148–152 [DOI] [PubMed] [Google Scholar]
- 33. Datta V, et al. 2005. Mutational analysis of the group A streptococcal operon encoding streptolysin S and its virulence role in invasive infection. Mol. Microbiol. 56:681–695 [DOI] [PubMed] [Google Scholar]
- 34. Chiang-Ni C, Wu JJ. 2008. Effects of streptococcal pyrogenic exotoxin B on pathogenesis of Streptococcus pyogenes. J. Formos. Med. Assoc. 107:677–685 [DOI] [PubMed] [Google Scholar]
- 35. Shelburne SA, et al. 2010. A combination of independent transcriptional regulators shapes bacterial virulence gene expression during infection. PLoS Pathog. 6:e1000817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shelburne SA, et al. 2006. Maltodextrin utilization plays a key role in the ability of group A Streptococcus to colonize the oropharynx. Infect. Immun. 74:4605–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roy DJ, Casabon I, Vaillancourt K, Huot JL, Vadeboncoeur C. 2008. Streptococci and lactococci synthesize large amounts of HPr(Ser-P) (His-P). Can. J. Microbiol. 54:941–949 [DOI] [PubMed] [Google Scholar]
- 38. Takase K, et al. 1994. Sequencing and characterization of the ntp gene cluster for vacuolar-type Na(+)-translocation ATPase of Enterococcus hirae. J. Biol. Chem. 269:11037–11044 [PubMed] [Google Scholar]
- 39. Lu YJ, Rock CO. 2006. Transcriptional regulation of fatty acid biosynthesis in Streptococcus pneumoniae. Mol. Microbiol. 59:551–566 [DOI] [PubMed] [Google Scholar]
- 40. Balemans W, et al. 2010. Essentiality of FASII pathway for Staphylococcus aureus. Nature 463:E3–E5 [DOI] [PubMed] [Google Scholar]
- 41. Fozo EM, Scott-Anne K, Koo H, Quivey RG. 2007. Role of unsaturated fatty acid biosynthesis in virulence of Streptococcus mutans. Infect. Immun. 75:1537–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pancholi V, Fontan PA, Jin H. 2003. Plasminogen-mediated group A streptococcal adherence to and pericellular invasion of human pharyngeal cells. Microb. Pathog. 35:293–303 [DOI] [PubMed] [Google Scholar]
- 43. Graham MR, et al. 2002. Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. U. S. A. 99:13855–13860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ribardo DA, McIver KS. 2009. Defining the Mga regulon: comparative tranascriptome analysis reveals both direct and indirect regulation by Mga in the group A Streptococcus. Mol. Microbiol. 62:491–508 [DOI] [PubMed] [Google Scholar]
- 45. Dmitriev AV, et al. 2006. The Rgg regulator of Streptococcus pyogenes influences utilization of nonglucose carbohydrates, prophage induction, and expression of the NAD-glycohydrolase virulence operon. J. Bacteriol. 188:7230–7241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosch J, Caparon M. 2004. A microdomain for protein secretion in gram-positive bacteria. Science 304:1513–1515 [DOI] [PubMed] [Google Scholar]
- 47. Pancholi V, Fischetti VA. 1998. Alpha-enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J. Biol. Chem. 273:14503–14515 [DOI] [PubMed] [Google Scholar]
- 48. van de Rijn I, Kessler RE. 1980. Growth characteristics of group A streptococci in a new chemically defined medium. Infect. Immun. 27:444–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pancholi V, Boël G, Jin H. 2010. Streptococcus pyogenes Ser/Thr kinase-regulated cell wall hydrolase is a cell division plane-recognizing and chain-forming virulence factor. J. Biol. Chem. 285:30861–30874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jin H, Pancholi V. 2006. Identification and biochemical characterization of a eukaryotic-type serine/threonine kinase and its cognate phosphatase in Streptococcus pyogenes: their biological functions and substrate identification. J. Mol. Biol. 357:1351–1372 [DOI] [PubMed] [Google Scholar]
- 51. Jones KF, Fischetti VA. 1987. Biological and immunochemical identity of M protein on group G streptococci with M protein on group A streptococci. Infect. Immun. 55:502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fontán PA, Pancholi V, Nociari MM, Fischetti VA. 2000. Antibodies to streptococcal surface enolase react with human alpha-enolase: implications in poststreptococcal sequelae. J. Infect. Dis. 182:1712–1721 [DOI] [PubMed] [Google Scholar]
- 53. Ravins M, et al. 2000. Characterization of a mouse-passaged, highly encapsulated variant of group A Streptococcus in in vitro and in vivo studies. J. Infect. Dis. 182:1702–1711 [DOI] [PubMed] [Google Scholar]
- 54. Collin M, Olsén A. 2000. Generation of a mature streptococcal cysteine proteinase is dependent on cell wall-anchored M1 protein. Mol. Microbiol. 36:1306–1318 [DOI] [PubMed] [Google Scholar]
- 55. Ma Y, et al. 2006. Identification and characterization of bicistronic speB and prsA gene expression in the group A Streptococcus. J. Bacteriol. 188:7626–7634 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of primers used in real-time PCR.
Primers used for cloning genes.
Microarray-based differential gene expression analysis for M1-SDHHBtail versus M1-SF370 (GSE 15231).
Comparison of microarray- and qRT-PCR-based gene-expression profiles.
Microarray-based differential gene expression profiles for PTS- and carbohydrate metabolism/transport-related genes.
qRT-PCR-based gene-expression profiles of selected genes for M1-SDHHBtail::sdh versus M1-SDHHBtail and M1-WT::sdhHBtail versus M1-WT.