Abstract
Alveolarization depends on circulating glucocorticoid (GC), retinoid (RA) and Vitamin D (VitD). Bronchopulmonary dysplasia (BPD), a leading cause of neonatal morbidity, is associated with arrested alveolarization. In hyperoxia-exposed rats displaying features of BPD, reduced levels of Lgl1 normalize during recovery. We show that GC (100nM) stimulates (7–115 fold) and VitD (100µM) suppresses (2 fold) Lgl1 expression. RA (all trans/9-cis, 10µM) effects are biphasic. From postnatal (PN) days 7–10, RA was stimulatory (2 fold) at 24h, after which effects were inhibitory (3–15 fold). Lgl1 promoter-luciferase reporter assays confirmed that these agents operated at the transcriptional level. Interestingly, the individual inhibitory effects of VitD and RA on GC induction of Lgl1 were abrogated when both agents were present, suggesting that steric hindrance may influence promoter accessibility. Analysis of the proximity (<50 base pairs) of binding sites for overlapping VitD and RA receptors to that of the GC receptor identified 81% of promoters in 66 genes (including Lgl1) important in human lung development compared to 48% in a random set of 1000 genes. Complex integration of the effects of GC, RA, and VitD on gene expression in the postnatal lung is likely to contribute to the timely advance of alveolarization without attendant inflammation.
Bronchopulmonary dysplasia (BPD), a leading cause of morbidity in preterm infants (1), is characterized by arrested alveolarization. Part of this process, secondary septation, involves subdivision of terminal air sacs into alveoli. Multiple transcription factors regulate alveolarization, including hormone receptors that activate or repress gene expression (2,3). Glucocorticoids (GCs) and retinoids (RAs) act through the GC and RA receptors (GR, RAR / RXR respectively). GR signaling inhibits secondary septation by establishing a single-layered capillary network (4). GCs have been used to treat or prevent BPD (5). However, the beneficial effects of GC are now considered to be outweighed by increased risk of impaired lung and brain growth (6).
RA has been implicated in alveolar development and regeneration (7). Postnatal(PN) RA treatment counteracts GC-induced inhibition of alveolarization (8). By contrast, RA potentiates GC stimulation of expression of mesenchymal mitogens of alveolar Type II cells. Vitamin D (VitD) is an important regulator of lung growth in utero (9).
In a search for downstream targets of GC that regulate lung maturation, we cloned Lgl1 (late gestation lung 1), a mesenchyme-specific gene (10,11) maximally expressed in late gestation and early postnatal life (10,12). Inhibition of Lgl1 impaired airway branching (13). In Lgl1 null mice, absence of Lgl1 is lethal prior to lung formation (14). Lgl1+/− heterozygotes display a complex respiratory phenotype including delayed histological maturation, features of inflammation in the newborn period and altered lung mechanics at maturity. In oxygen toxicity models of BPD, reduced levels of Lgl1 were restored during recovery (12). We therefore hypothesized that Lgl1 modulates alveolarization, subject to regulation by GC, RA, and VitD.
We report on the coordinate transcriptional regulation of Lgl1 by GC, RA, and VitD. Multiple genes important in human lung development are enriched for binding sites for GC, RA, and VitD that are in close proximity. Complex integration of the effects of GC, RA, and VitD on gene expression in the postnatal lung is likely to contribute to the timely advance of alveolarization without attendant inflammation.
MATERIALS AND METHODS
Fetal rat lung primary cell culture
All procedures involving animals were conducted according to criteria of the Animal Care Committees of the Canadian Council and the Montreal Children’s Hospital Research Institute, McGill University Health Centre. Timed pregnant Sprague-Dawley rats (day 0, mating; term, day 22) and postnatal (PN, day1, birth) were from Charles-River. Primary fibroblasts originating adjacent to the epithelium were isolated as described(10).
Cell treatment protocols
Lung primary cells reached 75–80% confluence in MEM-EBSS containing 10% FBS, penicillin (100units/mL), and streptomycin (100µg/mL). The culture conditions were changed for 24h to MEM-EBSS media containing 5% charcoal-stripped fetal bovine serum (FBS), penicillin (100units/mL) and streptomycin (100µg/mL) before being treated with hydrocortisone (cortisol, 10pM- 100nM), all-trans-retinoic acid (ATRA, 1µM–100µM), 9-cis-retinoic acid (9-cis-RA, 10µM), 1α, 25-dihydroxyvitamin D3 (VitD, 0.1 – 1000µM) for 2–72h in serum-free media. Control plates contained equal volumes of ethanol or DMSO in serum-free media (n ≥ 4).
RNA Isolation
Cells were lysed using 1ml Trizol. Total isolated RNA was resuspended in RNASecure and quantified using an ND-1000 spectrophotometer.
Quantitative Real-Time RT-PCR
Real-time/Quantitative RT-PCR (qRT-PCR) was performed in triplicate as described (12).
Transfection and Dual Reporter Gene Assays
A 901bp sequence of the 5’ flanking region of rat Lgl1 (analyzed using MatInspector® promoter inspector software (15)) was inserted between the Xho1 and Hind III sites upstream of the luciferase gene in the pGL3 basic plasmid to generate pLgl1-luc. Embryonic day (E) 20 and PN7 rat lung fibroblasts were seeded (2.5 × 105 cells/ well) in 24 well plates. The next day, cells were cotransfected in serum and antibiotics free DMEM using lipofectamine 2000 reagent, with 0.8µg pLgl1-luc or with the empty pGL3Basic plasmid (control), and 4ng of pRSV40-luc (Renilla). After 6h, media was changed to antibiotic-free DMEM containing 5% charcoal-stripped FBS and cells were grown for 24h. Transfected cells were treated with 1µM Dex, 100nM cortisol, 10µM ATRA, 10µM -9-cis-RA, 100µM VitD in DMEM containing 5% charcoal-stripped FBS (n = 3). After 24–72h, cells were washed with PBS and lysed in 100µl passive lysis buffer. Firefly- and Renilla-luciferase activities were measured using the Dual Luciferase Reporter assay system kit and lysates measured using a Monolight 3010 luminometer. Relative promoter activities are reported as ratio of firefly to renilla luciferase activity to normalize for transfection efficiency. Assays were performed in triplicate.
Generation of deletion constructs
Deletion constructs were generated using a reverse primer on the pGL3 basic plasmid and two forward primers GRE+ (−572/−552) and GRE-(−512/−492). Resulting PCR fragments were cloned in pGL3 basic plasmid using XhoI and HindIII sites. Plasmids obtained (GRE+, and GRE−) were transfected in NIH3T3 cells (in triplicate), and Luciferase activity assayed as above.
Analysis of proximity of vitamin D receptor (VDR)/RXR or VDR/RAR consensus binding sites to GR
Analysis was carried out for 66 genes (Table 1) with a previously reported role in lung development (16). One thousand randomly chosen Refseq genes were used as controls. The regulatory framework specified overlap of bindings sites of VDR and either RXR or RAR, and that the GR binding site lies within 50bp of the overlapping sites. Nineteen motifs were surveyed in (http://www.biobaseinternational.com/pages/index.php?id=transfac), and (http://thebrain.bwh.harvard.edu/uniprobe/about.php). Clover software (17) was used to search for consensus binding sites of VDR, RXR, RAR, and GR. The Regulatory Framework Enrichment Score (RFE) was computed as the proportion of selected genes that contained the regulatory framework. The Motif score threshold determines how well a motif-sequence match suggests a true binding site. RFE of 6 is the default.
Table 1.
Genes involved in human lung development
| Genes Enrichment Set | ||
|---|---|---|
| 1 | Bmp5 | bone morphogenetic protein 5 |
| 2 | Bmp7 | bone morphogenetic protein 7 |
| 3 | Crispld2 | cysteine-rich secretory protein LCCL domain containing 2 |
| 4 | Egf | epidermal growth factor |
| 5 | Eln | elastin |
| 6 | Fgf10 | fibroblast growth factor 10 |
| 7 | Fgf3 | fibroblast growth factor 3 |
| 8 | Fgf4 | fibroblast growth factor 4 |
| 9 | Fgf7 | fibroblast growth factor 7 |
| 10 | Fgfr1 | fibroblast growth factor receptor 1 |
| 11 | Fgfr2 | fibroblast growth factor receptor 2 |
| 12 | Fgfr3 | fibroblast growth factor receptor 3 |
| 13 | Fgfr4 | fibroblast growth factor receptor 4 |
| 14 | Fgfrl1 | fibroblast growth factor receptor-like 1 (FGFR-5) |
| 15 | Flt1 | FMS-like tyrosine kinase 1 |
| 16 | Foxa2 | forkhead box A2 (HNF-3β) |
| 17 | Foxf1a | forkhead box F1a (HFN-8) |
| 18 | Foxj2 | forkhead box J2 (HFH-4) |
| 19 | Gata5 | GATA binding protein 5 |
| 20 | Gars | glycyl-tRNA synthetase (SMAD1-MAD homolog 1) |
| 21 | Gli1 | GLI-Kruppel family member GLI1 |
| 22 | Kdr | kinase insert domain protein receptor |
| 23 | Mmp13 | matrix metallopeptidase 13 (Mmp1) |
| 24 | Mmp2 | matrix metallopeptidase 2 |
| 25 | Mmp9 | matrix metallopeptidase 9 |
| 26 | Nr3c1 | nuclear receptor subfamily 3, group C, member 1 |
| 27 | Pdgfa | platelet derived growth factor, alpha |
| 28 | Pdgfb | platelet derived growth factor, B polypeptide |
| 29 | Rara | retinoic acid receptor, alpha |
| 30 | Rarb | retinoic acid receptor, beta |
| 31 | Rarg | retinoic acid receptor, gamma |
| 32 | Ret | ret proto-oncogene (PTC) |
| 33 | Rxra | retinoid X receptor alpha |
| 34 | Rxrb | retinoid X receptor beta |
| 35 | Rxrg | retinoid X receptor gamma |
| 36 | Sftpb | surfactant associated protein B |
| 37 | Sftpc | surfactant associated protein C |
| 38 | Smad1 | MAD homolog 1 (GARS glycyl-tRNA synthetase) |
| 39 | Smad2 | MAD homolog 2 (forkhead box H1) |
| 40 | Smad4 | MAD homolog 4 |
| 41 | Tgfa | transforming growth factor, alpha |
| 42 | Tgfb1 | transforming growth factor, beta 1 |
| 43 | Tgfb2 | transforming growth factor, beta 2 |
| 44 | Tgfb3 | transforming growth factor, beta 3 |
| 45 | Tgfbr1 | transforming growth factor, beta receptor I |
| 46 | Timp1 | tissue inhibitor of metalloproteinase 1 |
| 47 | Timp3 | tissue inhibitor of metalloproteinase 3 |
| 48 | Tnc | tenascin C |
| 49 | Vdr | vitamin D receptor |
| 50 | Vegfa | vascular endothelial growth factor A |
| 51 | Wnt2 | wingless-related MMTV integration site 2 |
| 52 | Wnt5a | wingless-related MMTV integration site 5A |
| 53 | Wnt7b | wingless-related MMTV integration site 7B |
| Genes not in Enrichment Set | ||
| 54 | Bmp4 | bone morphogenetic protein 4 |
| 55 | Egfr | epidermal growth factor receptor |
| 56 | Gata6 | GATA binding protein 6 |
| 57 | Mmp12 | matrix metallopeptidase 12 |
| 58 | Pdgfra | platelet derived growth factor receptor, alpha polypeptide |
| 59 | Pdgfrb | platelet derived growth factor receptor, beta olypeptide |
| 60 | Shh | sonic hedgehog |
| 61 | Sftpa1 | surfactant associated protein A1 |
| 62 | Smad3 | MAD homolog 3 (Drosophila) |
| 63 | Smad7 | MAD homolog 7 |
| 64 | Sftpd | surfactant associated protein D |
| 65 | Tgfbr2 | transforming growth factor, beta receptor II |
| 66 | Nkx2-1 | NK2 homebox I |
In vivo GC-treatment of rats
Sprague-Dawley rat pups were injected i.p with 100ul of either saline or 3 mg/kg budesonide (Bud, Astra Zeneca, Lund, Sweden) on PN4, PN5, and PN6. After 72hrs, (at PN7) the lungs were collected and flash-frozen. RNA was isolated with Trizol and Lgl1 mRNA was measured by quantitative real-time PCR (QRT-PCR). n=6 for each group.
Statistical analysis
All data are presented as mean ± SEM. Statistical significance was determined by two-way Analysis of Variance (ANOVA). Pair-wise group comparisons were then assessed using Student-Neuman-Keuls test. Significance was defined as p < 0.05.
RESULTS
GC stimulation of Lgl1 in rat lung fibroblasts is temporally regulated
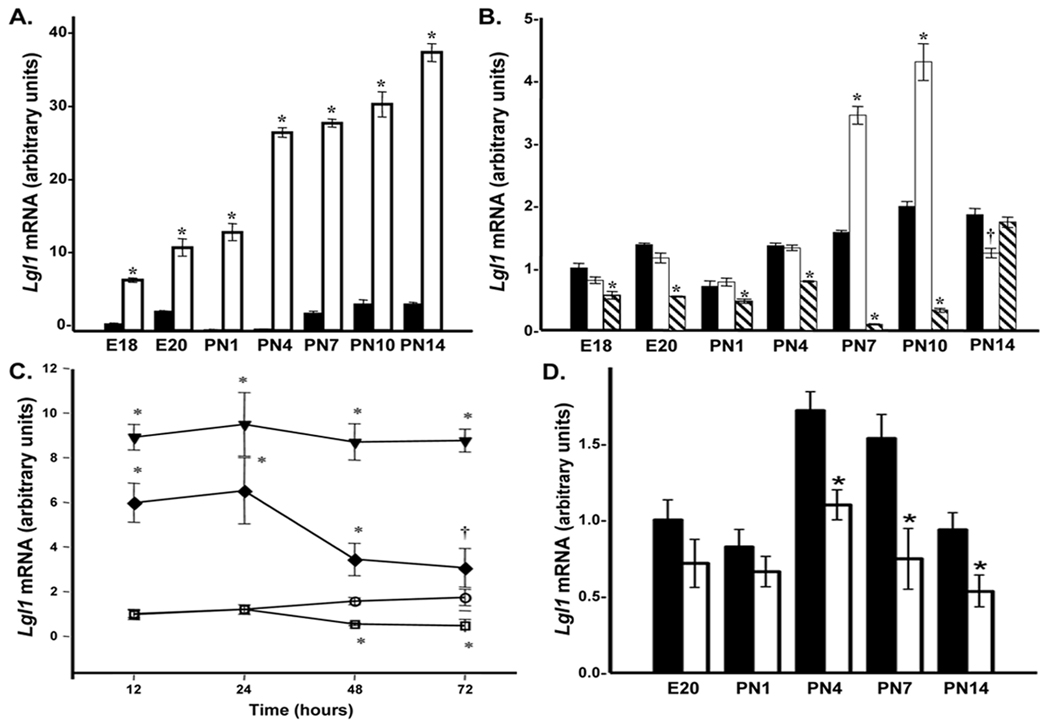
During maximal septation in rat, circulating levels of plasma corticosteroid are low. Given that lung Lgl1 levels are high during this period (12), we explored the temporal pattern of GC regulation of Lgl1 mRNA. Primary cultures of rat lung fibroblasts from E18 to PN14 were exposed to cortisol (100nM) for 24h and Lgl1 mRNA levels were determined by qRT-PCR. Cortisol stimulated Lgl1 mRNA levels at all time-points (Fig 1A) with maximal stimulation of 100fold at PN4.
Figure 1.
A. Temporal regulation of Lgl1by cortisol in rat lung fibroblasts. Rat lung fibroblasts isolated from E18 to PN14 were treated with 100nM cortisol for 24h as described in methods. Lgl1 mRNA levels were quantified by qRT-PCR (n=6). *p ≤ 0.01 Control (■) vs. GC-treated (□). B. Temporal regulation of Lgl1 by ATRA in rat lung fibroblasts. Rat lung fibroblasts isolated from E18 to PN14 were treated with 10µM ATRA for 24 and 48h as described in methods. Lgl1 mRNA levels were quantified by qRT-PCR (n=4). †p ≤ 0.05 and *p ≤ 0.01 control (■) vs. ATRA-treated 24hrs (□) and ( ) ATRA-treated 48hrs. C. Effects of coordinate treatment with cortisol and ATRA on Lgl1 mRNA in PN4 rat lung fibroblasts. Rat lung fibroblasts isolated at PN4 were treated with 100nM cortisol, 10µM ATRA or both for 24 to 72h as described in methods. Lgl1 mRNA levels were quantified by qRT-PCR (n=4). †p ≤ 0.05 and *p ≤ 0.01 control (
) ATRA-treated 48hrs. C. Effects of coordinate treatment with cortisol and ATRA on Lgl1 mRNA in PN4 rat lung fibroblasts. Rat lung fibroblasts isolated at PN4 were treated with 100nM cortisol, 10µM ATRA or both for 24 to 72h as described in methods. Lgl1 mRNA levels were quantified by qRT-PCR (n=4). †p ≤ 0.05 and *p ≤ 0.01 control ( ) vs. treated 100nM cortisol (
) vs. treated 100nM cortisol ( ), 10µM ATRA (■) and 100nM cortisol + 10µM ATRA (◆). D. Suppression of Lgl1 by VitD in rat lung fibroblasts at PN4, 7 and 14. Rat lung fibroblasts isolated from E20 to PN14 were cultivated for 24 hrs in media containing charcoal-stripped fetal bovine serum (FBS) and treated with 10µM VitD for 24h. Lgl1 mRNA levels were quantified by qRT-PCR (n=6). *p ≤ 0.01 control (■) vs. vitD-treated (□).
), 10µM ATRA (■) and 100nM cortisol + 10µM ATRA (◆). D. Suppression of Lgl1 by VitD in rat lung fibroblasts at PN4, 7 and 14. Rat lung fibroblasts isolated from E20 to PN14 were cultivated for 24 hrs in media containing charcoal-stripped fetal bovine serum (FBS) and treated with 10µM VitD for 24h. Lgl1 mRNA levels were quantified by qRT-PCR (n=6). *p ≤ 0.01 control (■) vs. vitD-treated (□).
Retinoids regulate Lgl1 expression in postnatal lung
Metabolically active forms of RA in lung fibroblasts increase after birth (7) with a parallel increase in retinoid receptors from PN8–PN12 (18). To determine whether retinoids regulate Lgl1 expression during this period via RARs (activated by ATRA and 9-cis-RA) or RXRs (activated by 9-cis-RA), we initially analyzed the effects of ATRA and 9-cis-RA on Lgl1 mRNA levels in PN7 and PN10 rat lung fibroblasts. At both time points, 9-cis-RA or ATRA had a biphasic effect on Lgl1 mRNA: weakly stimulatory after 24h but strongly inhibitory after 48h.
Primary rat lung fibroblasts from E18-PN14 were exposed to 10µM ATRA for 24 or 48h. At 24h ATRA stimulated Lgl1 mRNA 2-fold on PN7 and PN10 (Fig 1B). By contrast, after 48h exposure ATRA suppressed Lgl1 levels from E18 through PN10. Maximal inhibition by ATRA after 48h at PN10 was 15fold.
The effects on Lgl1 mRNA expression of GC and ATRA are antagonistic
Given GC stimulation and ATRA suppression of Lgl1 mRNA levels, we asked whether ATRA inhibits cortisol induced up regulation of Lgl1 mRNA. Primary rat lung fibroblasts isolated on PN4, when GC effects were maximal, were exposed to GC, ATRA, or both for 12–72h (Fig 1C). GC stimulated and ATRA suppressed Lgl1 mRNA expression. While ATRA alone had no effect on Lgl1 mRNA levels following 12–24h exposures, ATRA suppressed cortisol induced Lgl1 levels as early as 12h. ATRA suppression of cortisol-stimulated Lgl1 mRNA levels was stronger after 48h but did not completely abrogate GC stimulation. Maximal suppression of GC stimulation was from 5.5 to 2-fold.
Vitamin D suppression of Lgl1 in rat lung fibroblasts
VitD mediates maturation of late gestation alveolar Type II cells (9). To explore the effects of VitD on Lgl1 mRNA expression, rat lung fibroblasts isolated from E20-PN14 were exposed to VitD (10µM) for 24h and Lgl1 mRNA levels were determined by qRT-PCR (Fig 1D). VitD inhibited Lgl1 mRNA levels from PN4–PN14 (Fig 1D). Maximal inhibition at PN7 was 2-fold.
Combined treatment with RA and VitD does not suppress GC induction of Lgl1 mRNA
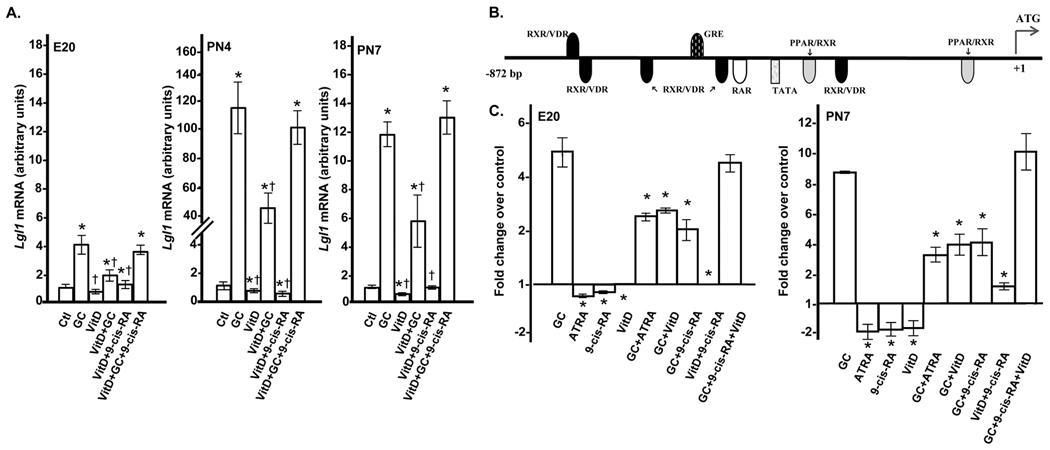
In the postnatal lung, synergistic action between VitD and RA stimulates growth of immature fibroblasts (19). As both of these agents are present in the lung during alveolarization, we determined the effects on Lgl1 mRNA of combined treatment with VitD and 9-cis-RA in the presence and absence of GC. Rat lung fibroblasts isolated at PN4 were exposed to VitD, 9-cis-RA or VitD plus 9-cis-RA for 24h. While RA alone did not impact Lgl1 mRNA expression at 24h, RA significantly suppressed GC induction of Lgl1 mRNA at this time (Fig 1C and data not shown). Combined treatment with VitD plus 9-cis-RA led to a modest inhibition of Lgl1 mRNA at PN4 and PN7 which was not additive (Fig 2A). VitD, like RA, suppressed GC induction of Lgl1 mRNA by ~50% (Fig 2A). Most interestingly, combined treatment with VitD and 9-cis-RA had no effect on GC induction of Lgl1 mRNA at all timepoints (Fig 2A).
Figure 2.
A. Effects of coordinate treatments with VitD, cortisol, and 9-cis-RA on Lgl1 expression in rat lung fibroblasts. Rat lung fibroblasts isolated at E20, PN4 and PN7 were treated with 100nM GC, 10µM VitD or combinations of 100nM GC, 10µM VitD and 10µM 9-cis-RA for 24h as described in methods. Lgl1 mRNA levels were quantified by qRT-PCR (n=6). *p ≤ 0.001, control vs. treated cells. † p ≤ 0.02, combined treatments vs. treatment with GC alone combination. B. Analysis of the 901bp 5’-flanking region of Lgl1. Representation of the Lgl1 5’-flanking promoter region showing binding sites of interest. C. Summary of luciferase assay results. Graph depicting the fold change in Lgl1 promoter activity at 48h in treated compared to control (untreated) cells at E20 andPN7. *p ≤ 0.003, combined treatments vs. treatment with GC alone.
GC, ATRA, 9-cis-RA, and VitD regulate Lgl1 promoter activity
We next explored whether GC, RA, and VitD regulate Lgl1 expression by effects on gene transcription. Consensus recognition sequences for putative binding sites for GR, RXR, RAR, and VDR are illustrated in Figure 2B. Five VDR/RXR heterodimer binding sites surround the single consensus binding site for GR with the most proximal located 38bp downstream. As a weak stimulatory effect of RA on Lgl1 expression occurred at PN7 and PN10, we carried out these experiments at E20 and PN7.
Fibroblasts isolated at E20 or PN7 were cotransfected with the luciferase expression plasmids pLgl1-Luc and pRSV40-luc (Renilla). Transfected cells were exposed to GC, ATRA, 9-cis-RA or VitD individually or in combination. Luciferase reporter activities were normalized to Renilla in two sets of experiments. In the first set, we confirmed the effects of GC and RA (ATRA and 9-cis-RA) individually or in combination. In the second set, we evaluated the effects of combined treatments with VitD and 9-cis-RA in the presence or absence of GC.
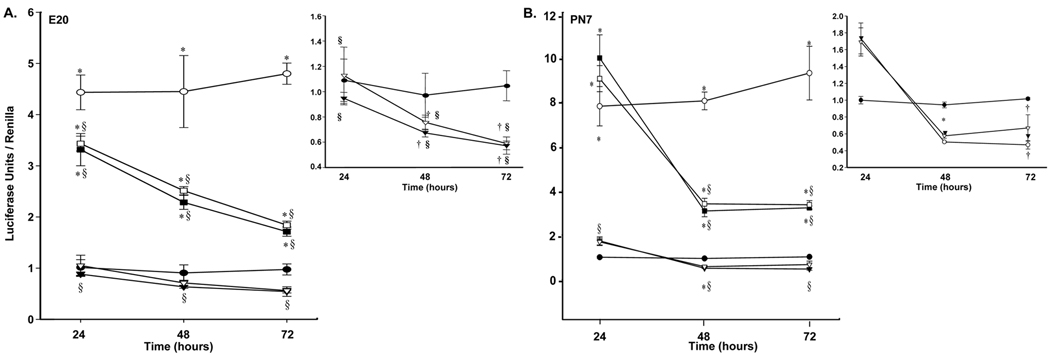
The Lgl1-reporter plasmid demonstrated significant basal activity (Fig 3A). GC, ATRA, and 9-cis-RA effects on Lgl1-promoter driven luciferase reporter activities were entirely consistent with the observed effects of these agents on Lgl1 mRNA expression levels. In E20 cells, cortisol activated the Lgl1 promoter at all time points. No significant effect on promoter activity was observed following 24h exposure to ATRA or 9-cis-RA. Exposure to cortisol plus ATRA or 9-cis-RA suppressed GC-induced promoter activity, which was time dependent.
Figure 3.
A. The effects of GC, ATRA and 9-cis-RA on Lgl1 promoter activity in E20 rat lung fibroblasts. Rat lung fibroblasts isolated at E20 were cotransfected with pLgl1-luc and pRSV40-luc (Renilla) were treated with 100nM cortisol, 10µM ATRA, 10µM 9-cis-RA or combinations of GC plus RA or GC for 24 to 72h as described in methods. Firefly- and Renilla- luciferase activities were measured. Relative promoter activities are reported as a ratio of firefly to Renilla luciferase activity to normalize for transfection efficiency (n=3). †p ≤ 0.05 and *p ≤ 0.01 control vs. treated. § p. ≤0.02, combined treatments vs. treatment with GC alone. B. The effects of GC, ATRA and 9-cis-RA on Lgl1 promoter activity in PN7 rat lung fibroblasts. Rat lung fibroblasts isolated at PN7 were cotransfected, treated and assessed as in Figure 3A (n=3). †p ≤ 0.05 and *p ≤ 0.01, control vs. treated. § p ≤ 6×10−4, combined treatments vs. treatment with GC alone. Control (●), 100nM cortisol ( ), 10µM 9-cis-RA (
), 10µM 9-cis-RA ( ), 10µM ATRA (▼), 100nM cortisol + 10µM 9-cis-RA (
), 10µM ATRA (▼), 100nM cortisol + 10µM 9-cis-RA ( ) and 100nM cortisol + 10µM ATRA (■).
) and 100nM cortisol + 10µM ATRA (■).
At PN7 (Fig 3B), GC effects on luciferase promoter activity were more pronounced. At 24hr, exposure to ATRA or 9-cis-RA for 24h was associated with weak activation of Lgl1 expression; combined treatment with GC plus RA augmented luciferase transcriptional activity above that seen with GC alone, consistent with our findings that 24h exposure to ATRA at PN7 or PN10 weakly stimulated Lgl1 mRNA expression.
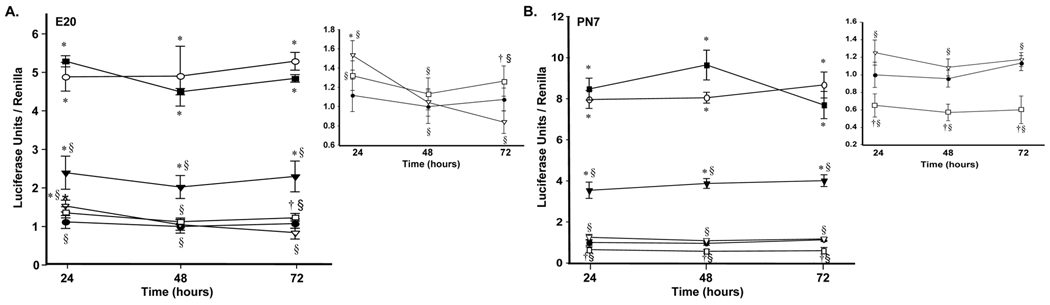
We next assessed the effects of VitD with or without RA on GC induction of Lgl1 promoter activity (Figs 4A, 4B). VitD effects on Lgl1 promoter driven luciferase reporter activity were consistent with those on Lgl1 mRNA expression levels. At E20, VitD alone had no significant effect and VitD plus 9-cis-RA weakly suppressed promoter activity at 72 h only (Fig 4A, insert). By contrast, VitD suppressed GC induction by ~50% at all time points. Similar to the case of Lgl1 mRNA, treatment with both VitD and 9-cis-RA failed to suppress GC induction of promoter activity. At PN7, VitD alone modestly inhibited Lgl1 promoter-driven luciferase activity (Fig 4B, insert). VitD plus 9-cis-RA failed to suppress GC induction of the Lgl1 promoter. The results of Lgl1 promoter-driven luciferase activity are summarized in Figure 2C.
Figure 4.
A.The effects of GC, 9-cis-RA and VitD on Lgl1 promoter activity in E20 rat lung fibroblasts. Rat lung fibroblasts isolated at E20 were cotransfected as in Figure 3A, and treated with 100nM cortisol, 10µM 9-cis-RA, 10µM VitD or combinations thereof for 24 to 72h and assessed as in Figure 3A (n=3). †p ≤ 0.05 and *p ≤ 0.01, control vs. treated. § p ≤0.009, combined treatments vs. treatment with GC alone. B. The effects of GC, 9-cis-RA and VitD on Lgl1 promoter activity in PN7 rat lung fibroblasts. Rat lung fibroblasts isolated at PN7 were cotransfected, treated and assessed as in Figure 3A (n=3). †p ≤ 0.05 and *p ≤ 0.01 control vs. treated. § p ≤6*10−4, combined treatments vs. treatment with GC alone. Control (●), 100nM cortisol ( ), 10µM VitD (
), 10µM VitD ( ), 100nM cortisol + 10µM VitD (▼), 10µM 9-cis-RA + 10µM VitD (
), 100nM cortisol + 10µM VitD (▼), 10µM 9-cis-RA + 10µM VitD ( ) and 100nM cortisol + 10µM 9-cis-RA + 10µM VitD (■).
) and 100nM cortisol + 10µM 9-cis-RA + 10µM VitD (■).
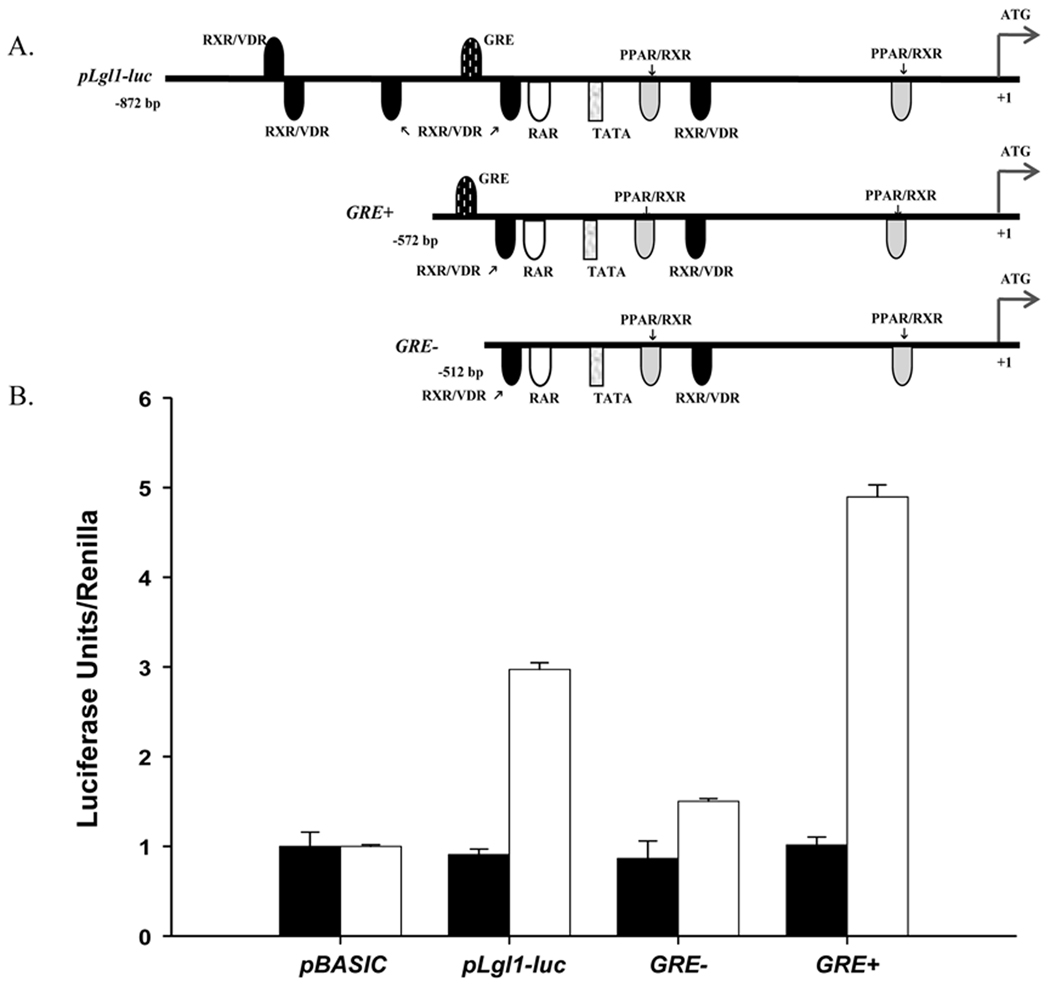
Deletion of the promoter binding site for GR suppresses GC regulation of Lgl1
As an independent confirmation of direct transcriptional regulation of Lgl1 by GC, we assessed the promoter activity of two constructs in which the binding site for GR was either eliminated (GRE−) or retained (GRE+) (Fig 5A). Lgl1-promoter driven luciferase activity was measured following transfection of constructs into NIH 3T3 cells in the absence or presence of GC (Fig 5A). In these cells, the Lgl1- reporter plasmid shows a 3-fold increase in luciferase activity over Renilla in the presence of GC (Fig 5B). When transfected with the GRE- vector, luciferase activity was suppressed 1.5-fold. Residual Lgl1 activity above that of pLgl1-Luc may reflect concomitant loss of inhibitory transcription factor effects such as the 3 upstream VDR/RXR sites. This interpretation is also supported by the finding that GRE+ shows a greater stimulation than pLgl1-Luc.
Figure 5.
Deletion of GRE in the promoter of Lgl1 abolishes the response to GC in NIH 3T3 cells. A. Schematic representation of luciferase reporter constructs. B. Effect of GC on Lgl1 promoter activity in NIH3T3 cells. NIH3T3 cells were cotransfected with pRSV40-luc (Renilla) and one of the constructs depicted in A as described in methods. Cells were treated with serum-free media with (□) or without (■) 100nM GC, for an additional 48 h. Firefly luciferase activity, normalized to Renilla, was measured.
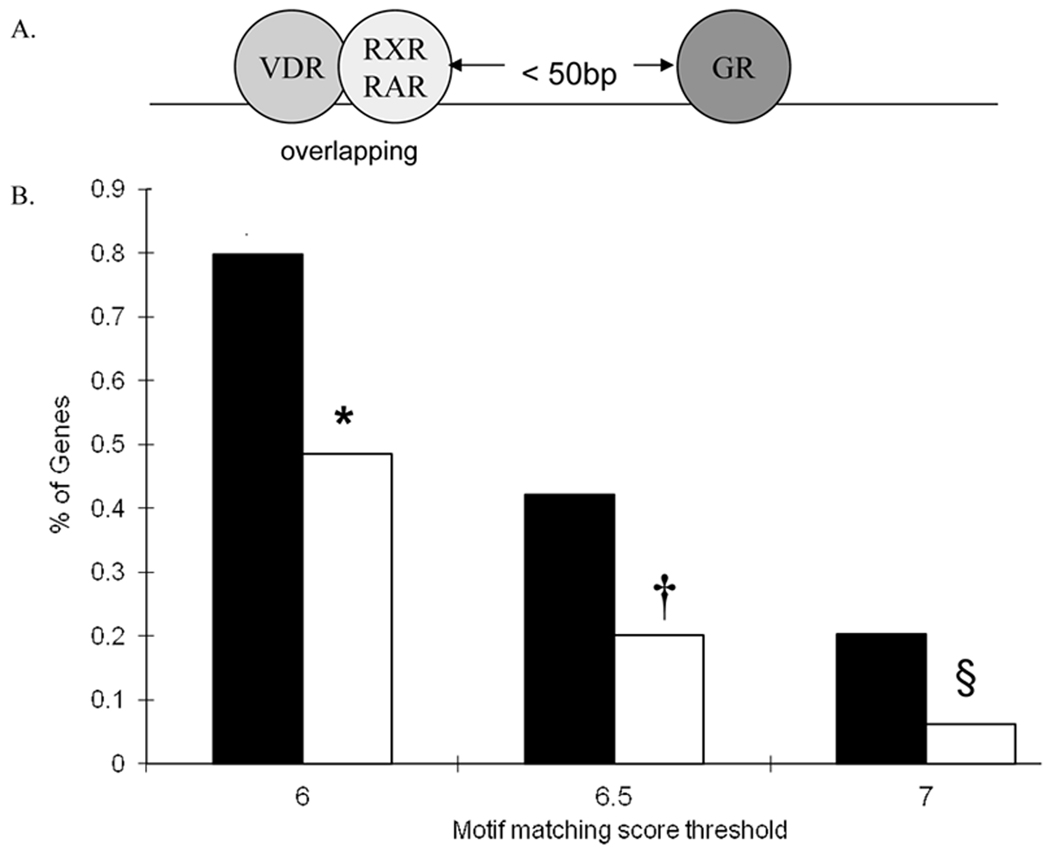
Enrichment of glucocorticoid responsive elements adjacent to overlapping VitD and RA binding sites in the promoters of lung development genes
Given that the individual effects of RA and VitD on GC stimulation of Lgl1 are abrogated when both were present, suggested that steric hindrance of transcription factor binding may occur when overlapping VDR/RXR sites closely abut GR binding sites. Since GC, RA, and VitD regulate multiple aspects of respiratory development, we interrogated the 5’ proximal promoters of 66 genes with known roles in respiratory development (Table 1) for GREs within a fixed distance of overlapping VDR/RXR (or VDR/RAR) compared to a set of 1000 random Refseq genes. The regulatory framework included genes for which the binding site of VDR and RXR (or RAR) are overlapping, and within 50bp of a GRE (Fig 6A). The Regulatory Framework Enrichment Score (RFE) describes the proportion of the 66 selected genes that satisfy the regulatory framework (Fig 6B). The “motif score threshold” is the likelihood that a motif represents a true binding site. Using a threshold of 6.0, 81 % (Table 1, Figure 6) of the Development Gene Set met the enrichment criteria compared to only 48% in the Random Set. When the threshold is raised to 7, 20% of the Development Set met the enrichment criteria compared to 6% in the Random Set.
Figure 6.
The promoters of genes important in lung development are enriched for VDR/RXR or VDR/RAR binding sites in close proximity to binding sites for GR. A. Depiction of the regulatory framework defining the gene enrichment set – VDR/RXR (or VDR/RAR) within 50bp of GR binding site. B. The percent of genes in the Gene Enrichment Set using motif matching score thresholds of 6, 6.5 and 7. Selected (■) and Random (□).
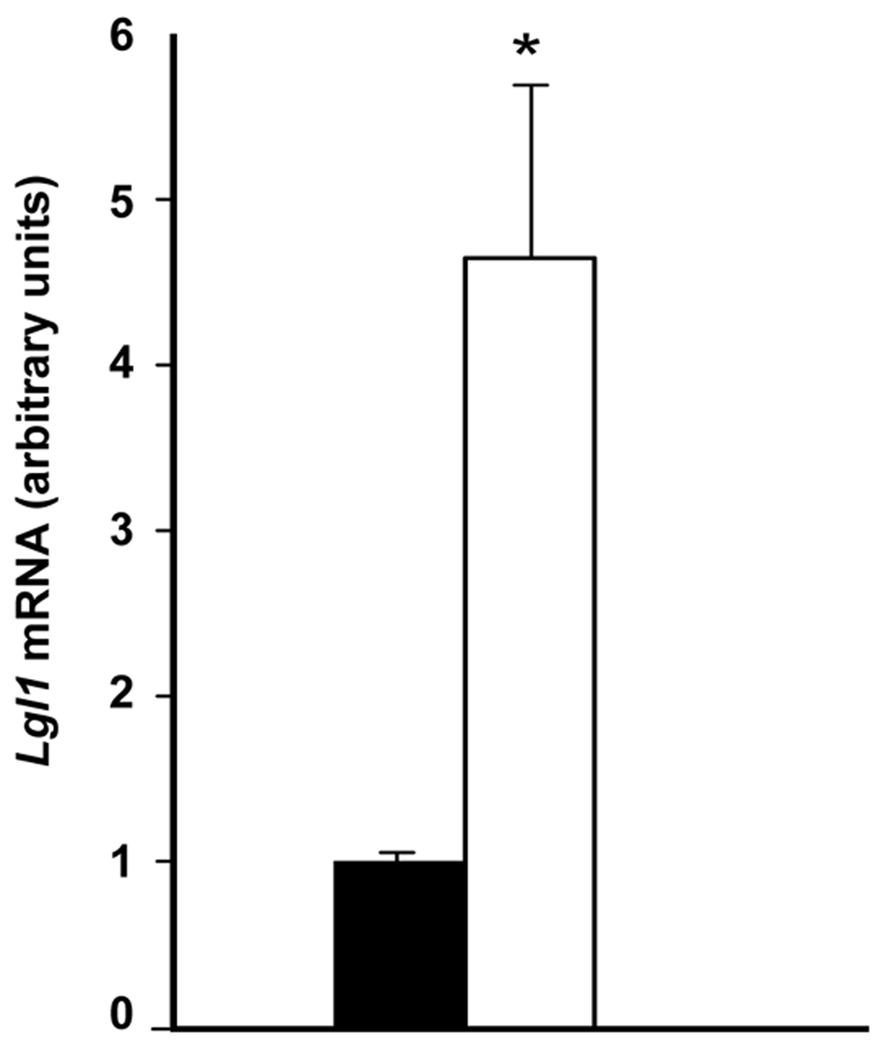
In vivo GC stimulation of Lgl1 expression in postnatal rat
To determine the stimulatory effects of GC on Lgl1 expression in developing lung in vivo, rat pups were injected daily with 3mg/kg budesonide from PN4–PN6. Lgl1 mRNA expression in whole lung RNA of GC-treated pups at PN7 was 4.5 fold (Fig 7).
Figure 7.
GC stimulation of Lgl1 mRNA in postnatal rat lung in vivo. Rat lungs isolated at PN7 following daily treatment with 3mk/kg budesonide from PN4–PN6 (n=6). Lgl1 mRNA levels were quantified by qRT-PCR. p≤ 0.01, control (■) and treated (□).
DISCUSSION
The alveolar stage of lung development represents a particularly vulnerable period in lung maturation. At birth, failure to successfully adapt to air leads to respiratory distress and may be associated with long-term sequelae (BPD). A growing body of evidence points to roles of GC, RA, and VitD in regulating both lung maturation and inflammation in the newborn period. Our studies focused on the regulation of a single gene, Lgl1, while at the same time providing novel information about the complex interactions of GC, RA, and VitD in the regulation of postnatal lung development.
Information regarding the direct molecular targets of GC and RA in lung development is limited. We were the first to report on the coincident regulation of midkine by GC and RA, providing a potential mechanism for the integration of GC and RA effects on fetal lung development (20). We now report on the coordinate transcriptional regulation of Lgl1 by GC, RA, and VitD in the postnatal lung. We show that the interaction of GC and RA on Lgl1 expression can be additive or antagonistic and that this interaction is dependent on both duration of exposure and developmental stage. Each of VitD and RA is inhibitory when present alone, but together they have no effect on the induction of Lg1l by GC, a finding with pharmacological implications.
GC is the most potent known modulator of Lgl1, stimulating expression over 100 fold at PN4. The sensitivity of Lgl1 to GC may account in part for the high levels of Lgl1 maintained in rat lung (12) from PN7–PN14 when circulating levels of GC are low (21). The biphasic effects of 9-cis-RA and ATRA between PN7 and PN10 are also of interest. During this period of increasing levels of active forms of retinoid, these agents can have opposing effects, stimulating Lgl1 expression after a short exposure (24h) and suppressing Lgl1 levels after longer exposure.
Postnatal GC treatment inhibits alveolarization (22). In animal models, some of the detrimental effects of GC on lung development may be ameliorated by simultaneous RA treatment (23,24). However, RA does not normalize Dex-induced changes in lung function (25). The potential role of VitD as a modulator of Lgl1 in postnatal lung is also of interest. To our knowledge, this is the first report of VitD modulating mesenchymal gene expression in developing lung. We speculate that Lgl1 may be a mesenchymal mediator of VitD effects on epithelial maturation.
GC, RA, and VitD regulation of pulmonary development likely operates via both direct and indirect mechanisms. We believe that this is the first example of coordinate temporal regulation of target gene promoter activity by GC, RA, and VitD during lung development. Interactions between RA and VitD increase combinatorial possibilities for gene regulation of Lgl1 by VDR, RXR, and RAR (26). In the presence of increased concentrations of these hormones, steric hindrance by VDR/RXR heterodimers may compromise the effects of either agent alone.
The enrichment of consensus binding sites for GR, VDR, RXR, and RAR in the promoter region of genes essential to normal human lung development suggests that these agents are likely to impact multiple critical developmental processes in the lung. The proximity of GR and VDR/RXR or VDR/RAR sites offers a context for complex fine-tuning of such regulatory mechanisms.
Deficiency of Lgl1 in the newborn period is associated with features of neonatal lung injury in rat (12). Lgl1 is an essential gene, required prior to lung formation (14). Haplo-insufficiency for Lgl1 in knockout mice is associated with a complex respiratory phenotype including delayed histological maturation, features of inflammation in the newborn period and altered lung mechanics at maturity. Hormones that regulate lung development also function as mediators of inflammation. Imbalance of these factors in the newborn period is likely to alter respiratory health. The effects of GC and RA in this period are well established. It is also now becoming clear that VitD is an important modulator of immune / inflammatory response (27,28). VDR expression in the lung microenvironment is required for maximal induction of lung inflammation (27).
Specific interventions that augment pulmonary maturation in a temporally appropriate manner have the potential to ameliorate hyperoxic lung injury. Abnormal lung development is also a susceptibility factor for respiratory diseases that present later in life, including COPD and asthma (29). As such, our findings may also have implications for long-term respiratory health.
Acknowledgments
Financial Support: This work was supported by grants from the Canadian Institutes of Health Research (FK and NBS), the National Heart Lung and Blood Institute of NIH (STW) and scholarships from the Montreal Children’s Hospital Research Institute (KN).
Abbreviations
- ATRA
All Trans-Retinoic Acid
- BPD
bronchopulmonary dysplasia
- E
embryonic day
- GC
glucocorticoid
- PN
postnatal
- qRT-PCR
quantitative real time- PCR
- RA
retinoic acid
- VitD
Vitamin D (1α, or 25-dihydroxyvitamin D3)
- VDR
vitamin D receptor
- RAR
retinoic acid receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jobe AH, Ikegami M. Lung development and function in preterm infants in the surfactant treatment era. Annu Rev Physiol. 2000;62:825–846. doi: 10.1146/annurev.physiol.62.1.825. [DOI] [PubMed] [Google Scholar]
- 2.Ballard PL. Hormonal regulation of pulmonary surfactant. Endocr Rev. 1989;10:165–181. doi: 10.1210/edrv-10-2-165. [DOI] [PubMed] [Google Scholar]
- 3.Chytil F. Retinoids in lung development. FASEB J. 1996;10:986–992. doi: 10.1096/fasebj.10.9.8801181. [DOI] [PubMed] [Google Scholar]
- 4.Massaro D, Massaro GD. Pre- and postnatal lung development, maturation, and plasticity. Invited review: Pulmonary alveoli: formation, the "call for oxygen," and other regulators. Am J Physiol Lung Cell Mol Physiol. 2002;282:L345–L358. doi: 10.1152/ajplung.00374.2001. [DOI] [PubMed] [Google Scholar]
- 5.Committee of Fetus and Newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109:330–338. doi: 10.1542/peds.109.2.330. [DOI] [PubMed] [Google Scholar]
- 6.Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, Donovan EF, Oh W, Bauer CR, Saha S, Poole WK, Stoll BJ National Institute of Child Health and Human Development Neonatal Research Network. Adverse Effects of Early Dexamethasone Treatment in Extremely-Low-Birth-Weight Infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 2001;344:95–101. doi: 10.1056/NEJM200101113440203. [DOI] [PubMed] [Google Scholar]
- 7.Maden M, Hind M. Retinoic acid in alveolar development, maintenance and regeneration. Philos Trans R Soc Lond B Biol Sci. 2004;359:799–808. doi: 10.1098/rstb.2004.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oshika E, Liu S, Singh G, Michalopoulos GK, Shinozuka H, Katyal SL. Antagonistic effects of dexamethasone and retinoic acid on rat lung morphogenesis. Pediatr Res. 1998;43:315–324. doi: 10.1203/00006450-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen TM, Guillozo H, Marin L, Tordet C, Koite S, Garabedian M. Evidence for a vitamin D paracrine system regulating maturation of developing rat lung epithelium. Am J Physiol. 1996;271:L392–L399. doi: 10.1152/ajplung.1996.271.3.L392. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan F, Ledoux P, Kassamali FQ, Gagnon S, Post M, Koehler D, Deimling J, Sweezey NB. A novel developmentally regulated gene in lung mesenchyme: homology to a tumor-derived trypsin inhibitor. Am J Physiol. 1999;276:L1027–L1036. doi: 10.1152/ajplung.1999.276.6.L1027. [DOI] [PubMed] [Google Scholar]
- 11.Oyewumi L, Kaplan F, Sweezey NB. Biochemical characterization of lgl1, a mesenchymal protein in fetal lung that regulates epithelial airway branching. Biochem J. 2003;376:61–69. doi: 10.1042/BJ20030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadeau K, Jankov RP, Tanswell AK, Sweezey NB, Kaplan F. Lgl1 is suppressed in oxygen toxicity animal models of bronchopulmonary dysplasia and normalizes during recovery in air. Pediatr Res. 2006;59:389–395. doi: 10.1203/01.pdr.0000198819.81785.f1. [DOI] [PubMed] [Google Scholar]
- 13.Oyewumi L, Kaplan F, Gagnon S, Sweezey NB. Antisense Oligodeoxynucleotides Decrease LGL1 mRNA and Protein Levels and Inhibit Branching Morphogenesis in Fetal Rat Lung. Am J Respir Cell Mol Biol. 2003;28:232–240. doi: 10.1165/rcmb.4877. [DOI] [PubMed] [Google Scholar]
- 14.Lan J, Ribeiro L, Mandeville I, Nadeau K, Bao T, Cornejo S, Sweezey N, Kaplan F. Inflammatory cytokines, goblet cell hyperplasia and altered lung mechanics in Lgl1+/− mice. Respir Res. 2009;10:83. doi: 10.1186/1465-9921-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quandt K, Frech K, Karas H, Wingender E, Werner T, Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23:4878–4884. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth-Kleiner M, Post M. Similarities and Dissimilarities of Branching and Septation During Lung Development. Pediatr Pulmonol. 2005;40:113–134. doi: 10.1002/ppul.20252. [DOI] [PubMed] [Google Scholar]
- 17.Frith MC, Fu Y, Yu L, Chen JF, Hansen U, Weng Z. Detection of functional DNA motifs via statistical over-representation. Nucleic Acids Res. 2004;32:1372–1381. doi: 10.1093/nar/gkh299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGowan S, Jackson S, Jenkins-Moore M, Dai H, Chambon P, Snyder J. Mice Bearing Deletions of Retinoic Acid Receptors Demonstrate Reduced Lung Elastin and Alveolar Numbers. Am J Respir Cell Mol Biol. 2000;23:162–167. doi: 10.1165/ajrcmb.23.2.3904. [DOI] [PubMed] [Google Scholar]
- 19.Liebeskind A, Srinivasan S, Kaetzel D, Bruce M. Retinoic acid stimulates immature lung fibroblast growth via a PDGF-mediated autocrine mechanism. Am J Physiol Lung Cell Mol Physiol. 2000;279:L81–L90. doi: 10.1152/ajplung.2000.279.1.L81. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan F, Comber J. The growth factor midkine is modulated by both glucocorticoid and retinoid in fetal lung development. Am J Respir Cell Mol Biol. 2003;28:33–41. doi: 10.1165/rcmb.2002-0047oc. [DOI] [PubMed] [Google Scholar]
- 21.Henning SJ. Plasma concentrations of total and free corticosterone during development in the rat. Am J Physiol. 1978;235:E451–E456. doi: 10.1152/ajpendo.1978.235.5.E451. [DOI] [PubMed] [Google Scholar]
- 22.Massaro GD, Massaro D. Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am J Physiol. 1996;270:L305–L310. doi: 10.1152/ajplung.1996.270.2.L305. [DOI] [PubMed] [Google Scholar]
- 23.Veness-Meehan KA, Pierce RA, Moats-Staats BM, Stiles AD. Retinoic acid attenuates O2-induced inhibition of lung septation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L971–L980. doi: 10.1152/ajplung.00266.2001. [DOI] [PubMed] [Google Scholar]
- 24.Massaro GD, Massaro D. Retinoic acid treatment partially rescues failed septation in rats and in mice. Am J Physiol Lung Cell Mol Physiol. 2000;278:L955–L960. doi: 10.1152/ajplung.2000.278.5.L955. [DOI] [PubMed] [Google Scholar]
- 25.Garber SJ, Zhang H, Foley JP, Zhao H, Butler SJ, Godinez RI, Godinez MH, Gow AJ, Savani RC. Hormonal regulation of alveolarization: structure-function correlation. Respir Res. 2006;7:47. doi: 10.1186/1465-9921-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatterjee M. Vitamin D and genomic stability. Mutat Res. 2001;475:69–87. doi: 10.1016/s0027-5107(01)00080-x. [DOI] [PubMed] [Google Scholar]
- 27.Wittke A, Chang A, Froicu M, Harandi OF, Weaver V, August A, Paulson RF, Cantorna MT. Vitamin D receptor expression by the lung micro-environment is required for maximal induction of lung inflammation. Arch Biochem Biophys. 2007;460:306–313. doi: 10.1016/j.abb.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–1035. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 29.Shi W, Bellusci S, Warburton D. Lung development and adult lung diseases. Chest. 2007;132:651–656. doi: 10.1378/chest.06-2663. [DOI] [PubMed] [Google Scholar]