Abstract
There are limited data as to the effectiveness of MMF plus high dose corticosteroids for the treatment of acute GVHD and even less data regarding the pharmacokinetic disposition and exposure-response relationship of mycophenolate in individuals with GVHD. Mycophenolate pharmacokinetics were studied in a multi-center CTN randomized phase II trial evaluating the effectiveness of MMF as one of four agents added to corticosteroids as treatment of acute GVHD. Thirty-two of the patients randomized to receive MMF underwent pharmacokinetic sampling in weeks 1 and 2 were studied. Mean±sd age was 41±13.6 years. Twenty one (65.6%), 5 (15.6%), 6 (18.8%) patients had a complete response (CR), partial response (PR) or lesser response by day 28, respectively. Twenty-five (78.1%), 2 (6.3%), 5 (15.6%) patients had a CR, PR, or other response by day 56 to treatment, respectively. Single mycophenolic acid (MPA) pharmacokinetic measurements from weeks 1 and 2 did not correlate with CR at either day 28 or 56 (p>0.07). However, if the mean of weeks 1 and 2 total MPA troughs was >0.5 mcg/mL or unbound trough >0.015 mcg/mL, a significantly greater proportion achieved a CR+PR at day 28 and 56. A CR+PR at day 28 was observed in 19/19 (100%) of patients if the mean total trough was >0.5 mg/mL, but in only 7/13 (54%) if ≤0.5 mcg/mL (p=0.002). Similarly, 15/15 (100%) individuals had a CR+PR at day 28 if their unbound MPA trough concentration was >0.015 mcg/mL while only 11/17 (65%) responded if trough was ≤0.015 mcg/mL (p=0.02). There was no association between the pharmacokinetic measures and risk of infection by day 90 or overall survival at day 180 post randomization. About one-half of subjects’ therapy did not achieve the favorable MPA total and unbound trough concentrations. The current practice of MMF 1 gm twice daily dosing provides low plasma concentrations in many patients. Increased dosing at 3 gm/day may improve the efficacy of MMF as acute GVHD therapy.
Introduction
Acute graft vs host disease (GVHD) is a common complication occurring in 35–50% of recipients of hematopoietic cell transplant (HCT) resulting in greater treatment related mortality and delayed patient recovery after transplantation. Standard initial therapy for acute GVHD is methylprednisolone 1– 2.5 mg/kg/day; however, only 50% of patients will have durable response.1 Numerous immunosuppressants have been investigated for the treatment of acute GVHD either in combination with steroid therapy or as single agents.2–8 Several newer agents, including mycophenolate mofetil (MMF), have emerged as potentially effective for acute GVHD, however, well controlled trials defining the most effective agent are lacking.9–15 Recently the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) conducted a multicenter, randomized, phase 2, trial (BMT CTN 0302) comparing corticosteroids combined with either etanercept, MMF, denileukin diftitox or pentostatin for treatment of newly diagnosed acute GVHD in 180 patients who had undergone allogeneic HCT.16 The MMF arm had a greater CR rate at day 28 (60%, 95% C.I.: 46 – 74) and day 56 (73%, 95% C.I.: 60 – 86), and fewer treatment failures at day 56 (9%, 95% C.I.: 0.6 – 17) than the other arms although no formal statistical comparisons were done due to the small numbers. These data suggest that MMF may have significant activity against acute GVHD and a follow-up phase III trial is planned to confirm this. Given these findings, understanding mycophenolate exposure-response relationships and defining the optimal dose for GVHD treatment is critical and may further improve efficacy of MMF.
MMF is a prodrug designed to enhance the bioavailability of the active form, mycophenolic acid (MPA). MMF is extensively hydrolyzed to MPA by esterases located in the systemic circulation, gut wall, liver and tissues.17, 18 Glucuronidation of MPA occurs in the kidney, liver and intestine by UDP glucuronosyltransferases (UGTs) to the main inactive metabolite, mycophenolic acid glucuronide (MPAG).19, 20 MPAG is then transported in the bile to the gastrointestinal tract by multi-drug resistance protein 2 or excreted into the urine by active tubular secretion and eliminated.18 Mycophenolate may also undergo enterohepatic recirculation where biliary MPAG is deglucuronidated to MPA by gut bacteria which is then reabsorbed into the systemic circulation.18, 21, 22
MPA pharmacokinetic disposition and exposure response relationships in the setting of acute GVHD treatment are poorly understood. It is possible that more intensive immunosuppression is required during GVHD treatment and that different MPA plasma targets may be required relative to GVHD prophylaxis. In addition, MPA is highly dependent on metabolism in the intestine and liver, and given that GVHD may involve these sites it is reasonable to speculate that glucuronidation, enterohepatic recirculation or bioavailability may be altered. Although debated, data in organ transplantation and HCT show that a minimal required plasma exposure of mycophenolate is required for optimal immunosuppression in the prophylactic setting.23–33 Because of concerns that exposure may be altered in the setting of acute GVHD,34 pharmacokinetics were performed in subjects on the MMF arm of BMT CTN 0302 and are reported here. The primary objective was to evaluate the association between mycophenolate pharmacokinetics and acute GVHD complete response (CR) and partial response (PR) at days 28 and 56 post-treatment. Secondary objectives were to assess the relationships between pharmacokinetics, and the incidence of severe infections and survival.
Methods
Study Design and Patients
This was a prespecified analysis in patients randomized to the MMF arm of CTN 0302. Forty-five patients with newly diagnosed acute GVHD ≥ 6 years of age who required systemic treatment were treated with MMF; pharmacokinetics were studied in 32 patients. Patients received treatment with MMF plus methylprednisolone 2 mg/kg/day IV divided in 2–3 daily doses (or prednisone 2.4 mg/kg/day orally) for a minimum of 7 days. Subjects could not have received prior systemic therapy for the treatment of acute GVHD except for a maximum of 48 hours of high dose steroid therapy (≥ 1 mg/kg/day of methylprednisolone). Patients had received a related or unrelated allogeneic HCT. Patients were ineligible if they had uncontrolled infections, absolute neutrophil counts <500 cells/μL, or creatinine clearance of ≤30 ml/min. Patients were also ineligible if they had received MMF for GVHD prophylaxis within the 7 days prior to study enrollment. The study was approved by each participating institutions institutional review board (IRB) and the IRB of the BMT CTN. All patients gave written, informed consent.
MMF Treatment
MMF (CellceptR) was given at a dose of 20 mg/kg twice daily orally or IV if body surface area (BSA) was >1.5 m2 (maximum 1 gm twice daily) and 750 mg twice daily orally or IV if BSA was <1.5 m2 at time of GVHD diagnosis. The choice of oral or IV administration was determined clinically at each site. Patients with gastrointestinal (GI) GVHD that were unable to tolerate more than 500 mL of oral fluids/day received IV mycophenolate for at least the first 3 days of therapy. Patients with suspected mycophenolate related GI toxicity received MMF dose reductions of 50%. MMF was discontinued in those with GI symptoms that were either persistent or severe. Likewise the MMF dose was reduced by 50% in patients with an absolute neutrophil count (ANC) between 1000 and 500 cells/μL the dose was reduced by 50% and discontinued if the ANC was <500 cells/μL. Patients with GVHD in CR at day 28 continued their MMF through the completion of the steroid taper. MMF was then tapered over the following 4 weeks.
Acute GVHD Response and Toxicity Assessment
Acute GVHD was clinically diagnosed or biopsy proven. GVHD was scored and overall grade assigned as defined by the Consensus Criteria35 at days 28, 56 and then monthly for 9 months following initiation of treatment. A CR in GVHD was a complete resolution of all signs and symptoms of GVHD in all evaluable organs without additional therapy. A PR was an improvement by at least one stage in one or more organs involved without progression. A mixed response (MR) was an improvement in at least one involved organ with progression or newly developed GVHD in one or more organs. No response (NR) was the lack of improvement or worsening in any organ within 14 days of start of therapy. Progression was worsening by one or more stages in ≥ 1 organ without improvement in any organ. Failure of therapy was a PR, MR, NR, progression, death or development of chronic GVHD. Severe, fatal or life threatening infections were recorded through day 90. Infections were reported regardless of attribution to study treatment and were graded according to NCI Common Terminology Criteria for Adverse Events (version 3.0). Death due to any cause was considered an event in the survival analysis.
Mycophenolate Bioanalysis and Pharmacokinetics
Total and unbound MPA and total MPAG in the plasma was measured on each sample on an Agilent 1100 series (Agilent Technologies, Wilmington, DE) HPLC system with UV detection as previously described.36, 37 The validated assays were linear in the range of 0.025–20 mcg/ml for total MPA, 1–100 mcg/ml for total MPAG, and 0.001–0.5 mcg/ml for unbound MPA. Assay accuracy, intraday and interday variability as % relative standard deviation were 97–111%, 0.6–4.4%, and 1.0–6.0% for total MPA; 97–101%, 0.9–1.4%, and 0.7–4.8% for MPAG; and 96–118%, 0.7–5.4%, and 0.5–7.8% for unbound MPA, respectively.
Patients were evaluated at steady state with pharmacokinetics during weeks 1 and 2 after initiation of MMF treatment. Steady state was defined as >5 half lives from initiation of MMF. A limited sampling strategy was used where 4 blood samples were obtained over 6 hours following dose administration. Blood samples (5 mL) were collected in purple top tubes containing EDTA and taken at times 0 (C0), 1 (C1), 2 (C2) and 6 (C6) hours following the administration of the oral dose and at 0, 2, 4 (C4) and 6 hours after the start of the IV infusion. Intravenous MMF was administered at a constant rate over 2 hours. Blood samples were spun and plasma removed and aliquoted into two 1.5 ml Eppendorf screw cap vials and frozen at −20 degrees Celsius. Samples were shipped to a central laboratory the University of Minnesota for analysis. For each patient, a total and unbound MPA area under the curve (AUC)0–12 was determined from a limited sampling model previously developed in HCT recipients.38 If orally administered; total AUC0–12 mcg hr/mL = 4.43 + 2.76*C0 + 0.51*C1 +1.97*C2 + 4.27*C6 and unbound AUC0–12 ng hr/mL = 63.92 + 2.01*C0+0.67*C1 +2.05*C2 + 4.26*C6. If intravenously administered; total AUC0–12 mcg hr/mL = −0.49 + 1.58*C2 + 0.41*C4 +13.88*C6 and unbound AUC0–12 ng hr/mL = 7.99 + 1.40*C2 + 2.47*C4 + 9.54*C6. Total and unbound MPA AUC0–6 and MPAG AUC0–6 were calculated using noncompartmental analysis using WinNonLin (Version 5.2, Pharsight Corporation). Steady state trough was the concentration at time zero. The free fraction of MPA was determined by calculating the ratio of unbound MPA AUC0–6 to total MPA AUC0–6. MPAG to MPA ratio was determined from the trough concentration.
Statistical Analysis
The distribution of pharmacokinetic measures (total and unbound trough, AUC0–6 and AUC0–12, Cmax) was evaluated. Comparison of week 1 and week 2 pharmacokinetics measures were conducted using the Wilcoxon signed rank test or McNemar’s test on patients with both measurements. Comparisons of IV and PO pharmacokinetics were conducted using the Wilcoxon rank sum test or the Fisher’s Exact test. The association among various pharmacokinetic measures was assessed using Spearman’s rho. The association between pharmacokinetic measures (AUCs and troughs) and clinical outcomes (CR, PR, CR or PR, infection, survival) was conducted by dichotomizing the outcome at select time points. Pharmacokinetic measures were explored as continuous variables using non-parametric tests to test the association between outcomes and the continuous MPA pharmacokinetic measures. We postulated dichotomized values using published data for each pharmacokinetic measurement and performed chi-square tests or Fisher’s exact tests to test the association between dichotomized values and clinical endpoints. We analyzed the pharmacokinetic measurements at week 1 and week 2 separately, as well as the mean of weeks 1 and 2 to stabilize the measurement; the results were generally consistent, so except as noted the mean of weeks 1 and 2 data are presented. We also examined the association between non-pharmacokinetic variables (conditioning regimen, donor and graft source, GVHD prophylaxis at transplantation and grade of GVHD at enrollment) and outcome. Survival analysis was performed to evaluate the association between pharmacokinetic measures and overall survival. Dichotomized results are reported since they provide interpretations that can be used in clinical care. Only p-values ≤ 0.05 were considered significant. Multiple regression analyses were not performed due to limited sample size. SAS 9.1 statistical software was used for data analysis.
Results
Pharmacokinetics
Mycophenolate pharmacokinetics were studied in 32 of the 45 patients randomized to MMF. Thirteen patients were not studied because the patient was unable or declined to undergo additional blood sampling for pharmacokinetics, or the patient was removed from study drug prior to sampling. Characteristics and response to MMF treatment in the 32 patients were not different from the whole MMF group (data not shown). Patient characteristics are given in Table 1. Five subjects were studied only once resulting in a total of 59 pharmacokinetic profiles. Thirty patients received MMF 1gm twice daily and two patients received 0.75gm twice daily. Mycophenolate was administered orally or IV depending on clinical status. Twenty patients received oral MMF during both sampling periods. Median MPA pharmacokinetic measures were similar in weeks 1 and 2 of treatment (Table 2) except for total troughs which were higher in week 2. Oral administration yielded 4–6 times higher trough concentrations than IV administration, while IV administration provided higher AUCs (Table 3). In all pharmacokinetic sets, median (range) unbound MPA fraction was 2.2% (0.9–5.6) and was significantly higher with IV compared to PO treatment. The median MPAG to MPA ratio was higher in week 1 [66.0 (17.6–447.1)] compared to week 2 [45.7 (14.9–576.7)].
Table 1.
Patient Characteristics
| Age (yrs), mean±SD | 41±13.6 |
| Weight (kg) at time of study entry, mean±SD | 82.6±25.4 |
| Primary Disease | n (%) |
| Acute myelogenous leukemia | 15 (46.9%) |
| Acute lymphoblastic leukemia | 6 (18.8%) |
| Chronic myelogenous leukemia | 2 (6.3%) |
| Myelodysplastic Syndrome | 1 (3.1%) |
| Lymphoma | 4 (12.5%) |
| Other | 4 (12.5%) |
| Donor source | |
| Related | 16 (50%) |
| Unrelated | 16 (50%) |
| Stem cell source | |
| Bone marrow | 11 (34.3%) |
| Peripheral blood | 19 (59.4%) |
| Cord blood | 2 (6.3%) |
| GVHD prophylaxis at time of transplantation | |
| Tacrolimus/methotrexate | 16 (50%) |
| Cyclosporine/other | 6 (19%) |
| Cyclophosphamide | 6 (19%) |
| Tacrolimus/other | 4 (12%) |
| Conditioning regimen | |
| Nonmyeloablative or reduced intensity | 4 (12.5%) |
| Myeloablative | 28 (87.5% |
| Grade of acute GVHD at time of enrollment | |
| I | 1 (3.1%) |
| II | 19 (59.4%) |
| III | 12 (37.5%) |
| IV | 0 (0%) |
Table 2.
MPA Pharmacokinetics in weeks 1 and 2 following initiation of MMF treatment1
| Week 1 (n=29) | Week 2 (n=30) | p-value2 | |
|---|---|---|---|
| IV/PO (n) | 11/18 | 7/23 | 0.383 |
| Day posttransplant | 36.0 [17 – 81] | 46 [22 – 88] | |
| Dose 1000mg/750mg (n) | 27/2 | 28/2 | |
| Serum creatinine mg/dL | 0.90 [0.4 – 1.7] | 0.90 [0.4 – 2.8] | 0.12 |
| Total bilirubin mg/dL | 0.70 [0.2 – 19.7] | 0.70 [0.3 – 21.7] | 0.35 |
| Serum albumin g/dL | 2.95 [1.6 – 4.3] | 3.30 [1.5 – 3.9] | 0.21 |
| MPA Total MPA PK | |||
| Trough, mcg/mL | 0.465 [0.036 – 2.54] | 0.782 [0.0 –5.10] | 0.05 |
| AUC0–6, mcg*hr/mL | 20.11 [5.86 – 44.1] | 18.70 [3.8 – 53.7] | 0.61 |
| AUC0–12, mcg*hr/mL | 23.97 [7.9 – 44.4] | 21.90 [11.6 – 76.2] | 0.98 |
| MPA Unbound MPA PK | |||
| Trough, mcg/mL | 0.006 [0.0 – 0.106] | 0.015 [0.0 – 0.149] | 0.14 |
| AUC0–6, mcg*hr/mL | 0.445 [0.093 – 0.126] | 0.384 [0.099 – 2.80] | 0.80 |
| AUC0–12, mcg*hr/mL | 0.467 [0.169 – 1.33] | 0.444 [0.176 – 3.257] | 0.28 |
| MPAG trough, mcg/mL | 32.88 [3.89–163.2] | 33.76 [5.65–209.3] | 0.27 |
| MPA unbound fraction | 0.0195 [0.012–0.044] | 0.023 [0.009–0.056] | 0.44 |
Data are median [range], p is comparison of week 1 and week 2 pharmacokinetics for only those subjects with an assessment at both week 1 & week 2 (paired analysis, n=27).
Wilcoxon Signed Rank Test except where otherwise noted.
McNemar’s Test (exact p-value)
Table 3.
Mycophenolate Pharmacokinetics During IV and PO Administration in week 1 of treatment1
| Route of Administration | IV (n=11) | PO (n=18) | p-value2 |
|---|---|---|---|
| MMF dose 1000mg BID/750mg BID, n/n | 9/2 | 18/0 | 0.143 |
| Day post transplantation of pharmacokinetics | 35 [21–81] | 38.5 [17 – 74 ] | 0.67 |
| Total MPA | |||
| Trough (mcg/mL) | 0.22 [0.04 – 0.62] | 0.96 [0.04 – 2.54] | 0.0028 |
| AUC0–6, (mcg*hr/mL) | 20.36 [9.1–37.0] | 18.76 [5.86 – 44.06] | 0.57 |
| AUC0–12, (mcg *hr/mL) | 21.12 [7.9 – 32.57] | 24.0 [11.4 – 44.41] | 0.80 |
| Unbound MPA | |||
| Trough (mcg/mL) | 0.005 [0.0 – 0.027] | 0.014 [0 – 0.106] | 0.03 |
| AUC0–6, (mcg*hr/mL) | 0.612 [0.262 – 1.262] | 0.367 [0.093 – 1.15] | 0.02 |
| AUC0–12, (mcg*hr/mL) | 0.502 [0.235– 1.018] | 0.450 [0.17 – 1.33] | 0.20 |
| MPAG trough, mcg/mL | 39.29 [3.89 – 89.99] | 32.80 [4.6 – 163.23] | 0.51 |
| MPA unbound fraction | 0.029 [0.017 – 0.042] | 0.017 [0.012–0.044] | 0.005 |
Data are median [range]
p-value is comparison of IV and PO pharmacokinetics by Wilcoxon Rank Sum Test (Mann-Whitney), unless otherwise noted.
Fisher Exact Test
Total and unbound troughs were highly correlated in weeks 1 and 2 (Spearman’s rho=0.88 and 0.87, respectively, p<0.001). Total trough and total AUC0–12 were only moderately correlated in week 1 (rho=0.53, p=0.003) and week 2 (rho=0.75, p<0.001). Correlations between unbound trough and unbound AUC0–12 in weeks 1 and 2 were rho=0.49, p=0.006 and rho=0.40, p=0.028, respectively.
GVHD Response and MPA Exposure
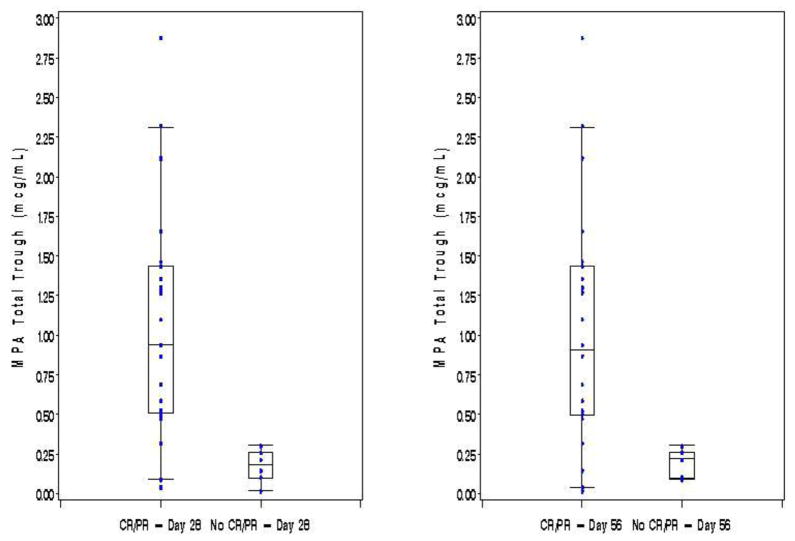
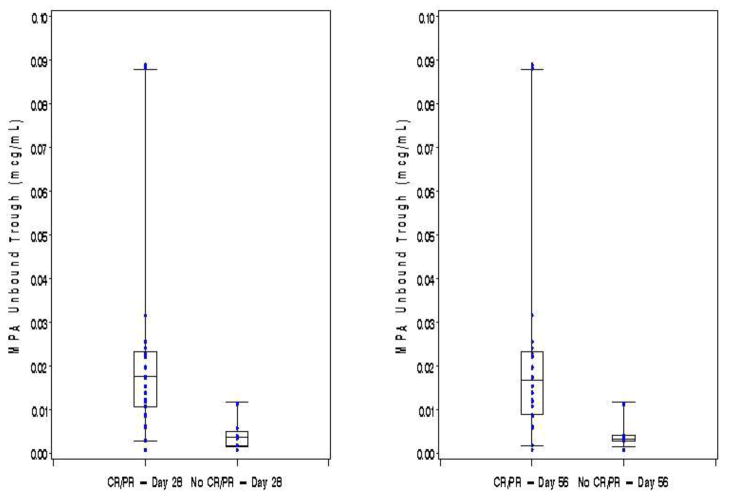
The majority of patients achieved either a CR (n=21, 66%) or PR (n=5, 15%) by day 28 after initiation of therapy while the remaining 6 patients (19%) had either NR or MR or GVHD progression. At day 56 the response rates were 78% (n=25) with CR, 6% (n=2) with PR, and 16% (n=5) with NR, MR or progression. Single time point pharmacokinetic measurements at either 1 or 2 weeks did not correlate with achieving CR at day 28 or 56. However, higher mean trough concentrations (taken by averaging the trough levels from both weeks 1 and 2) were associated with a significantly better combined CR+PR at both days 28 and 56. As shown in Figure 1, patients whose mean total trough of weeks 1 and 2 was >0.5 mcg/ml were more likely to achieve CR+PR at day 28 than patients with lower mean total troughs (19/19, 100% vs 7/13, 54%, p=0.002). At day 56, a CR+PR was also higher in those with a mean week 1 and 2 total trough >0.5 mcg/mL than those with lower troughs (19/19, 100% vs 8/13, 62%, p=0.006). Not surprisingly, higher mean unbound troughs also correlated with response to treatment. As shown in Figure 2, patients with a mean of weeks 1 and 2 unbound troughs >0.015 mcg/ml were more likely to achieve CR+PR at day 28 than patients with lower mean unbound troughs (15/15, 100% vs 11/17, 65%, p=0.02). At day 56, a CR+PR was also higher in those with a mean week 1 and 2 unbound trough >0.015 mcg/mL than those with a lower trough (15/15, 100% vs 12/17, 71%, p=0.04).
Figure 1. Total MPA Troughs by Response at Day 28 and 56 Posttreatment.
Troughs are the mean of weeks 1 and 2 in each subject. Only subjects with both week 1 and 2 pharmacokinetics are included. Box whisker plots extend to the 5th and 95th percentiles.
Figure 2. Unbound MPA Troughs by Response at Day 28 and 56 Posttreatment.
Troughs are the mean of weeks 1 and 2 in each subject. Only subjects with both week 1 and 2 pharmacokinetics are included. Box whisker plots extend to the 5th and 95th percentiles.
Substantial numbers of patients had MPA exposure below these determined trough thresholds. In week 1, 16/29 (55%) had a total trough <0.5 mcg/mL and 20/29 (69%) had unbound troughs <0.015 mcg/ml. In week 2, 11/30 (37%) patients had a total trough <0.5 mcg/ml and 15/30 (50%) had an unbound trough <0.015 mcg/mL. Interestingly, other variables including donor type, graft source, conditioning regimen, GVHD prophylaxis, and grade of GVHD at enrollment were not associated with response (all p>0.09).
Site of GVHD and MPA Exposure
GVHD organ involvement did not predict MPA exposure although the number of observations in our study was limited. In patients with only skin GVHD (n=10), the median (range) MPA total and unbound trough concentrations were 1.18 (0.02–2.11) and 0.017 (0.002–0.032) mcg/mL, respectively. Individuals with liver involvement (n=6) had total and unbound trough concentrations of 0.54 (0.10–0.99) and 0.015 (0.004–0.021) mcg/mL, respectively, whereas those without liver involvement (n=26) had similar trough concentrations of 0.81 (0.021–2.88) and 0.012 (0–0.088) mcg/mL, respectively. In patients, with lower GI involvement (n=13) total and unbound MPA concentrations were somewhat lower at 0.38 (0.04–2.88) and 0.007 (0.000–0.088) mcg/mL, respectively while in those without lower GI involvement (n=19) concentrations were generally higher at 0.99 (0.021–2.31) and 0.015 (0.0018–0.087) mcg/mL, respectively.
Infections, Overall Survival and MPA Exposure
Severe, life threatening or fatal infections (n=12, 8 viral, 3 bacterial, 1 fungal) occurred in 10 individuals by day 90 post-randomization. MPA exposure measures were not significantly different between those with and without a serious infectious event (all p>0.19). There was no association between the pharmacokinetic measures and overall survival at day 180 post randomization (p≥0.23).
Discussion
The pharmacokinetics and pharmacodynamics of MMF have been extensively studied in solid organ transplantation; however, relatively limited data are available in HCT. In organ transplant the relationship between mycophenolate pharmacokinetics and development of acute allograft rejection has been reported in numerous studies and although controversial, most support an association between higher MPA exposure and protection from acute rejection.33, 39–42 More recent data in kidney transplant showed that prophylactic calcineurin inhibitors could be safely minimized or corticosteroids withdrawn if total MPA trough concentrations were maintained >1.2 or 1.6 mcg/ml, or if AUC0–12 was ≥ 40 mcg hr/mL.27, 43 To achieve these targets, oral MMF doses of 2.5–3 gm/day were typically required.27 Higher starting doses of MMF in kidney transplant are now under investigation.33
The optimal dose of MMF in HCT remains debated. Most centers have adopted the common prophylactic dose for kidney transplant of 1 gm twice daily. However, plasma concentrations are typically lower in HCT recipients relative to organ transplantation at this dose, especially when combined with cyclosporine.18, 22, 23, 28, 34, 44–51 We have previously studied mycophenolate pharmacokinetics early post transplant after nonmyeloablative conditioning and found that low total MPA trough concentrations are associated with poorer engraftment and low unbound MPA AUCs are associated with higher rates of acute GVHD.23 Similarly, Giaccone et al showed that low total MPA exposure was associated with lower donor T-cell chimerism after nonmyeloablative HCT.28 Doses of 3 gm/day are typically required to achieve the target concentrations defined in these studies when combined with cyclosporine.37
MMF use for the treatment of acute GVHD is growing and data from BMT CTN trial 0302 suggests that MMF plus corticosteroids may have significant activity against GVHD.16 In the treatment setting, MPA pharmacokinetic disposition, exposure-response relationship and optimal dosing are unclear. Two previous studies evaluated the use of MMF for the treatment of acute and chronic GVHD and measured MPA concentrations.34, 52 There was a suggestion in both studies that individuals with higher MPA exposure had better GVHD response; however; both studies were small. In the current pharmacokinetic analysis conducted with BMT CTN 0302, we found that subjects with total MPA trough concentrations >0.5 mcg/mL or unbound troughs >0.015 mcg/mL were more likely to achieve a CR+PR at day 28 and 56 than those below these thresholds. In week 1 of MMF treatment, 55–69% of subjects and in week 2, 37–50% of subjects had MPA exposure below these therapeutically favorable thresholds. These data suggest that MMF doses >2 gm/day may be required in a substantial number of patients.
Higher doses of MMF (3 gm/day) are increasingly common in the prophylactic setting. Significantly higher donor T-cell chimerism was observed in a group of patients receiving prophylactic MMF 3 gm/day compared to 2 gm/day.53 Although there was a slightly higher rate of infection in the 3 gm/day group, no difference in overall or progression free survival was observed. Studies in kidney transplant and HCT have shown a higher incidence of leukopenia or CMV reactivation when the MPA exposure is high.28, 30, 31, 54 In our analysis, only a small number of patients achieved exposure levels potentially associated with these events. Although higher MMF doses might result in more patients’ MPA exposure in the range associated with higher risk of infectious complications, no data in the context of GVHD treatment supports this as yet. This risk may be mitigated by better GVHD control and more rapid discontinuation of immunosuppression. In the current study, no increase in infections followed higher MPA exposure.
Several factors that affect MMF pharmacokinetics warrant discussion. First, the potential effect of intestinal and liver GVHD on MPA disposition is of concern. MPA is metabolized in the intestine and liver, common sites of GVHD involvement. A previous pilot study in 14 patients treated with MMF for acute GVHD found that total MPA concentrations were lower in individuals with intestinal GVHD relative to those with skin or liver involvement.34 This was further supported in our analysis where patients with lower GI or liver GVHD had trough concentrations lower than those with skin only involvement. Larger studies with pharmacokinetic analyses will be required to determine if the site of GVHD involvement affects dosing recommendations. Second, steroids are potent inducers of glucuronidation of many substrates in vitro and may affect MPA metabolism which is highly dependent on glucuronidation to form its metabolites including the major metabolite, MPAG.55 MPAG is known to displace MPA from protein binding sites thereby increasing unbound MPA. It is possible that steroids induce glucuronidation thereby enhancing MPAG formation and lowering MPA concentrations. The effect of corticosteroids on MPA metabolism has been studied in organ transplantation with conflicting conclusions.56, 57 In our analysis, MPAG to MPA exposure ratio was high, 45–66, while ratios of around 30 have been found in other HCT studies.23, 58 This suggests that MPAG formation may be enhanced or MPAG excretion reduced. MPAG readily accumulates in renal dysfunction; however, the mean serum creatinine in our subjects was 0.9 mg/dL; therefore it is unlikely that MPAG accumulated as a result of poor renal clearance alone. Therefore, high dose corticosteroids may affect the formation of MPAG and MPA concentrations; however, this requires confirmation in formal drug interaction studies.
Interestingly, total MPA trough concentrations were higher in subjects receiving oral MMF compared to IV. We have observed this finding in our other MMF pharmacokinetic studies and suspect it may be secondary to enterohepatic recirculation of MPAG or delayed absorption of the oral solid formulations. MPA metabolites, in particular MPAG, are available in the gut and subject to deglucuronidation back to MPA which is then reabsorbed into the systemic circulation. Although CSA is known to block enterohepatic recirculation of MPA, recirculation may still occur to some extent, albeit limited, resulting in slightly higher MPA trough concentrations after oral administration.
In conclusion, MPA total trough concentrations >0.5 mcg/mL or unbound concentrations >0.015 mcg/mL may be associated with better acute GVHD response at day 28 and 56 post treatment. There was no association between MPA pharmacokinetics and infections or survival. MMF 1 gm every 12 hours achieves these threshold concentrations in approximately 50% of patients and higher doses are required in many patients to achieve these therapeutically effective targets. These data should be confirmed in future independent trials.
Footnotes
This trial is registered at http://www.clinicaltrials.gov as NCT00224874.
Financial Disclosure: This work was supported, in part, by grants from Roche Laboratories, Inc. and the NHLBI/NCI funded Blood and Marrow Transplant Clinical Trials Network
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin PJ, Schoch G, Fisher L, Byers V, Anasetti C, Appelbaum FR, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76(8):1464–1472. [PubMed] [Google Scholar]
- 2.Cragg L, Blazar BR, Defor T, Kolatker N, Miller W, Kersey J, et al. A randomized trial comparing prednisone with antithymocyte globulin/prednisone as an initial systemic therapy for moderately severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6(4A):441–447. doi: 10.1016/s1083-8791(00)70036-x. [DOI] [PubMed] [Google Scholar]
- 3.Van Lint MT, Uderzo C, Locasciulli A, Majolino I, Scime R, Locatelli F, et al. Early treatment of acute graft-versus-host disease with high- or low-dose 6-methylprednisolone: a multicenter randomized trial from the Italian Group for Bone Marrow Transplantation. Blood. 1998;92(7):2288–2293. [PubMed] [Google Scholar]
- 4.Martin PJ, Nelson BJ, Appelbaum FR, Anasetti C, Deeg HJ, Hansen JA, et al. Evaluation of a CD5-specific immunotoxin for treatment of acute graft-versus-host disease after allogeneic marrow transplantation. Blood. 1996;88(3):824–830. [PubMed] [Google Scholar]
- 5.Mayer J, Krejci M, Doubek M, Pospisil Z, Brychtova Y, Tomiska M, et al. Pulse cyclophosphamide for corticosteroid-refractory graft-versus-host disease. Bone Marrow Transplant. 2005;35(7):699–705. doi: 10.1038/sj.bmt.1704829. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter PA, Lowder J, Johnston L, Frangoul H, Khoury H, Parker P, et al. A phase II multicenter study of visilizumab, humanized anti-CD3 antibody, to treat steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11(6):465–471. doi: 10.1016/j.bbmt.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Patriarca F, Sperotto A, Damiani D, Morreale G, Bonifazi F, Olivieri A, et al. Infliximab treatment for steroid-refractory acute graft-versus-host disease. Haematologica. 2004;89(11):1352–1359. [PubMed] [Google Scholar]
- 8.Lee SJ, Zahrieh D, Agura E, MacMillan ML, Maziarz RT, McCarthy PL, Jr, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104(5):1559–1564. doi: 10.1182/blood-2004-03-0854. [DOI] [PubMed] [Google Scholar]
- 9.Berger M, Biasin E, Saglio F, Fagioli F. Innovative approaches to treat steroid-resistant or steroid refractory GVHD. Bone Marrow Transplant. 2008;42 (Suppl 2):S101–105. doi: 10.1038/bmt.2008.294. [DOI] [PubMed] [Google Scholar]
- 10.Ho VT, Zahrieh D, Hochberg E, Micale E, Levin J, Reynolds C, et al. Safety and efficacy of denileukin diftitox in patients with steroid-refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104(4):1224–1226. doi: 10.1182/blood-2004-01-0028. [DOI] [PubMed] [Google Scholar]
- 11.Bolanos-Meade J, Jacobsohn DA, Margolis J, Ogden A, Wientjes MG, Byrd JC, et al. Pentostatin in steroid-refractory acute graft-versus-host disease. J Clin Oncol. 2005;23(12):2661–2668. doi: 10.1200/JCO.2005.06.130. [DOI] [PubMed] [Google Scholar]
- 12.Levine JE, Paczesny S, Mineishi S, Braun T, Choi SW, Hutchinson RJ, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111(4):2470–2475. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basara N, Blau WI, Romer E, Rudolphi M, Bischoff M, Kirsten D, et al. Mycophenolate mofetil for the treatment of acute and chronic GVHD in bone marrow transplant patients. Bone Marrow Transplant. 1998;22(1):61–65. doi: 10.1038/sj.bmt.1701281. [DOI] [PubMed] [Google Scholar]
- 14.Shapira MY, Resnick IB, Dray L, Aker M, Stepensky P, Elad S, et al. A new induction protocol for the control of steroid refractory/dependent acute graft versus host disease with alefacept and tacrolimus. Cytotherapy. 2009;11(1):61–67. doi: 10.1080/14653240802644669. [DOI] [PubMed] [Google Scholar]
- 15.Shaughnessy PJ, Bachier C, Grimley M, Freytes CO, Callander NS, Essell JH, et al. Denileukin diftitox for the treatment of steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11(3):188–193. doi: 10.1016/j.bbmt.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Alousi AM, Weisdorf DJ, Logan BR, Bolanos-Meade J, Carter S, Difronzo N, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114(3):511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bullingham RE, Nicholls A, Hale M. Pharmacokinetics of mycophenolate mofetil (RS61443): a short review. Transplant Proc. 1996;28(2):925–929. [PubMed] [Google Scholar]
- 18.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet. 2007;46(1):13–58. doi: 10.2165/00003088-200746010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Shipkova M, Strassburg CP, Braun F, Streit F, Grone HJ, Armstrong VW, et al. Glucuronide and glucoside conjugation of mycophenolic acid by human liver, kidney and intestinal microsomes. Br J Pharmacol. 2001;132(5):1027–1034. doi: 10.1038/sj.bjp.0703898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowalgaha K, Miners JO. The glucuronidation of mycophenolic acid by human liver, kidney and jejunum microsomes. Br J Clin Pharmacol. 2001;52(5):605–609. doi: 10.1046/j.0306-5251.2001.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Gelder T, Klupp J, Barten MJ, Christians U, Morris RE. Comparison of the effects of tacrolimus and cyclosporine on the pharmacokinetics of mycophenolic acid. Ther Drug Monit. 2001;23(2):119–128. doi: 10.1097/00007691-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet. 1998;34(6):429–455. doi: 10.2165/00003088-199834060-00002. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson P, Rogosheske J, Barker JN, Green K, Ng J, Weisdorf D, et al. Relationship of mycophenolic acid exposure to clinical outcome after hematopoietic cell transplantation. Clin Pharmacol Ther. 2005;78(5):486–500. doi: 10.1016/j.clpt.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 24.van Gelder T, Hilbrands LB, Vanrenterghem Y, Weimar W, de Fijter JW, Squifflet JP, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999;68(2):261–266. doi: 10.1097/00007890-199907270-00018. [DOI] [PubMed] [Google Scholar]
- 25.Weber LT, Shipkova M, Armstrong VW, Wagner N, Schutz E, Mehls O, et al. The pharmacokinetic-pharmacodynamic relationship for total and free mycophenolic Acid in pediatric renal transplant recipients: a report of the german study group on mycophenolate mofetil therapy. J Am Soc Nephrol. 2002;13(3):759–768. doi: 10.1681/ASN.V133759. [DOI] [PubMed] [Google Scholar]
- 26.Hale MD, Nicholls AJ, Bullingham RE, Hene R, Hoitsma A, Squifflet JP, et al. The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther. 1998;64(6):672–683. doi: 10.1016/S0009-9236(98)90058-3. [DOI] [PubMed] [Google Scholar]
- 27.Le Meur Y, Buchler M, Thierry A, Caillard S, Villemain F, Lavaud S, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007;7(11):2496–2503. doi: 10.1111/j.1600-6143.2007.01983.x. [DOI] [PubMed] [Google Scholar]
- 28.Giaccone L, McCune JS, Maris MB, Gooley TA, Sandmaier BM, Slattery JT, et al. Pharmacodynamics of mycophenolate mofetil after nonmyeloablative conditioning and unrelated donor hematopoietic cell transplantation. Blood. 2005;106(13):4381–4388. doi: 10.1182/blood-2005-06-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Gelder T, Silva HT, de Fijter JW, Budde K, Kuypers D, Tyden G, et al. Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: the fixed-dose concentration-controlled trial. Transplantation. 2008;86(8):1043–1051. doi: 10.1097/TP.0b013e318186f98a. [DOI] [PubMed] [Google Scholar]
- 30.Kuypers DR, Claes K, Evenepoel P, Maes B, Vanrenterghem Y. Clinical efficacy and toxicity profile of tacrolimus and mycophenolic acid in relation to combined long-term pharmacokinetics in de novo renal allograft recipients. Clin Pharmacol Ther. 2004;75(5):434–447. doi: 10.1016/j.clpt.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Kuypers DR, de Jonge H, Naesens M, de Loor H, Halewijck E, Dekens M, et al. Current target ranges of mycophenolic acid exposure and drug-related adverse events: A 5-year, open-label, prospective, clinical follow-up study in renal allograft recipients. Clin Ther. 2008;30(4):673–683. doi: 10.1016/j.clinthera.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Kiberd BA, Lawen J, Fraser AD, Keough-Ryan T, Belitsky P. Early adequate mycophenolic acid exposure is associated with less rejection in kidney transplantation. Am J Transplant. 2004;4(7):1079–1083. doi: 10.1111/j.1600-6143.2004.00455.x. [DOI] [PubMed] [Google Scholar]
- 33.van Gelder T. Mycophenolate blood level monitoring: recent progress. Am J Transplant. 2009;9(7):1495–1499. doi: 10.1111/j.1600-6143.2009.02678.x. [DOI] [PubMed] [Google Scholar]
- 34.Kiehl MG, Shipkova M, Basara N, Blau IW, Schutz E, Armstrong VW, et al. Mycophenolate mofetil in stem cell transplant patients in relation to plasma level of active metabolite. Clin Biochem. 2000;33(3):203–208. doi: 10.1016/s0009-9120(00)00053-9. [DOI] [PubMed] [Google Scholar]
- 35.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 36.Jacobson PA, Green KG, Hering BJ. Mycophenolate mofetil in islet cell transplant: variable pharmacokinetics but good correlation between total and unbound concentrations. J Clin Pharmacol. 2005;45(8):901–909. doi: 10.1177/0091270005278599. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson P, El-Massah SF, Rogosheske J, Kerr A, Long-Boyle J, DeFor T, et al. Comparison of two mycophenolate mofetil dosing regimens after hematopoietic cell transplantation. Bone Marrow Transplant. 2009;44(2):113–120. doi: 10.1038/bmt.2008.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng J, Rogosheske J, Barker J, Weisdorf D, Jacobson PA. A limited sampling model for estimation of total and unbound mycophenolic acid (MPA) area under the curve (AUC) in hematopoietic cell transplantation (HCT) Ther Drug Monit. 2006;28(3):394–401. doi: 10.1097/01.ftd.0000211821.73231.8a. [DOI] [PubMed] [Google Scholar]
- 39.Jeong H, Kaplan B. Therapeutic monitoring of mycophenolate mofetil. Clin J Am Soc Nephrol. 2007;2(1):184–191. doi: 10.2215/CJN.02860806. [DOI] [PubMed] [Google Scholar]
- 40.van Gelder T, Le Meur Y, Shaw LM, Oellerich M, DeNofrio D, Holt C, et al. Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Ther Drug Monit. 2006;28(2):145–154. doi: 10.1097/01.ftd.0000199358.80013.bd. [DOI] [PubMed] [Google Scholar]
- 41.Knight SR, Morris PJ. Does the evidence support the use of mycophenolate mofetil therapeutic drug monitoring in clinical practice? A systematic review. Transplantation. 2008;85(12):1675–1685. doi: 10.1097/TP.0b013e3181744199. [DOI] [PubMed] [Google Scholar]
- 42.van Hest RM, Hesselink DA, Vulto AG, Mathot RA, van Gelder T. Individualization of mycophenolate mofetil dose in renal transplant recipients. Expert Opin Pharmacother. 2006;7(4):361–376. doi: 10.1517/14656566.7.4.361. [DOI] [PubMed] [Google Scholar]
- 43.Gaston RS, Kaplan B, Shah T, Cibrik D, Shaw LM, Angelis M, et al. Fixed- or controlled-dose mycophenolate mofetil with standard- or reduced-dose calcineurin inhibitors: the opticept trial. Am J Transplant. 2009;9(7):1607–1619. doi: 10.1111/j.1600-6143.2009.02668.x. [DOI] [PubMed] [Google Scholar]
- 44.van Hest RM, Doorduijn JK, de Winter BC, Cornelissen JJ, Vulto AG, Oellerich M, et al. Pharmacokinetics of mycophenolate mofetil in hematopoietic stem cell transplant recipients. Ther Drug Monit. 2007;29(3):353–360. doi: 10.1097/FTD.0b013e31805d8816. [DOI] [PubMed] [Google Scholar]
- 45.Bornhauser M, Schuler U, Porksen G, Naumann R, Geissler G, Thiede C, et al. Mycophenolate mofetil and cyclosporine as graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Transplantation. 1999;67(4):499–504. doi: 10.1097/00007890-199902270-00001. [DOI] [PubMed] [Google Scholar]
- 46.Jenke A, Renner U, Richte M, Freiberg-Richter J, Platzbecker U, Helwig A, et al. Pharmacokinetics of intravenous mycophenolate mofetil after allogeneic blood stem cell transplantation. Clin Transplant. 2001;15(3):176–184. doi: 10.1034/j.1399-0012.2001.150306.x. [DOI] [PubMed] [Google Scholar]
- 47.Basara N, Blau WI, Kiehl MG, Schmetzer B, Bischoff M, Kirsten D, et al. Mycophenolate mofetil for the prophylaxis of acute GVHD in HLA-mismatched bone marrow transplant patients. Clin Transplant. 2000;14(2):121–126. doi: 10.1034/j.1399-0012.2000.140204.x. [DOI] [PubMed] [Google Scholar]
- 48.Nash RA, Johnston L, Parker P, McCune JS, Storer B, Slattery JT, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11(7):495–505. doi: 10.1016/j.bbmt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Renner U, Platzbecker U, Freiderg-Richter J, Plettig R, Helwig A, Schafer K, et al. Intravenous mycophenolate mofetil (MMF) after allogeneic blood stem cell transplanation. Results of a dose-finding study. Blood. 1999;94:156a . [abstract 681] [Google Scholar]
- 50.Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102(6):2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 51.Okamura A, Yamamori M, Shimoyama M, Kawano Y, Kawano H, Kawamori Y, et al. Pharmacokinetics-based optimal dose-exploration of mycophenolate mofetil in allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2008;88(1):104–110. doi: 10.1007/s12185-008-0093-4. [DOI] [PubMed] [Google Scholar]
- 52.Baudard M, Vincent A, Moreau P, Kergueris MF, Harousseau JL, Milpied N. Mycophenolate mofetil for the treatment of acute and chronic GVHD is effective and well tolerated but induces a high risk of infectious complications: a series of 21 BM or PBSC transplant patients. Bone Marrow Transplant. 2002;30(5):287–295. doi: 10.1038/sj.bmt.1703633. [DOI] [PubMed] [Google Scholar]
- 53.Maris MB, Sandmaier BM, Storer B, Shizuru J, Maloney D, Agura E, et al. Unrelated peripheral blood stem cell (PBSC) transplantation using nonmyeloablative conditioning and mycophenoate mofetil (MMF) TID results in high engraftment rates. Blood. 2004;104(11):503a. [Google Scholar]
- 54.Borrows R, Chusney G, Loucaidou M, James A, Lee J, Tromp JV, et al. Mycophenolic acid 12-h trough level monitoring in renal transplantation: association with acute rejection and toxicity. Am J Transplant. 2006;6(1):121–128. doi: 10.1111/j.1600-6143.2005.01151.x. [DOI] [PubMed] [Google Scholar]
- 55.Kanou M, Usui T, Ueyama H, Sato H, Ohkubo I, Mizutani T. Stimulation of transcriptional expression of human UDP-glucuronosyltransferase 1A1 by dexamethasone. Mol Biol Rep. 2004;31(3):151–158. doi: 10.1023/b:mole.0000043582.35335.ff. [DOI] [PubMed] [Google Scholar]
- 56.Cattaneo D, Perico N, Gaspari F, Gotti E, Remuzzi G. Glucocorticoids interfere with mycophenolate mofetil bioavailability in kidney transplantation. Kidney Int. 2002;62(3):1060–1067. doi: 10.1046/j.1523-1755.2002.00531.x. [DOI] [PubMed] [Google Scholar]
- 57.Lam S, Partovi N, Ting LS, Ensom MH. Corticosteroid interactions with cyclosporine, tacrolimus, mycophenolate, and sirolimus: fact or fiction? Ann Pharmacother. 2008;42(7):1037–1047. doi: 10.1345/aph.1k628. [DOI] [PubMed] [Google Scholar]
- 58.Jacobson P, Green K, Rogosheske J, Brunstein C, Ebeling B, Defor T, et al. Highly variable mycophenolate mofetil bioavailability following nonmyeloablative hematopoietic cell transplantation. J Clin Pharmacol. 2007;47(1):6–12. doi: 10.1177/0091270006295064. [DOI] [PubMed] [Google Scholar]