INTRODUCTION
Fetal Alcohol Spectrum Disorders (FASD) and related disorders occur in approximately 1% of live births in the United States and represent the most common cause of mental retardation (May et al., 2009). FASD is caused by ethanol induced neurodegeneration in the developing central nervous system (CNS). Pathology in the cerebellum, hippocampus, cerebrum, corpus callosum, basal forebrain, and other brain regions persists throughout life and underlies the dysfunction associated with this disorder (Guerri et al., 2009). FASD is commonly associated with significant lifetime disability, and thus the prevention and treatment of this syndrome is greatly needed (Stratton, 1996).
The effects of ethanol on the developing cerebellum have been extensively investigated. Brain imaging of children exposed to prenatal ethanol reveals defects throughout the cerebellum (O'Hare et al., 2005). Cerebellar maturation that occurs during the third trimester of human development has been well modeled in the neonatal rat with developmental equivalence during the first 2 postnatal weeks (Clancy et al., 2001; Cudd, 2005). In the neonatal rat, the cerebellum has been studied as a prototype for ethanol effects on neuronal development. The two major neuronal populations in the cerebellum, Purkinje cells and granule cells, are susceptible to ethanol with high vulnerability on postnatal days 4–6 (Goodlett and Eilers, 1997; Napper and West, 1995; Pierce et al., 1999), with deficits in these neuronal populations persisting in the adult animal (Goodlett et al., 1991; Green et al., 2002). Ethanol toxicity in cerebellar granule cells has been particularly well studied in culture. Granule cells require trophic support which can be provided by neurotrophins or NMDA receptor activation in culture. Ethanol interferes with normal neurotrophic support resulting in death of granule cells (Bhave et al., 1999; Bonthius et al., 2003; Snell et al., 2001).
Under normal physiological conditions, microglia promote neuronal development and survival by secreting factors including neurotrophins and protective cytokines, as well as by sequestering neurotransmitters (Nakajima et al., 2001; Streit et al., 2008). However, upon CNS insult, microglia can become pathologically activated leading to neuropathology, neuroinflammation, and/or neurodegeneration [reviewed in (Kaur et al., 2010; Lucin and Wyss-Coray, 2009; Lynch, 2009; Rivest, 2009)]. Studies in adult animal models of alcohol abuse also provide clues to the effects of ethanol on microglia [reviewed in (Crews and Nixon, 2009)]. In these models, ethanol administration induces an activated morphological phenotype and stimulates production of inflammatory and neurotoxic molecules. Collectively, these studies suggest that ethanol may stimulate neuron cell death, at least in part, through stimulation of neuroinflammatory and neurodegenerative processes in the CNS.
Peroxisome proliferator-activated receptor-γ (PPAR-γ) is a member of the nuclear receptor family of proteins. The role of PPAR-γ in glucose metabolism and adipogenesis is well established (Bajaj et al., 2007). More recently, PPAR-γ has been demonstrated to modulate inflammatory responses, including those in the CNS (Bernardo et al., 2000; Diab et al., 2002; Diab et al., 2004; Drew et al., 2008; Heneka et al., 2001; Niino et al., 2001; Petrova et al., 1999; Xu and Drew, 2007). PPAR-γ is expressed on cells of the central nervous system including microglia, astrocytes, oligodendrocytes, and neurons (Bernardo and Minghetti, 2008). The cyclopentenone prostaglandin 15-deoxy-Δ12,15 prostaglandin J2 (PGJ) is an endogenous ligand of PPAR-γ, while synthetic ligands include thiazolidinediones such as pioglitazone (PIO) and rosiglitazone, which are commonly prescribed for the treatment of type II diabetes (Stumvoll and Haring, 2002; Wagstaff and Goa, 2002). Interestingly, PPAR-γ agonists inhibit the activation of primary microglia and suppress the production of pro-inflammatory cytokines and chemokines by these cells (Bernardo et al., 2000; Diab et al., 2002; Petrova et al., 1999). We and others have demonstrated that PPAR-γ agonists, including the thiazolidinediones (Heneka et al., 2001; Niino et al., 2001) and PGJ (Diab et al., 2002), inhibit the development of experimental autoimmune encephalomyelitis, an animal model of the CNS inflammatory disease multiple sclerosis. This suggests that PPAR-γ agonists may be used therapeutically to limit pathology in neuroinflammatory and neurodegenerative disease, including that resulting from ethanol exposure.
The proposed studies were designed to test the general hypotheses that ethanol induces death of microglia and neurons in vivo and in primary cultures, and that PPAR-γ agonists protect against ethanol mediated cytotoxicity. Accumulating evidence suggests that ethanol induced inflammation contributes to ethanol induced neurodegeneration. The current studies demonstrate that PPAR-γ agonists protect primary microglia and cerebellar granule cell neurons from ethanol induced toxicity. Furthermore, these studies demonstrate that PPAR-γ agonists also protect microglia and cerebellar Purkinje cell neurons from the toxic effects of ethanol using a novel in vivo mouse model of FASD. Collectively, these studies suggest that PPAR-γ agonists may be effective in protecting against the development of FASD in humans.
METHODS
Animals
C57BL/6 strain mice were obtained from Harlan Laboratories (Indianapolis, IN) and housed in the federally approved campus Division of Laboratory Animal Medicine. Adult mice were bred to produce pups for either cell cultures or experimental treatment. All animal procedures and protocols were approved by the Institutional Animal Care and Use Committee.
Culture of microglia and neurons
Primary mouse microglia cultures were prepared as described (Drew and Chavis, 2001) with modifications. Briefly, meninges were removed from the cerebral hemispheres of 1- to 4- day-old C57BL/6 mice. The cortical tissue was minced and cells were separated by trypsinization, followed by trituration. Debris was removed by filtering the cell suspension through a 70 µm cell strainer. Cells were centrifuged at 153 ×g for 5 min at 4°C and then resuspended in DMEM medium containing 10% fetal bovine serum (Hyclone; Logan, UT), 1.4 mm L-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin, OPI medium supplement, and 0.5 ng/ml mouse GM-CSF (BD Pharmingen; San Diego, CA). Except where noted otherwise, tissue culture medium and reagents were obtained from BioWhittaker (Walkersville, MD). The cells were plated into tissue culture flasks and cultured at 37°C/5% CO2 until confluent, generally 7–10 days following plating of cells. Flasks were then shaken overnight (200 rpm, 37°C) to release microglia and oligodendrocytes from the more adherent astrocytes. The suspended cells were replated for 2–3 h and flasks were shaken gently to separate oligodendrocytes from the more adherent microglia. Purified microglia were plated at 1.25 × 10 5/ cm2 in 96-well plates and cultured in standard medium modified to contain 1% fetal bovine serum and treated with vehicle or 110 mM ethanol for 4 days. In a subset of cultures, 50 µM PIO or 2.5 µM PGJ was added. Culture medium served as vehicle control. During the period of ethanol treatment, ethanol treated cultures were incubated at 37°C in closed chambers equilibrated with 5% CO2 and 110 mM ethanol in water to stably maintain the concentration of ethanol in the medium (experimentally determined to be 105–110 mM). Control cultures that were not treated with ethanol were incubated at 37°C in closed chambers equilibrated with 5% CO2 and water.
Cerebellar granule cells were prepared from 4-day-old mice as we have described previously (Kane et al., 1996) with minor modification. Cells were cultured at 2.5 × 105 cells/cm2 in poly-L-lysine coated 24-well plates in DMEM:Ham’s F12 (90:10) with 10% Hyclone calf serum, 5 mM KCl, 100 µM NMDA, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. Ara-C was added to the cultures at 3 µM from day 2–6 in order to remove contaminating proliferating cells. Cultures were returned to standard medium for 2 days. Granule cells were treated with 50 µM PIO (Cayman Chemical; Ann Arbor, MI) or 5 µM PGJ (Cayman Chemical; Ann Arbor, MI) for 24 h, which continued as cells were subsequently treated with vehicle or 110 mM ethanol for an additional 48 h. Fresh culture medium served as vehicle control. Closed, equilibrated chambers were used for both vehicle and ethanol treated granule cell cultures as described for microglial cultures above.
The purity of microglia and neuron cultures was evaluated by immunohistochemical staining. Microglia were selectively stained with isolectin B4 (Sigma; St. Louis, MO). Neurons were specifically identified with anti-MAP2 antibody (Sigma; St. Louis, MO). Astrocytes were specifically identified with glial fibrillary acidic protein antibody (Dako; Carpinteria, CA). Microglial cultures were determined to be >96% pure and granule cell cultures were determined to be >98% pure by quantification of stained cells (data not shown).
Cell viability of cultured cell populations
Cell viability of cultured microglia were determined by MTT reduction assay, as described previously (Xu and Drew, 2007). Optical density was determined using a Spectromax 190 microplate reader (Molecular Devices) at 570 nm. Results were reported as percentage of viability relative to untreated control cultures.
Cell viability of cultured cerebellar granule cell neurons were determined by unbiased counting of surviving MAP2 positive neurons. At the conclusion of experimental treatment, cells were fixed with methanol at room temperature. Cells were stained with mouse anti-MAP2 primary antibody at 1:500 dilution in PBS + 0.2% Tween-20 overnight at 4°C and Alexa 488 labeled anti-mouse secondary antibody. Cell counts were performed in photomicrographs taken with a Nikon E600 microscope. Four photomicrographs were obtained from each of 4 replicate wells in each treatment condition. Cell counts were calculated as the mean ± standard deviation. Three independent experiments were performed and the mean ± standard error of these experiments was calculated.
Animal treatment
Mice were used as a neonatal model of FASD paralleling the previously described neonatal rat model (Kane et al., 1997; Light et al., 1998; Sonderegger et al., 1982). Pups (2 days old) were separated randomly into the following treatment groups: handled control, vehicle control, ethanol treated, and agonist + ethanol treated. Ethanol treated animals were administered 3.5 g/kg/day ethanol in 15% w/v solution of vehicle by gavage as we have previously described (Kane et al., 1997; Light et al., 2002b; Light et al., 1998; Pierce et al., 1997). Blood alcohol concentrations were determined to be 250–325 mg/dl. Gavage controls were given vehicle containing isocaloric dextrose. PPAR-γ agonists were dissolved in saline and were administered by i.p. injection at the following concentrations: PIO (0.5 mg/kg/day) (Victor et al., 2006) or PGJ (0.5 mg/kg/day) (Diab et al., 2002; Natarajan and Bright, 2002). Vehicle control animals were administered the same volume of saline as the agonists and the same volume of vehicle (isocaloric dextrose) as ethanol. Handled control animals were removed from the dam for the same period as the other animals and handled only for weighing. Preliminary analysis indicated that the cell counts for the handled control and isocaloric vehicle groups were not significantly different and the isocaloric vehicle group is reported as vehicle control.
Tissue preparation and staining
Animals were anesthetized with halothane, and perfused with saline followed by 4% periodate-lysine-paraformaldehyde fixative (Whiteland et al., 1995). Serial sagittal sections of the cerebellar vermis with attached brainstem were prepared at 50 μm thickness. Immunohistochemistry was performed with calbindin D28k antibody (Light et al., 2002a) to permit identification and counting of Purkinje cells, which are the only cells to stain with anti-calbindin D28K antibody in the cerebellar vermis. Tissue sections were incubated with primary antibody at 1:500 dilution for 4 h at room temperature followed by detection with biotin-labeled anti-mouse secondary antibody and the Vectastain ABC kit streptavidin-horeseradish peroxidase complex (Vector Laboratories, Burlingame, CA). Isolectin B4 histochemistry was performed to permit identification and counting of total microglia. Tissue sections were incubated with peroxidase labeled isolectin B4 reagent at 1:50 dilution for 1 h at room temperature then 24 h at 4°C and processed with diaminobenzidine for peroxidase reaction. Every other serial section of cerebellum was processed for detection of either Purkinje cells or microglia. Each stain was performed on 9–10 sections sampled in an even distribution across the tissue, providing appropriate sampling to apply the stereological cell count method.
Cell counts with stereology
Stained cells were counted in lobule IX of the cerebellar vermis by unbiased stereology (West et al., 1991) using the Bioquant Nova® Stereology software program (Bioquant Image Analysis Corporation; Nashville, TN,) and a Nikon E600 microscope, ASI motorized stage with XYZ encoder, and Sony video camera. The limits of lobule IX were defined in two dimensions by the pial surface and a straight line defined by the nadir of the sulcus between lobule VIII and IX and the nadir of the sulcus between lobule IX and X. The counting frame was specific for each cell type based on our previous experience: microglia (75 µm × 75 µm), and Purkinje cells (120 µm × 120 µm). The dissector height was 20–30 µm and 15–20 dissectors were positioned randomly by the stereology program over the defined tissue area. Cell counts were performed by an experienced individual who was blinded to the experimental conditions. Data were tabulated in the Bioquant® database. The estimated total number of cells was calculated using the reference volume, according to the Cavalieri Principle, and the numerical cell density determined with the optical fractionator within the Bioquant® program. The coefficient of error approximated 0.08 and the nugget percent variance approximated 0.2 across measurements.
Statistical analysis of data
Statistical analysis was performed with Statview® (SAS Institute Inc., Cary, NC) software. Data derived from in vitro culture studies were analyzed by one-way ANOVA followed by the Bonferroni/Dunn post-hoc test. Data derived from in vivo animal studies were analyzed by one-way ANOVA followed by the Fisher PLSD post-hoc test.
RESULTS
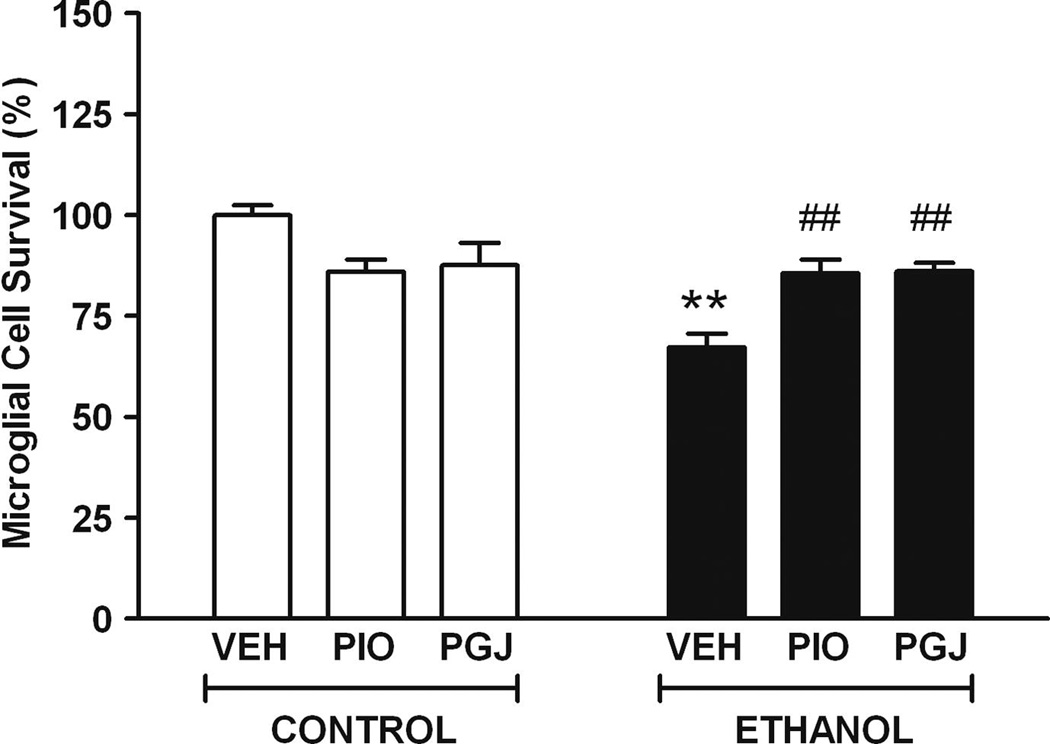
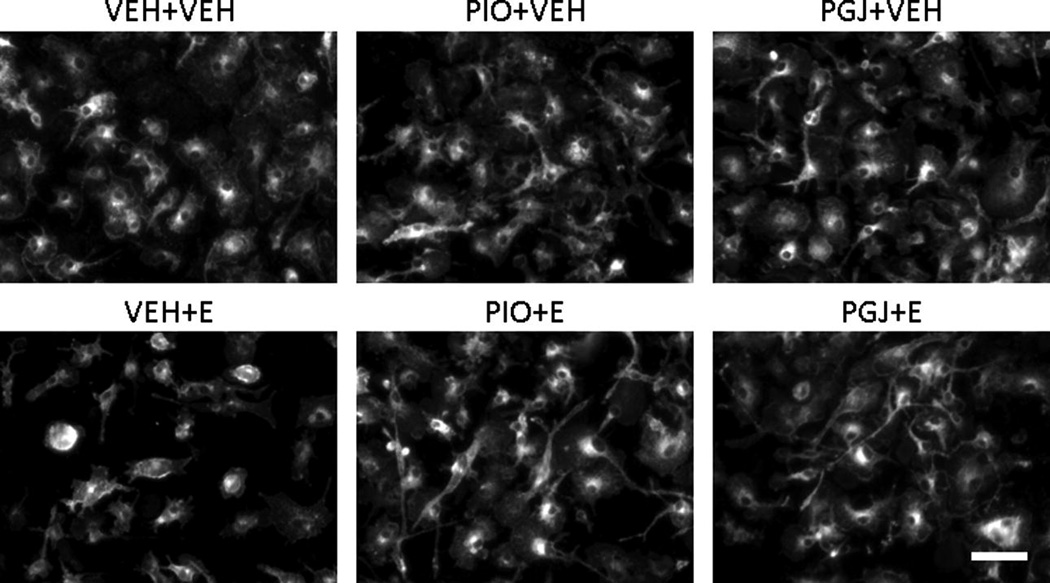
The effect of ethanol on viability and survival of microglial cells from the developing brain was modeled in cell culture. Compared to vehicle treated cultures, survival in ethanol treated cultures was 67.2 ± 3.4% (Figure 1). The surviving cells often exhibited smaller cell bodies and shorter, broader processes compared to vehicle treated control cultures (Figure 2). To determine if PPAR-γ agonists have the ability to protect against ethanol cytotoxicity, selected agonists were added to microglial cultures. In control studies, in the absence of ethanol, PPAR-γ agonists did not alter microglial cell viability or morphology relative to vehicle treated cultures. However, importantly, the thiazolidinedione PIO and the natural endogenous agonist PGJ protected microglia against ethanol mediated cell death. Collectively, these studies demonstrate that ethanol decreases viability of microglia and PPAR-γ agonists protect against ethanol mediated microglial cell death.
Figure 1. Microglial cell survival in vitro.
Primary cultures of purified microglial cells were treated with vehicle (VEH) or 110 mM ethanol (E) for 4 days, without or with 50 µM PIO or 2.5 µM PGJ. Cell survival was determined using the MTT viability assay. Data was normalized to 100% survival in vehicle treated control cultures. Results shown are the mean ± SE of three independent experiments with six replicates in each experiment. Statistical analysis was performed with one-way ANOVA and the Bonferroni/Dunn post hoc test. [F (5,12) = 8.887, p = 0.001]. (** p<0.005 compared to each of the three control conditions; ## p<0.005 compared to the VEH + ethanol condition).
Figure 2. Morphology of microglial cells in vitro.
Representative images of isolectin-stained cultures of microglial cells in primary culture. Cells were treated as described in Figure 1. Scale bar = 50 microns.
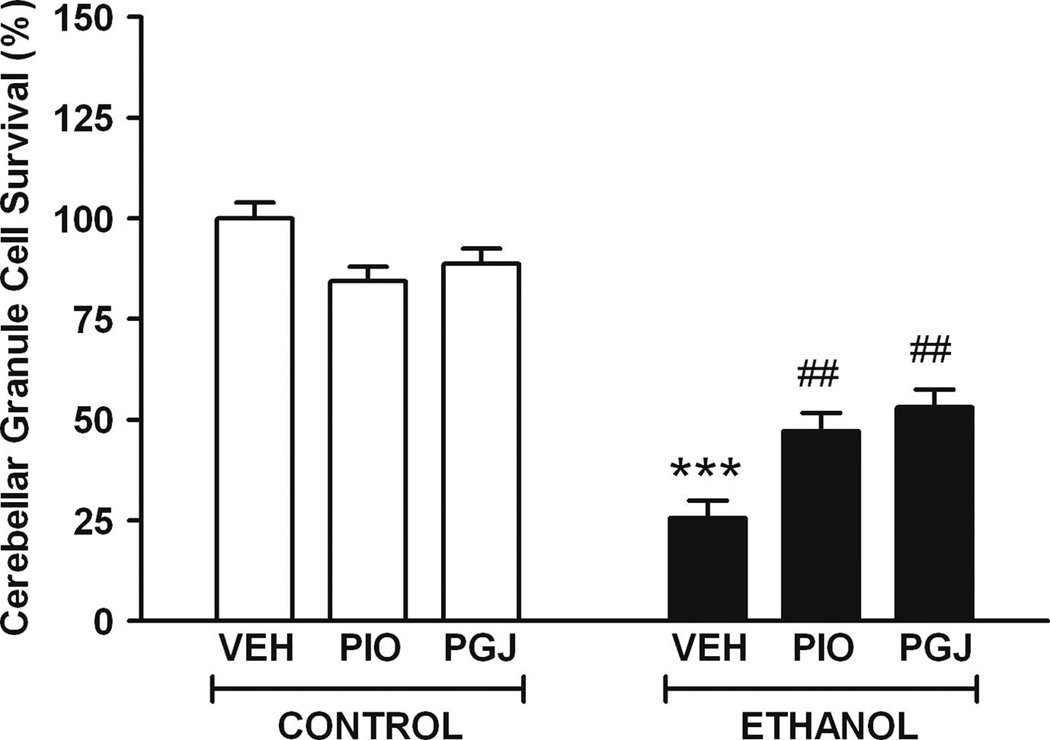
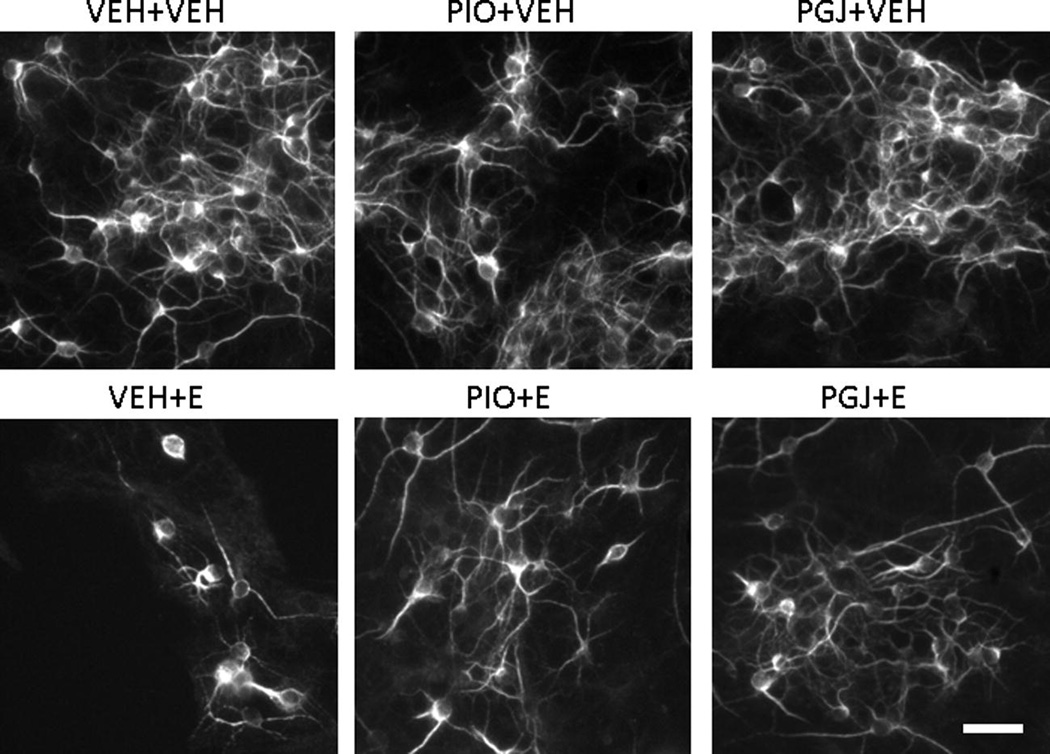
The impact of ethanol on viability and survival of granule cells from the developing cerebellum was also modeled in cell culture. Neuron cultures were treated for a shorter period of time (2 days vs. 4 days) than microglial cultures because these neurons are more sensitive to the toxic effects of ethanol under these culture conditions. Neuron survival was determined by counting cells stained by immunocytochemistry with anti-MAP2 antibody. This allowed specific positive identification and counting of granule neurons, which was particularly important since ethanol was demonstrated to be toxic to microglia (Figure 1) and microglia are known to be present, although not abundantly, in primary cerebellar granule cell cultures. Compared to vehicle treated cultures, survival in ethanol treated cultures was 25.6 ± 4.3% (Figure 3). The surviving cells often exhibited beaded processes compared to cells in vehicle treated control cultures (Figure 4). The ability of PPAR-γ agonists to protect against ethanol toxicity was also determined in the neuron cultures. In control cultures in the absence of ethanol, there was no significant difference in cell survival or morphology in the presence of either PPAR-γ agonist. However, addition of PPAR-γ agonists to cultures provided a significant level of protection against ethanol induced cell death. These studies demonstrate that ethanol decreases viability of neurons and that PPAR-γ agonists protect against ethanol mediated granule cell death.
Figure 3. Cerebellar granule cell survival in vitro.
Primary cultures of granule cells isolated from the cerebellum were treated without or with 50 µM PIO or 5 µM PGJ for 24 h followed by addition of vehicle (VEH) or 110 mM ethanol (E) for 48 h. Cell counts of MAP2-stained neurons were performed from 4 replicate photomicrographs obtained from each of 4 replicate wells per treatment condition. Data was normalized to 100% survival in vehicle treated control cultures. Results shown are the mean ± SE of three independent experiments. Statistical analysis was performed with one-way ANOVA and the Bonferroni/Dunn post hoc test. [F (5,12) = 49.450, p <0.0001]. (*** p<0.001 compared to each of the three control conditions; ## p<0.005 compared to the VEH + ethanol condition).
Figure 4. Morphology of cerebellar granule cells in vitro.
Representative images of MAP2-stained cultures of cerebellar granule cells. Cells were treated as described in Figure 3. Scale bar = 30 microns.
Neonatal rat models of FASD have been employed for some time and have productively established the current understanding of the mechanisms underlying the neuroanatomical and cellular pathology that is associated with alcohol consumption during the last trimester of brain development. Further advancement would be facilitated by development of a corresponding mouse model in order to probe and manipulate molecular signaling mechanisms during ethanol exposure. For this purpose, neonatal mice were treated in parallel to the method published for neonatal rats of the same age (Kane et al., 1997; Light et al., 1998; Sonderegger et al., 1982) in order to reproduce the developmental neuropathology produced by ethanol in the cerebellum (Goodlett and Eilers, 1997; Light et al., 2002a; Napper and West, 1995; Pierce et al., 1999).
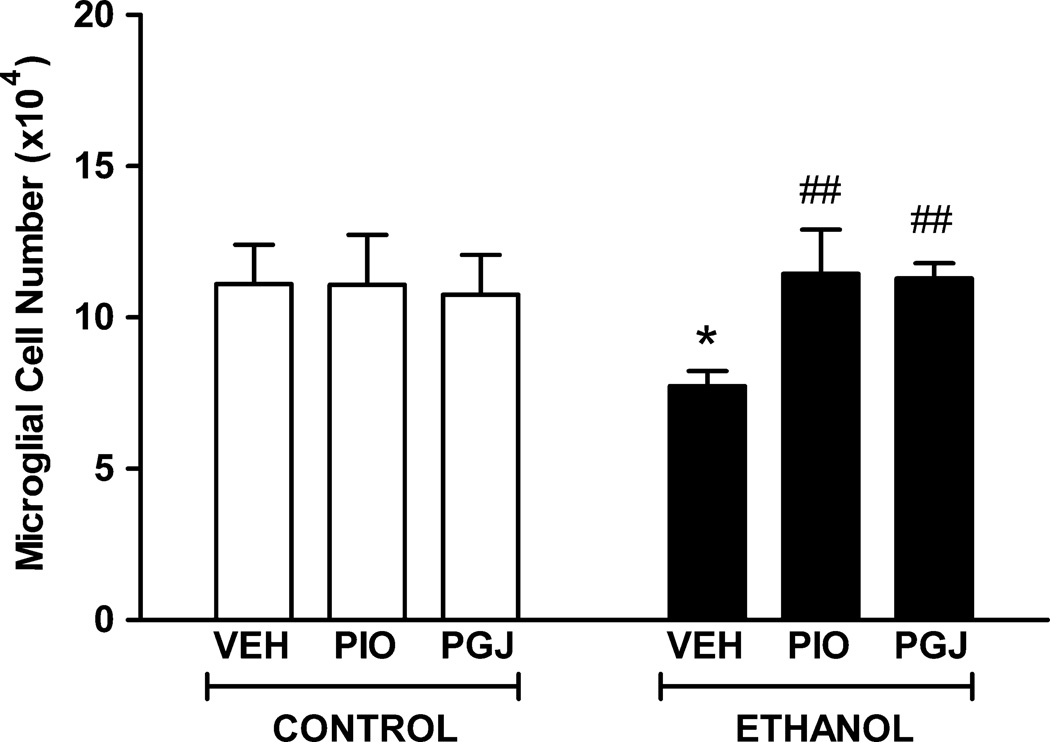
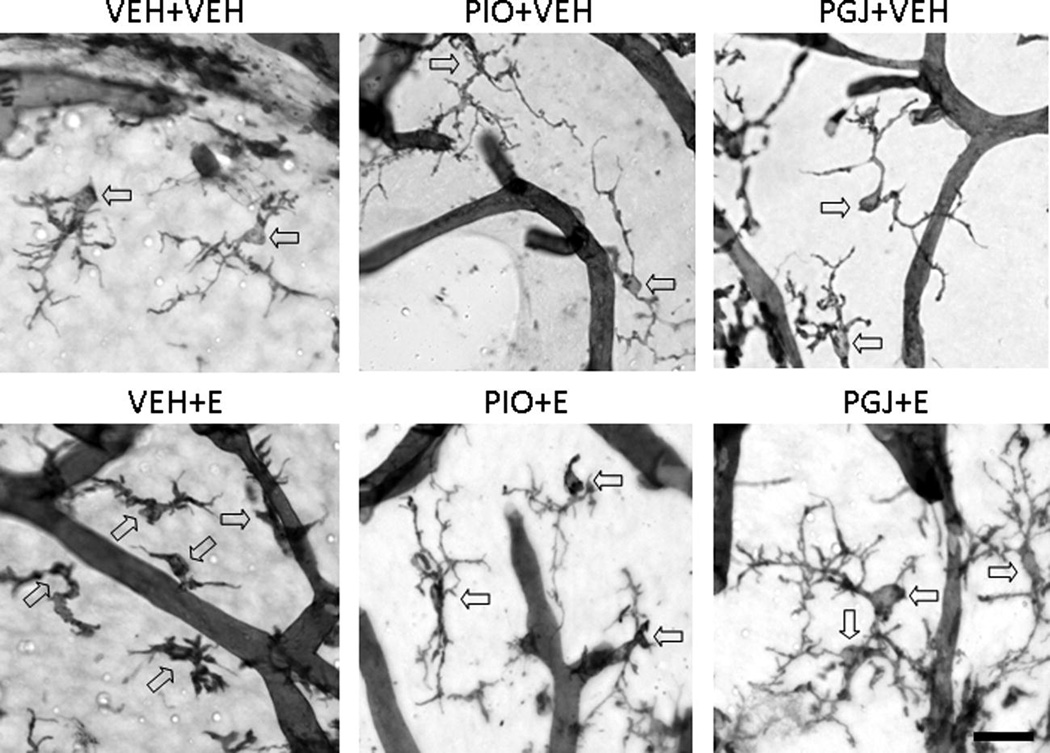
Interactions between microglia and neurons are critical to neuron development and survival. However, the impact of ethanol on microglia in the developing brain is unknown. Ethanol is cytotoxic to immature neurons in the developing cerebellum causing significant apoptosis of both Purkinje cells and granule cells when ethanol exposure occurs during the early postnatal period. To investigate whether ethanol is toxic to developing microglial cells, neonatal mice were administered ethanol by gavage. The microglial population in vehicle treated control animals was estimated by stereology to be 1.11 (± 0.13) × 105 cells in lobule IX of the vermis (Figure 5). In ethanol treated animals, the number of microglia was 0.77 (± 0.12) × 105 cells. This reflects a significant 30.4% loss of microglia due to ethanol exposure during postnatal days 3–5. The surviving microglia exhibited an altered morphology with shorter, thicker, and less elaborately branched processes compared to the thin, highly branched appearance of cell processes in control animals administered vehicle (Figure 6).
Figure 5. Protection of microglial cells in the postnatal mouse cerebellum in vivo.
Mouse pups were administered PIO or PGJ on postnatal days 2–5. Animals were given ethanol at 3.5 g/kg on postnatal days 3–5 and sacrificed on postnatal day 6. Control animals were administered vehicle (VEH) in lieu of agonist or ethanol (E). Sagittal tissue sections were stained with isolectin B4 and the number of microglial cells in the cerebellar vermis was quantified using stereological methods. Results shown are the mean ± SE of six animals in each treatment group. Statistical analysis was performed with one-way ANOVA and the Fisher PLSD post hoc test. [F (5,30) = 3.261, p = 0.0181]. (* p<0.01 compared to each of the three control conditions; ## p<0.005 compared to the VEH + ethanol condition).
Figure 6. Morphology of microglial cells in the postnatal mouse cerebellum in vivo.
Representative micrographs of isolectin B4 stained microglial cells in the cerebellum. Animals were treated as described in Figure 5. Representative microglial cells are indicated with arrows; the isolectin B4 positive tubular structures are blood vessels. Scale bar = 25 microns.
In the culture studies of primary microglia, ethanol cytotoxicity was blocked by treatment with the PPAR-γ agonists, PIO or PGJ. These agents were tested for their effectiveness to also protect microglia against ethanol in the animal model. In control studies, treatment of animals with agonist alone, in the absence of ethanol, did not alter the size of the microglial population or microglial morphology. Importantly, PPAR-γ agonists abrogated ethanol induced loss of microglia and morphological change in the developing cerebellum. The cerebellar vermes of animals treated with ethanol in conjunction with PIO contained 1.14 (± 0.36) × 105 microglia. Similarly, the cerebellar vermes of animals treated with ethanol in conjunction with PGJ contained 1.13 (± 0.12) × 105 microglia. These studies demonstrate that PPAR-γ agonists provide meaningful protection of microglia against ethanol mediated cell loss in the developing cerebellum. These studies correlate with the in vitro studies indicating that PPAR-γ agonists protect cerebral microglia from the toxic effects of ethanol. It should be noted that cerebral microglia were used in these studies instead of cerebellar microglia due to our extensive experience in characterization of cerebral microglia and due to the fact that few laboratories have successfully prepared and characterized primary cerebellar microglia.
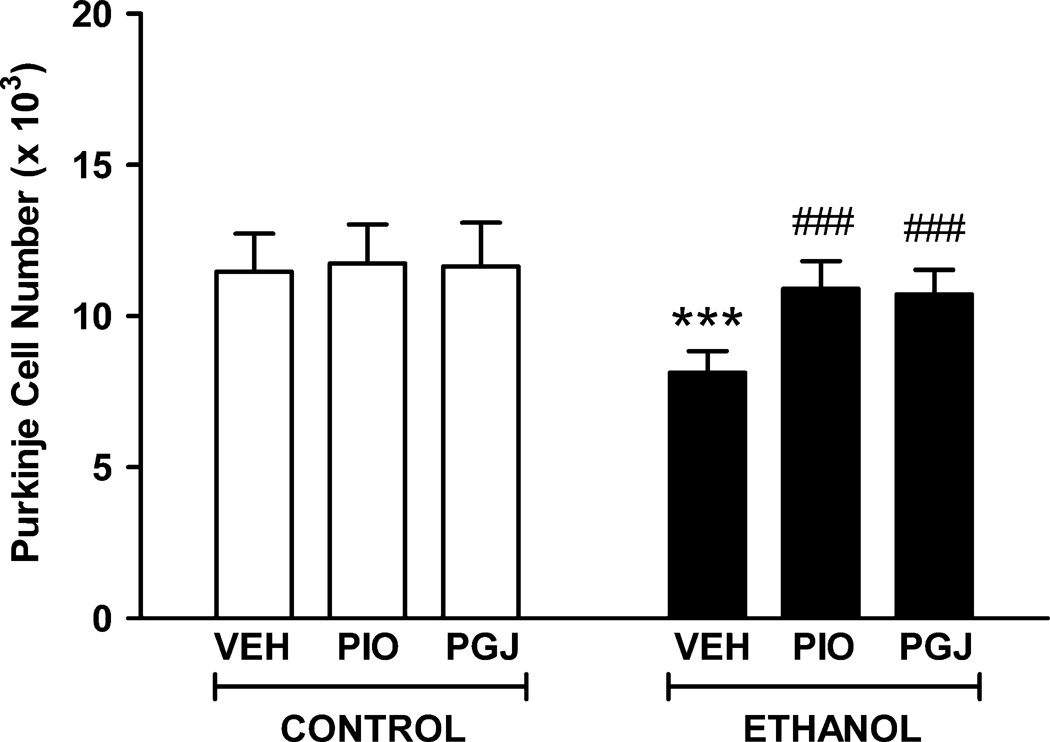
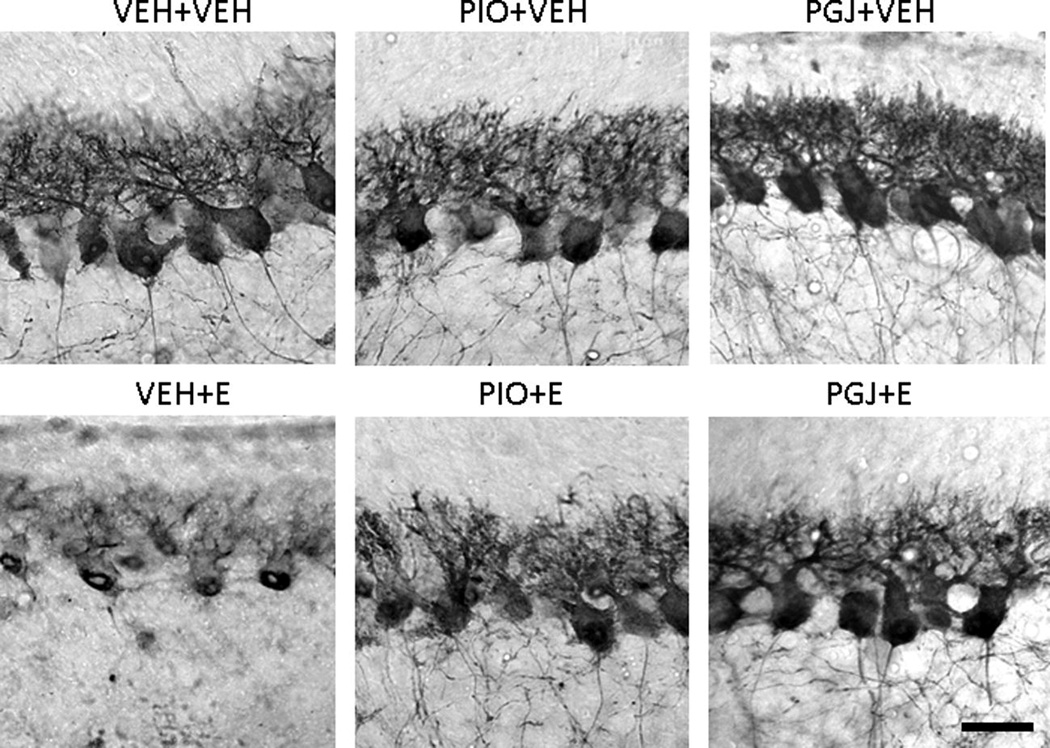
We investigated whether ethanol is toxic to developing Purkinje cells in the mouse model as we and others have previously shown in the rat model. Purkinje neurons are more sensitive to ethanol in this exposure paradigm than other cerebellar neurons and ethanol induced apoptosis of neurons is particularly well characterized in this population. The Purkinje cell population in vehicle treated control animals was estimated by stereology to be 1.15 (± 0.12) × 104 cells in lobule IX of the vermis (Figure 7). In ethanol treated animals, the number of Purkinje cells was 0.81 (± 0.07) × 104. This reflects a significant 29.1% loss of neurons due to ethanol exposure during postnatal days 3–5. There was reduced calbindin staining in the soma and dendrites of the surviving Purkinje cells in ethanol treated animals compared to control animals administered vehicle (Figure 8).
Figure 7. Protection of Purkinje cell neurons in the postnatal mouse cerebellum in vivo.
Mouse pups were administered PIO or PGJ on postnatal days 2–5. Animals were given ethanol at 3.5 g/kg on postnatal days 3–5 and tissue was harvested on postnatal day 6. Control animals were administered vehicle (VEH) in lieu of agonist or ethanol (E). Sagittal tissue sections were stained with anti-calbindin D28K antibody to identify Purkinje cells. The number of these cells in lobule IX of the cerebellar vermis was quantified using stereological methods. Results shown are the mean ± SE of six animals in each treatment group. Statistical analysis was performed with one-way ANOVA and the Fisher PLSD post hoc test. [F (5,30) = 8.932, p < 0.0001]. (*** p<0.001 compared to each of the three control conditions; ### p<0.001 compared to the VEH + ethanol condition).
Figure 8. Morphology of Purkinje cells in the postnatal mouse cerebellum in vivo.
Representative tissue sections stained with anti-calbindin D28K antibody to identify Purkinje cells in lobule IX. Animals were treated as described in Figure 7. Scale bar = 30 microns.
In the culture studies of cerebellar granule cells, the PPAR-γ agonists protected against ethanol induced neurotoxicity. The animal model was then utilitized to investigate whether neuronal vulnerability to ethanol could be blocked by these agonists in vivo. In control studies, treatment of animals with agonist alone, in the absence of ethanol, did not alter the size of the Purkinje cell population in lobule IX of the cerebellar vermis. Importantly, PPAR-γ agonists significantly reduced ethanol mediated loss of these cells in the developing cerebellum. The cerebellar vermes of animals treated with ethanol in conjunction with PIO contained 1.07 (± 0.82) × 104 Purkinje cells. Similarly, the cerebellar vermes of animals treated with ethanol in conjunction with PGJ contained 1.09 (± 0.92) × 104 Purkinje cells. These studies demonstrate that PPAR-γ agonists provide meaningful protection of neurons against ethanol mediated cell loss in the developing cerebellum. Future studies will be performed to determine if PPAR-γ agonists protect other cerebellar neurons including granule cells.
DISCUSSION
FASD is caused by exposure of the developing fetus to ethanol. It is associated with a variety of neurodevelopmental pathologies that persist throughout life resulting in devastating consequences both at an individual and a societal level. Throughout gestation, ethanol can cause dramatic effects on neuron viability and function with different neuronal populations exhibiting vulnerability at different times. For example, ethanol exposure during the later phases of brain development causes specific consequences in the cerebellum, including neuronal apoptosis (Light et al., 2002a), disrupted formation of dendrites and synapses, and dysregulated expression of neurotransmitters and their receptors, and neurotrophins and their receptors (Karacay et al., 2008; Light et al., 2002b; Light et al., 1989a, b; Pierce et al., 2010). These discoveries were possible because of the development of neonatal rat models of FASD which mimic the third trimester of brain development in the human. In the course of the present investigation, the paradigm we established previously in development of the neonatal rat model (Kane et al., 1997; Light et al., 1998) was used to establish a parallel mouse model. Accordingly, herein, we validate that the mouse model described reproduced the loss of neurons that has been reported in the rat. This was quantified by stereological cell counts of Purkinje cells in lobule IX of the vermis, which represents one of the most ethanol vulnerable regions of the brain in the neonatal rodent (Pierce et al., 1989; Pierce et al., 1999). Because the effects of ethanol on glia in the developing nervous system are less well characterized, the mouse model was then utilized to probe the impact of ethanol on microglial cells under the same in vivo treatment conditions that generated neuron cell death.
Microglia are resident immune cells in the CNS and function in innate immune responses. It is well understood that microglia have dual roles in the CNS (Kaur et al., 2010; Lucin and Wyss-Coray, 2009; Lynch, 2009; Rivest, 2009). Under normal conditions they provide trophic support to neurons. However, in response to CNS infection or injury, they undergo a progressing gradient of activation and play an important role in ridding the CNS of pathogens or removing debris resulting from injury. The effects of ethanol in stimulating an immune response by microglia is complex and appears to be determined by a variety of factors including age of the experimental organism, as well as the paradigm used in the treatment and withdrawal of ethanol (Crews and Nixon, 2009). For example, a single short term binge exposure to ethanol did not induce expression of pro-inflammatory cytokines in the CNS (Zahr et al., 2010). In contrast, intermittent binge exposure to alcohol induced an activated microglial morphological phenotype and stimulated production of inflammatory and neurotoxic molecules including NO, COX2, cytokines such as TNFα and IL1β, and chemokines including MCP1 and MIP1α and β (Alfonso-Loeches et al., 2010; He and Crews, 2008; Pascual et al., 2009; Qin et al., 2008). In other studies, ethanol induction of pro-inflammatory cytokine and chemokine expression in the CNS was cooperatively enhanced by subsequent exposure to the bacterial surface protein lipopolysaccharide, suggesting that ethanol may prime microglia to convert to an activated phenotype (Qin et al., 2008). Although molecules including NO, TNFα, and IL1β play an important role in immune responses against pathogens, these molecules can also be toxic to host cells including neurons. Collectively, these studies suggest that agents that limit ethanol induced immune responses by microglia may be effective in limiting ethanol induced neurodegeneration.
PPARs are members of the nuclear receptor family originally characterized as modulators of glucose and lipid metabolism (Bajaj et al., 2007; Desvergne and Wahli, 1999). In addition, the role of these receptors in altering immune responses has more recently become appreciated. PPAR-γ agonists have been demonstrated to suppress the development of experimental autoimmune encephalitis, an animal model of multiple sclerosis, which is characterized by CNS inflammation and associated demyelination (Diab et al., 2002; Heneka et al., 2001; Niino et al., 2001). These agonists have also been demonstrated to potently suppress the production of pro-inflammatory molecules by microglia, suggesting that agonist suppression of experimental autoimmune encephalitis occurs, at least in part, through suppression of glial cell activation (Bernardo et al., 2000; Diab et al., 2002; Diab et al., 2004; Drew et al., 2008; Heneka et al., 2001; Niino et al., 2001; Petrova et al., 1999; Xu and Drew, 2007). Currently, we demonstrate that PPAR-γ agonists protect neurons and microglia in vitro and in vivo from the toxic effects of ethanol. Since ethanol has been demonstrated to induce immune activity in the CNS, this suggests that PPAR-γ agonists may limit ethanol induced neuroinflammation and associated neurodegeneration.
Mechanistically, PPAR-γ is believed to regulate genes that encode proteins important in lipid and glucose metabolism by binding to peroxisome proliferator-activated response elements found in the promoters of these genes. In contrast, PPAR-γ agonists principally limit inflammation by suppressing the expression of genes encoding pro-inflammatory cytokines and chemokines through a process termed receptor-dependent transrepression. This process is believed to involve physical interaction between PPAR-γ and other transcription factors which normally activate genes encoding pro-inflammatory molecules resulting in suppression of the activity of these genes (Kamei et al., 1996; Kerppola et al., 1993). Examples of transcription factors that associate with PPAR-γ to suppress activation of genes encoding pro-inflammatory molecules by receptor dependent transrepression include NF-κB, AP-1, and STAT-1 (Li et al., 2000). In addition to acting through receptor-dependent mechanisms, PPAR-γ agonists have been demonstrated to suppress inflammation through receptor independent mechanisms. For example, these agonists can suppress specific steps in NF-κB signaling pathways through receptor independent mechanisms (Castrillo et al., 2000; Rossi et al., 2000; Straus et al., 2000). In the future, it will be important to determine the mechanisms by which PPAR-γ agonists protect neurons and microglia from the toxic effects of ethanol.
Previous studies have demonstrated that PPAR-γ agonists can alter the viability of neurons. This receptor is expressed by neurons suggesting that these agonists may regulate neuron viability through receptor dependent mechanisms (Inestrosa et al., 2005; Smith et al., 2003). These agonists can protect neurons from the toxic effects of agents including NMDA (Zhao et al., 2006) and apolipoprotein E4 (Brodbeck et al., 2008). The mechanisms by which PPAR-γ agonists alter neuron viability have not been completely elucidated. However, studies indicate that these agonists may modulate apoptosis of neurons through altering the expression of the anti-apoptotic factor Bcl-2. The mechanisms by which PPAR-γ induction of Bcl-2 protects neurons is not completely clear but may result from maintaining mitochondrial function and limiting the toxic effects of reactive oxygen species on these cells (Fuenzalida et al., 2007). PPAR-γ agonists could also protect neurons through production of neurotrophic factors. This possibility has not been extensively investigated and there is some indication that PPAR-γ agonists may actually reduce the expression of neurotrophic factors (Lee et al., 2010). However, ethanol has been demonstrated to increase translocation of the pro-apoptotic protein bax to the mitochondrial membrane, which is associated with neuron cell death. Interestingly, the neurotrophic factor brain derived neurotrophic factor blocks translocation of Bax and protects neurons from the toxic effects of ethanol (Heaton et al., 2006; Heaton et al., 2011). In addition to directly altering neuron cell viability, PPAR-γ agonists are capable of modulating neuron viability indirectly by suppressing glial cell activation (Combs et al., 2000; Kim et al., 2002; Zhao et al., 2006). Interestingly, in addition to altering neuron viability, PPAR-γ agonists are also capable of modulating the differentiation and proliferation of neural stem cells (Morales-Garcia et al., 2011; Wada et al., 2006). Future studies are needed to determine the mechanisms by which PPAR-γ agonists protect neurons in animal models of FASD.
The current studies tested the hypothesis that ethanol induces death of microglia and neurons in vivo and in vitro and that the ethanol neuropathology could be blocked by agonists to PPAR-γ. We determined that ethanol causes loss of microglia as well as neurons in the cerebellum in a mouse model of FASD. Further, we established in the mouse model that either synthetic or endogenous PPAR-γ agonists limit this ethanol mediated cell loss. The phenomena of ethanol induced cell death and protection by PPAR-γ agonists was reproduced in primary cultures of microglia and neurons. This suggests that these agonists may be effective therapeutically in limiting the neuropathology associated with FASD. Importantly, PPAR-γ agonists including the thiazolidinediones PIO and rosiglitazone are commonly used for the treatment of type II diabetes and have good safety profiles. This should facilitate evaluation of the therapeutic efficacy of PPAR-γ agonists in the treatment of neuroinflammatory and neurodegenerative disorders, perhaps including ethanol mediated disease. To this end, additional basic research is needed to better determine the mechanisms by which PPAR-γ agonists protect neuronal cells from the toxic effects of ethanol, which would facilitate a rational approach to treatment of ethanol induced neuropathology.
ACKNOWLEDGMENTS
This research was funded by the National Institutes of Health awards AA12756, AA14888, AA14645, and AA18834.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
REFERENCES
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj M, Suraamornkul S, Hardies LJ, Glass L, Musi N, DeFronzo RA. Effects of peroxisome proliferator-activated receptor (PPAR)-alpha and PPAR-gamma agonists on glucose and lipid metabolism in patients with type 2 diabetes mellitus. Diabetologia. 2007;50:1723–1731. doi: 10.1007/s00125-007-0698-9. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Levi G, Minghetti L. Role of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) and its natural ligand 15-deoxy-Delta12, 14-prostaglandin J2 in the regulation of microglial functions. Eur J Neurosci. 2000;12:2215–2223. doi: 10.1046/j.1460-9568.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Minghetti L. Regulation of Glial Cell Functions by PPAR-gamma Natural and Synthetic Agonists. PPAR Res. 2008;2008:864140. doi: 10.1155/2008/864140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave SV, Ghoda L, Hoffman PL. Brain-derived neurotrophic factor mediates the anti-apoptotic effect of NMDA in cerebellar granule neurons: signal transduction cascades and site of ethanol action. J Neurosci. 1999;19:3277–3286. doi: 10.1523/JNEUROSCI.19-09-03277.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, Karacay B, Dai D, Pantazis NJ. FGF-2, NGF and IGF-1, but not BDNF, utilize a nitric oxide pathway to signal neurotrophic and neuroprotective effects against alcohol toxicity in cerebellar granule cell cultures. Brain Res Dev Brain Res. 2003;140:15–28. doi: 10.1016/s0165-3806(02)00549-7. [DOI] [PubMed] [Google Scholar]
- Brodbeck J, Balestra ME, Saunders AM, Roses AD, Mahley RW, Huang Y. Rosiglitazone increases dendritic spine density and rescues spine loss caused by apolipoprotein E4 in primary cortical neurons. Proc Natl Acad Sci U S A. 2008;105:1343–1346. doi: 10.1073/pnas.0709906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo A, Diaz-Guerra MJ, Hortelano S, Martin-Sanz P, Bosca L. Inhibition of IkappaB kinase and IkappaB phosphorylation by 15-deoxy-Delta(12,14)-prostaglandin J(2) in activated murine macrophages. Mol Cell Biol. 2000;20:1692–1698. doi: 10.1128/mcb.20.5.1692-1698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer's disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci. 2000;20:558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood) 2005;230:389–393. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, Drew PD, Racke MK. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- Diab A, Hussain RZ, Lovett-Racke AE, Chavis JA, Drew PD, Racke MK. Ligands for the peroxisome proliferator-activated receptor-gamma and the retinoid X receptor exert additive anti-inflammatory effects on experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:116–126. doi: 10.1016/j.jneuroim.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Drew PD, Chavis JA. The cyclopentone prostaglandin 15-deoxy-Delta(12,14) prostaglandin J2 represses nitric oxide, TNF-alpha, and IL-12 production by microglial cells. J Neuroimmunol. 2001;115:28–35. doi: 10.1016/s0165-5728(01)00267-3. [DOI] [PubMed] [Google Scholar]
- Drew PD, Xu J, Racke MK. PPAR-gamma: Therapeutic Potential for Multiple Sclerosis. PPAR Res. 2008;2008:627463. doi: 10.1155/2008/627463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuenzalida K, Quintanilla R, Ramos P, Piderit D, Fuentealba RA, Martinez G, Inestrosa NC, Bronfman M. Peroxisome proliferator-activated receptor gamma up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J Biol Chem. 2007;282:37006–37015. doi: 10.1074/jbc.M700447200. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Eilers AT. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: a stereological study of temporal windows of vulnerability. Alcohol Clin Exp Res. 1997;21:738–744. [PubMed] [Google Scholar]
- Goodlett CR, Thomas JD, West JR. Long-term deficits in cerebellar growth and rotarod performance of rats following "binge-like" alcohol exposure during the neonatal brain growth spurt. Neurotoxicol Teratol. 1991;13:69–74. doi: 10.1016/0892-0362(91)90029-v. [DOI] [PubMed] [Google Scholar]
- Green JT, Tran T, Steinmetz JE, Goodlett CR. Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eyeblink conditioning in adult rats. Brain Res. 2002;956:302–311. doi: 10.1016/s0006-8993(02)03561-8. [DOI] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP. Foetal Alcohol Spectrum Disorders and alterations in brain and behaviour. Alcohol Alcohol. 2009;44:108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Madorsky I, Siler-Marsiglio K, Shaw G. Effect of bax deletion on ethanol sensitivity in the neonatal rat cerebellum. J Neurobiol. 2006;66:95–101. doi: 10.1002/neu.20208. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Siler-Marsiglio K. Ethanol influences on Bax translocation, mitochondrial membrane potential, and reactive oxygen species generation are modulated by vitamin E and brain-derived neurotrophic factor. Alcohol Clin Exp Res. 2010;35:1–12. doi: 10.1111/j.1530-0277.2011.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Landreth GE, Feinstein DL. Role for peroxisome proliferator-activated receptor-gamma in Alzheimer's disease. Ann Neurol. 2001;49:276. doi: 10.1002/1531-8249(20010201)49:2<276::aid-ana53>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Godoy JA, Quintanilla RA, Koenig CS, Bronfman M. Peroxisome proliferator-activated receptor gamma is expressed in hippocampal neurons and its activation prevents beta-amyloid neurodegeneration: role of Wnt signaling. Exp Cell Res. 2005;304:91–104. doi: 10.1016/j.yexcr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin SC, Heyman RA, Rose DW, Glass CK, Rosenfeld MG. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- Kane CJM, Pierce DR, Nyamweya NN, Yang H, Kasmi Y, Mosby R, Serbus DC, Light KE. Nutritional factors modify the inhibition of CNS development by combined exposure to methadone and ethanol in neonatal rats. Pharmacol Biochem Behav. 1997;56:399–407. doi: 10.1016/s0091-3057(96)00239-0. [DOI] [PubMed] [Google Scholar]
- Karacay B, Li S, Bonthius DJ. Maturation-dependent alcohol resistance in the developing mouse: cerebellar neuronal loss and gene expression during alcohol-vulnerable and-resistant periods. Alcohol Clin Exp Res. 2008;32:1439–1450. doi: 10.1111/j.1530-0277.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- Kaur G, Han SJ, Yang I, Crane C. Microglia and central nervous system immunity. Neurosurg Clin N Am. 2010;21:43–51. doi: 10.1016/j.nec.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Kerppola TK, Luk D, Curran T. Fos is a preferential target of glucocorticoid receptor inhibition of AP-1 activity in vitro. Mol Cell Biol. 1993;13:3782–3791. doi: 10.1128/mcb.13.6.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kwon KJ, Park JY, Lee SH, Moon CH, Baik EJ. Effects of peroxisome proliferator-activated receptor agonists on LPS-induced neuronal death in mixed cortical neurons: associated with iNOS and COX-2. Brain Res. 2002;941:1–10. doi: 10.1016/s0006-8993(02)02480-0. [DOI] [PubMed] [Google Scholar]
- Lee CH, Choi JH, Yoo KY, Park OK, Moon JB, Sohn Y, Cho JH, Hwang IK, Won MH. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, decreases immunoreactivity of markers for cell proliferation and neuronal differentiation in the mouse hippocampus. Brain Res. 2010;1329:30–35. doi: 10.1016/j.brainres.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Li M, Pascual G, Glass CK. Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol Cell Biol. 2000;20:4699–4707. doi: 10.1128/mcb.20.13.4699-4707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light KE, Belcher SM, Pierce DR. Time course and manner of Purkinje neuron death following a single ethanol exposure on postnatal day 4 in the developing rat. Neuroscience. 2002a;114:327–337. doi: 10.1016/s0306-4522(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Light KE, Brown DP, Newton BW, Belcher SM, Kane CJ. Ethanol-induced alterations of neurotrophin receptor expression on Purkinje cells in the neonatal rat cerebellum. Brain Res. 2002b;924:71–81. doi: 10.1016/s0006-8993(01)03224-3. [DOI] [PubMed] [Google Scholar]
- Light KE, Kane CJ, Pierce DR, Jenkins D, Ge Y, Brown G, Yang H, Nyamweya N. Intragastric intubation: important aspects of the model for administration of ethanol to rat pups during the postnatal period. Alcohol Clin Exp Res. 1998;22:1600–1606. doi: 10.1111/j.1530-0277.1998.tb03954.x. [DOI] [PubMed] [Google Scholar]
- Light KE, Serbus DC, Santiago M. Exposure of rats to ethanol from postnatal days 4 to 8: alterations of cholinergic neurochemistry in the cerebral cortex and corpus striatum at day 20. Alcohol Clin Exp Res. 1989a;13:29–35. doi: 10.1111/j.1530-0277.1989.tb00279.x. [DOI] [PubMed] [Google Scholar]
- Light KE, Serbus DC, Santiago M. Exposure of rats to ethanol from postnatal days 4 to 8: alterations of cholinergic neurochemistry in the hippocampus and cerebellum at day 20. Alcohol Clin Exp Res. 1989b;13:686–692. doi: 10.1111/j.1530-0277.1989.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. The multifaceted profile of activated microglia. Mol Neurobiol. 2009;40:139–156. doi: 10.1007/s12035-009-8077-9. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Morales-Garcia JA, Luna-Medina R, Alfaro-Cervello C, Cortes-Canteli M, Santos A, Garcia-Verdugo JM, Perez-Castillo A. Peroxisome proliferator-activated receptor gamma ligands regulate neural stem cell proliferation and differentiation in vitro and in vivo. Glia. 2011;59:293–307. doi: 10.1002/glia.21101. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Honda S, Tohyama Y, Imai Y, Kohsaka S, Kurihara T. Neurotrophin secretion from cultured microglia. J Neurosci Res. 2001;65:322–331. doi: 10.1002/jnr.1157. [DOI] [PubMed] [Google Scholar]
- Napper RM, West JR. Permanent neuronal cell loss in the cerebellum of rats exposed to continuous low blood alcohol levels during the brain growth spurt: a stereological investigation. J Comp Neurol. 1995;362:283–292. doi: 10.1002/cne.903620210. [DOI] [PubMed] [Google Scholar]
- Natarajan C, Bright JJ. Peroxisome proliferator-activated receptor-gamma agonists inhibit experimental allergic encephalomyelitis by blocking IL-12 production, IL-12 signaling and Th1 differentiation. Genes Immun. 2002;3:59–70. doi: 10.1038/sj.gene.6363832. [DOI] [PubMed] [Google Scholar]
- Niino M, Iwabuchi K, Kikuchi S, Ato M, Morohashi T, Ogata A, Tashiro K, Onoe K. Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by an agonist of peroxisome proliferator-activated receptor-gamma. J Neuroimmunol. 2001;116:40–48. doi: 10.1016/s0165-5728(01)00285-5. [DOI] [PubMed] [Google Scholar]
- O'Hare ED, Kan E, Yoshii J, Mattson SN, Riley EP, Thompson PM, Toga AW, Sowell ER. Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport. 2005;16:1285–1290. doi: 10.1097/01.wnr.0000176515.11723.a2. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: down-regulation of inducible nitric-oxide synthase by 15-deoxy-Delta12,14-prostaglandin J2. Proc Natl Acad Sci U S A. 1999;96:4668–4673. doi: 10.1073/pnas.96.8.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce DR, Goodlett CR, West JR. Differential neuronal loss following early postnatal alcohol exposure. Teratology. 1989;40:113–126. doi: 10.1002/tera.1420400205. [DOI] [PubMed] [Google Scholar]
- Pierce DR, Hayar A, Williams DK, Light KE. Developmental alterations in olivary climbing fiber distribution following postnatal ethanol exposure in the rat. Neuroscience. 2010;169:1438–1448. doi: 10.1016/j.neuroscience.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce DR, Kane CJ, Serbus DC, Light KE. Microencephaly and selective decreases in cerebellar Purkinje cell numbers following combined exposure to ethanol and methadone during rat brain development. Dev Neurosci. 1997;19:438–445. doi: 10.1159/000111241. [DOI] [PubMed] [Google Scholar]
- Pierce DR, Williams DK, Light KE. Purkinje cell vulnerability to developmental ethanol exposure in the rat cerebellum. Alcohol Clin Exp Res. 1999;23:1650–1659. [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- Smith SA, Monteith GR, Holman NA, Robinson JA, May FJ, Roberts-Thomson SJ. Effects of peroxisome proliferator-activated receptor gamma ligands ciglitazone and 15-deoxy-delta 12,14-prostaglandin J2 on rat cultured cerebellar granule neuronal viability. J Neurosci Res. 2003;72:747–755. doi: 10.1002/jnr.10613. [DOI] [PubMed] [Google Scholar]
- Snell LD, Bhave SV, Tabakoff B, Hoffman PL. Chronic ethanol exposure delays the 'developmental switch' of the NMDA receptor 2A and 2B subunits in cultured cerebellar granule neurons. J Neurochem. 2001;78:396–405. doi: 10.1046/j.1471-4159.2001.00424.x. [DOI] [PubMed] [Google Scholar]
- Sonderegger T, Colbern D, Calmes H, Corbitt S, Zimmermann E. Methodological note: intragastric intubation of ethanol to rat pups. Neurobehav Toxicol Teratol. 1982;4:477–481. [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. National Academy Press. Washington, DC: Institute of Medicine; 1996. [Google Scholar]
- Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Glass CK. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Miller KR, Lopes KO, Njie E. Microglial degeneration in the aging brain--bad news for neurons? Front Biosci. 2008;13:3423–3438. doi: 10.2741/2937. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Haring HU. Glitazones: clinical effects and molecular mechanisms. Ann Med. 2002;34:217–224. [PubMed] [Google Scholar]
- Victor NA, Wanderi EW, Gamboa J, Zhao X, Aronowski J, Deininger K, Lust WD, Landreth GE, Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur J Neurosci. 2006;24:1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- Wada K, Nakajima A, Katayama K, Kudo C, Shibuya A, Kubota N, Terauchi Y, Tachibana M, Miyoshi H, Kamisaki Y, Mayumi T, Kadowaki T, Blumberg RS. Peroxisome proliferator-activated receptor gamma-mediated regulation of neural stem cell proliferation and differentiation. J Biol Chem. 2006;281:12673–12681. doi: 10.1074/jbc.M513786200. [DOI] [PubMed] [Google Scholar]
- Wagstaff AJ, Goa KL. Rosiglitazone: a review of its use in the management of type 2 diabetes mellitus. Drugs. 2002;62:1805–1837. doi: 10.2165/00003495-200262120-00007. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Whiteland JL, Nicholls SM, Shimeld C, Easty DL, Williams NA, Hill TJ. Immunohistochemical detection of T-cell subsets and other leukocytes in paraffin-embedded rat and mouse tissues with monoclonal antibodies. J Histochem Cytochem. 1995;43:313–320. doi: 10.1177/43.3.7868861. [DOI] [PubMed] [Google Scholar]
- Xu J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists suppress the production of IL-12 family cytokines by activated glia. J Immunol. 2007;178:1904–1913. doi: 10.4049/jimmunol.178.3.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Luong R, Sullivan EV, Pfefferbaum A. Measurement of serum, liver, and brain cytokine induction, thiamine levels, and hepatopathology in rats exposed to a 4-day alcohol binge protocol. Alcohol Clin Exp Res. 2010;34:1858–1870. doi: 10.1111/j.1530-0277.2010.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Ou Z, Grotta JC, Waxham N, Aronowski J. Peroxisome-proliferator-activated receptor-gamma (PPARgamma) activation protects neurons from NMDA excitotoxicity. Brain Res. 2006;1073–1074:460–469. doi: 10.1016/j.brainres.2005.12.061. [DOI] [PubMed] [Google Scholar]