Abstract
Aim:
The effects of Guanabenz, an agonist of α2-adrenergic receptors routinely used in human medicine as an antihypertensive drug, were studied on NaIO3-induced retinal pigment epithelium (RPE) degeneration, laser-induced choroidal neovascularization (CNV) and choroidal blood flow, in animal models.
Methods:
The 35mg/kg NaIO3-induced RPE degeneration rat eyes were instilled with 1% Guanabenz eye drops 3 times a day for 7 days before NaIO3 injection, and then 2 to 4 weeks thereafter. RPE function was measured with c-wave of electroretinogram (ERG). Male Brown Norway rats were anesthetized to receive Nd:YAG laser to break the Bruch’s membrane. One percent Guanabenz eye drops were given likewise. The development of CNV was determined by fluorescein angiography performed on week 2 and week 4 using sodium fluorescein (FA) or fluorescein isothiocyanatedextran (FD70-FA). Colored microsphere technique was used for in vivo experiments to determine the choroidal blood flow in ocular hypertensive (40 mmHg) rabbit eyes.
Results:
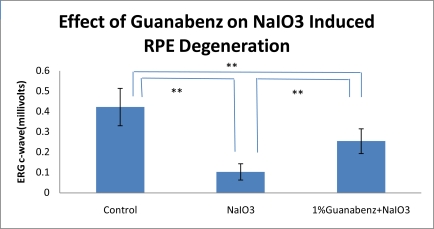
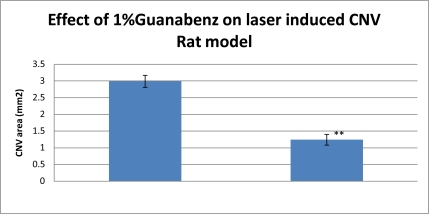
The RPE function was protected significantly by Guanabenz according to the c-wave of ERG. Four weeks after NaIO3 injection, the amplitude of ERG c-wave was 0.422±0.092 millivolts in the control group, 0.103±0.04 millivolts in the NaIO3 group, and 0.254±0.061 millivolts in the Guanabenz+NaIO3 group. There was a significant protection of the ERG c-wave by Guanabenz as compared to NaIO3 group (P<0.01). The angiograms of FD70-FA showed decreased lesion size in the Guanabenz group. Four weeks after laser treatment, the size of the CNV lesion was 2.99±0.18 mm2 in the control group, and 1.24±0.16 mm2 in the Guanabenz group (P<0.01). The choroidal blood flow was significantly increased at 30 and 60 minutes after Guanabenz instillation as compared to corresponding controls.
Conclusions:
Guanabenz significantly protected RPE from NaIO3-induced degeneration, inhibited the development of CNV in laser-induced rat AMD model and increased choroidal blood flow markedly in vivo.
Keywords: Guanabenz, sodium iodate, retinal pigment epithelium, age-related macular degeneration, choroidal blood flow.
INTRODUCTION
Age-related macular degeneration (AMD) was first described in the medical literature in 1875 as “symmetrical central choroidoretinal disease occurring in senile persons” [1]. It’s the main cause of legal blindness among those over 65 years of age in the United States [2]. AMD was reported to account for 54% of all current cases of blindness among the Caucasian population in the United States [3]. The study predicted that as a result of the rising prevalence of AMD, the number of blind people in the US could increase by as much as 70% by 2020.
The RPE forms a monolayer between the neurosensory retina and the choroid. Its main functions are to supply nutrients to the adjacent photoreceptors and to dispose of shed photoreceptor outer segments by phagocytosis [4]. The RPE disabled to remove the metabolic waste results in the accumulation of drusen. RPE dysfunction causes the breakdown of the Bruch’s membrane. The breaks in Bruch’s membrane under the detached RPE serve as an entrance for new and immature choroidal vessels into the subretinal space that lead to the formation of choroidal neovascularization. Furthermore, RPE loss may cause loss of choriocapillaris [5]. Therefore, RPE is a target for therapeutic approaches aimed at enhancement of photoreceptor survival in such ocular disease.
Guanabenz is a phenylacetyl-guanidine derivative, widely used as a centrally acting alpha2-adrenoceptor agonist, with an antihypertensive action similar to that of clonidine. It also has been reported to have protective effects against oxidative cytotoxicity in astrocytes [6, 7]. This study is to observe the effects of Guanabenz on NaIO3-induced retinal pigment epithelium (RPE) degeneration, laser-induced choroidal neovascularization (CNV), and choroidal blood flow in animal models.
METHODS
Materials
Guanabenz (purity≥98%), NaIO3 (purity≥99.5%), and dimethyl sulfoxide (DMSO, purity≥99.9%) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
Eight-week-old male Brown-Norway (BN) rats were purchased through LARR (Texas A&M University, USA). All rats had free access to a standard diet and drinking water and were housed in a standard animal room maintained at 24±0.5℃,with relative humidity of 14±5%, and with a 12:12 h cyclic lighting schedule. All procedures were conformed to the ARVO Resolution on the use of animals in ophthalmic and vision research. Rats were anesthetized for all procedures with intramuscular injection of ketamine (35mg/kg) and xylazine (5mg/kg). The pupils were dilated with 1% atropine and 2.5% phenylephrine.
New Zealand white rabbits, weighing 2.5-3.0 kg, were purchased through LARR (Texas A&M University, USA). They were anesthetized with 35 mg/kg ketamine and 5 mg/kg xylazine i.m, and half of the initial dose was given each 1 hour thereafter.
NaIO3-Induced AMD Rat Model
Thirty BN rats were randomly divided into 3 groups, 10 rats in normal group, 10 rats in NaIO3 group and 10 rats in Guanabenz+ NaIO3 group. Control group was instilled with solvent (Sigma-Aldrich) alone without NaIO3 injection. NaIO3 group was instilled with solvent plus 35mg/kg NaIO3 injection through hypoglossal vein, whereas Guanabenz+ NaIO3 group was instilled with 1% Guanabenz eye drops plus 35mg/kg NaIO3 injection [8, 9]. Both eyes of all rats were instilled with 1 drop of solvent or 1% Guanabenz for 3 times a day for 1 week before and 2 and 4 weeks after NaIO3 injection [10]. At the end of 2 and 4 weeks, c-wave of ERG was recorded.
BN rats were dark adapted for 2 hours, pupils were dilated and then anesthetized. Before recording, one drop of 0.5% tetracaine was given for surface anesthesia. All rats were kept warm during ERG measurement. DC-ERG recording methods developed by Peachey were followed [11]. Briefly, a 1-mm diameter glass capillary tube with filament (Sutter Instruments, Novato, CA) that was filled with Hanks balanced salt solution (Invitrogen, Carlsbad, CA) was used to connect with a Ag/AgCl wire electrode with a attached connector. The tubes were positioned to the corneal surface. Responses were amplified (dc-100 Hz; gain=1000X; DP-31, Warner Instruments, Hamden, CT) and digitized at 10 Hz or 1000Hz. Data were analyzed by iWORX LabScribe Data Recording Software (iWorx0CB Sciences, Dover, NH). Light stimuli was derived from an optical channel using a fiber-lite high intensity illuminator (Dolan-Jenner Industries, Inc., MA), with neutral density filters (Oriel, Stratford, CT) placed in the light path to adjust stimulus luminance. The stimulus luminance used in this experiment was 3.22 log cd/m2 and stimulated for 30 seconds. Luminance calibration was made by a Minolta (Ramsey, NJ) LS-110 photometer focused on the output side of the fiber optic bundle where the rat eye was located.
Laser-Induced CNV Rat Model
Twenty rats were divided into 2 groups, 10 rats in normal group and 10 in Guanabenz group. The fundus was visualized with a VOLK super Pupil XL Biomicroscopy Lens ( Keeler Instrument Inc., Broomall, PA, USA). A double-frequency Nd:YAG laser (Laserex LP3532; Lumenis Inc., Salt Lake City, UT) was used at 532-nm wavelength. Laser parameters used were 100-μ m spot size, 0.15-second exposure and 150-200mw powers. Five laser spots were made to the ocular fundus at approximately equal distances around the optic nerves. Acute vapor bubbles suggested rupture of Bruch’s membrane [12]. Only laser spots with bubble formation were included in the study. Lesions with subretinal hemorrhage were excluded.
FA was performed 2 and 4 weeks after laser treatment with a digital fundus camera (TRC-50EX; TOPCON, Tokyo, Japan). Ten percent fluorescein isothiocyanate-dextran solution was injected through hypoglossal vein at 1.4mg/kg. Angiograms were taken continuously until 20 min after i.v. injection. The lesion size was evaluated with Imagenet2000 digital imaging system (Topcon Medical Systems, Inc., Paramus, NJ, USA).
Measurement of Choroid Blood Flow in Ocular Hypertensive Rabbit Eyes
An ocular hypertensive model was created by raising the intraocular pressure of the left eye to 40 mmHg, which reduced the ocular blood flow to approximately one third of the normal valued [13]. The left ventricle was cannulated through the right carotid artery for the injection of colored microspheres and the femoral artery was cannulated for blood sampling. One percent drug solution (50 μL) or vehicle (50 μL) was instilled topically to the left eye, and the ocular blood flow of the ocular hypertensive rabbits was measured with colored microspheres at 0, 30, 60 and 120 minutes thereafter. At each time point, 2 million microspheres in 0.2 mL were injected as a reference, and blood samples were taken from the femoral artery for exactly 1 minute following injection of the microspheres. The blood sample was collected in a heparinized tube and the volume was recorded. The rabbits were euthanized with an injection of 100 mg/kg pentobarbital sodium after the last blood sampling. The left eyes were enucleated and dissected into the retina, choroid, iris and ciliary body. The tissue samples were weighed.
The details of sample processing and microsphere counting were provided by E-Z Trac (Los Angeles, CA). In brief, a hemolysis reagent was added to the microfuge tubes with the blood sample, then vortexed and centrifuged for 30 minutes at 6000 rpm. The supernatant was removed, and tissue/blood digest reagents I and II were added. The tubes were capped, vortexed, and centrifuged for 30 minutes again. The supernatant was removed, and the counting reagent was added, then vortexed, and centrifuged for 15 minutes at the same resolutions as above. The supernatant was removed, and the microspheres were resuspended in a precise volume of the counting reagent, The number of microspheres was counted with a hemocytometer.
Tissue/blood digest reagent I was added to the microfuge tubes with the tissue samples, sealed, and heated at 95℃ for 15 minutes. The tubes were vortexed for 30seconds, then reheated and revortexed until all tissue samples were dissolved. The tissue/blood digest reagent II was added while the tissue samples were still hot, then the tubes were capped, vortexed, and centrifuged for 30 minutes. The protocol thereafter was the same as that used to process the blood sampled, and the microspheres were counted.
The blood flow of each tissue at a certain time point was calculated from the following equation:
Qm = (Cm × Qr)/Cr
in which Qm is the blood flow of a tissue in terms of μL/minute per mg, Cm is the microsphere count per mg of tissue, Qr is the flow rate of blood sample in terms of μL per minute, and Cr is the total microsphere count in the referenced blood sample.
Statistical Analysis
All data were presented as mean±SEM. One-way analysis of variance (ANOVA) and nonpaired Student’s t-test was performed to analyze the significance between groups at a certain time point. The differences were considered significant at P<0.05.
RESULTS
Effect of Guanabenz on NaIO3-Induced RPE Degeneration
NaIO3-induced RPE degeneration was considered as dry AMD rat model and ERG c-wave was used to evaluate the function of RPE. Four weeks after NaIO3 injection, the amplitude of ERG c-wave was 0.422±0.092 millivolts in the control group, 0.103±0.04 millivolts in the NaIO3 group, and 0.254±0.061 millivolts in the Guanabenz+NaIO3 group. ANOVA was used for statistical analysis. The least significant difference (LSD) post hoc test was used to examine whether there was any difference between groups with equal variance. There was a significant protection of the ERG c-wave by Guanabenz as compared to NaIO3 group (P<0.01).
Effect of Guanabenz on Laser-induced Rat AMD Model
Angiography was widely used to measure the CNV area in laser-induced AMD rat model. The angiograms of FD70-FA showed diminished lesion size in the Guanabenz group. Four weeks after laser treatment, the size of the CNV lesion was 2.99±0.18 mm2 in the control group, and 1.24±0.16 mm2 in the Guanabenz group (P<0.01).
Effect of Guanabenz on Choroid Blood Flow of Ocular Hypertensive Rabbits
The choroid blood flow declined gradually in the vehicle control group. Guanabenz significantly increased the choroid blood flow at 30 and 60 min after drug instillation compared with the corresponding vehicle control group (P<0.05).
DISCUSSION
AMD is a debilitating disease of the eye, which manifests clinically with loss of central vision and pathologically with the accumulation of drusen, RPE degeneration, photoreceptor atrophy, and in wet AMD cases, with CNV formation. There are several risk factors, including age, race, smoking, and diet [14]. The etiology and pathogenesis of the disease include, but are not limited to oxidative stress, subclinical inflammation, complements formation, cytokine release, choroid atrophy, and the like.
The direct toxic effect of NaIO3 on RPE cells with secondary effects on photoreceptors and the choriocapillaries in vivo is well known [15]. The mechanisms of the toxicity of NaIO3 to RPE cells are as follows: first, NaIO3 can increase the ability of melanin to convert glycine into glyoxylate, a potential cell toxic compound [16]; second, NaIO3 could denaturant retinal proteins by changes of –SH levels in retina [17]; third, NaIO3 could cause considerable structure changes by breakdown of RPE diffusion barrier or by reduction of adhesion between RPE and photoreceptor cells [18-21]; finally, NaIO3 inhibits various enzyme activities, such as triose phosphate dehydragenase, succinodehydrogenase and lactate dehydrogenase [20, 22].
The results of ERG c-wave measurement showed that Guanabenz significantly protected RPE cells from NaIO3-induced injury. Slowing down of the RPE cells degeneration indicates prevention or slowing down of the AMD process. Imidazoline drugs, including guanabenz, were reported to show protective effects against naphthazarin-induced oxidative cytotoxicity, as evidenced by LDH release and Hoechst 33342/propidium iodide staining [6, 7]. The imidazoline drugs stabilized lysosomes and inhibited naphthazarin-induced lysosomal destabilization, as evidenced by acridine orange relocation. Guanabenz inhibited, the leakage of lysosomal cathepsin D to cytosol, the decreased mitochondrial potential, and the release of mitochondrial cytochrome c, which were induced by naphthazarin. The lysosomal destabilization by oxidative stress and other apoptotic signals and subsequent cathepsin D leakage to the cytosol can induce apoptotic changes of mitochondria and eventually cell death. Therefore, lysosomal stabilization by imidazoline drugs may be ascribed to their protective effects against oxidative cytotoxicity. However, how Guanabenz exactly expresses its protective effect on RPE remains unclear. Further study is needed to reveal the mechanism of Guanabenz in RPE protection.
In general, degeneration of RPE cells is the first step of dry-AMD, and the CNV formation is the key point of dry-AMD transformation to wet-AMD. FA diagraphs showed that Guanabenz significantly decreased the intensity of fluorescein leakage from the photocoagulated lesions and the size of CNV induced by laser treatment on Brown Norway rats. Thus, Guanabenz showed its protection ability in converting dry-AMD to wet-AMD.
The results of choroid blood flow showed a significant increase in choroid blood flow by Guanabenz. The pathological studies have highlighted the role of oxidative stress and inflammatory changes in the pathogenesis of AMD [23]. The increase of choroid blood flow may facilitate removal of metabolic wastes, inflammatory factors and replenish nutrients to RPE and photoreceptors. Consequently, it may change the microenviroment, the balance between pro- and antiangiogenesis factors, and the change of the process of angiogenesis [24].
Guanabenz has been used for many years to treat hypertension on a daily basis, without major side-effects. The results suggest that it could be used to prevent and treat AMD in the future.
Fig. (1).
Effect of Guanabenz on NaIO3-induced RPE degeneration in rat eyes. Significant differences are indicated by ** (P<0.01).
Fig. (2).
Effect of 1%Guanabenz on Laser-induced CNV Rat model. Significant differences are indicated by ** (P<0.01).
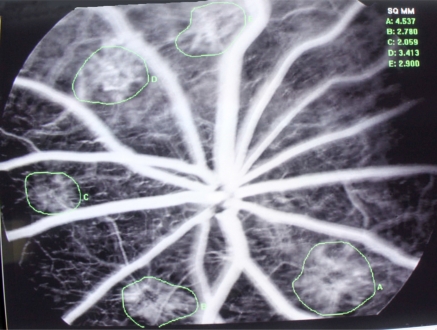
Fig. (3).
Angiogram of Laser-induced CNV Rat model, pic of Control group.
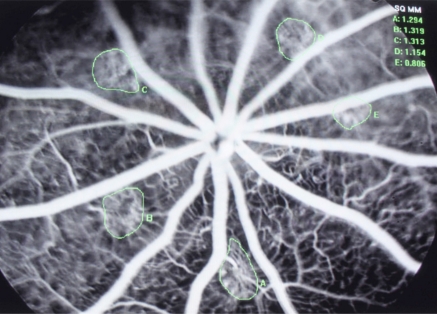
Fig. (4).
Angiogram of Laser-induced CNV Rat model, pic of 1% Guanabenz group.
Fig. (5).
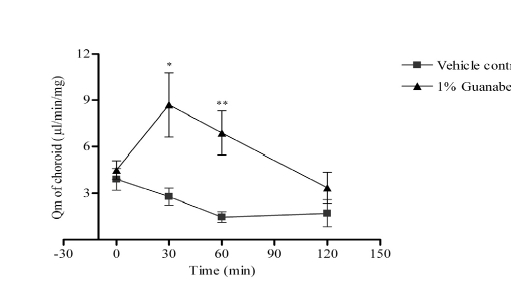
Effect of guanabenz on ocular blood flow of ocular hypertensive rabbits. Data were expressed as means ± SEM. n=6 in each group; * was P < 0.05 and ** was P<0.01 as compared with the vehicle control group.
REFERENCES
- 1.Tay HW. Symmetrical central choroidoretinal disease occurring in senile persons R. Lond. Ophthal Hosp Rep. 1875;8:231–44. [Google Scholar]
- 2.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 3.Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 4.Engelmann K, Valtink M. RPE cell cultivation. Graefes Arch Clin Exp Ophthalmol. 2004;242(1):65–7. doi: 10.1007/s00417-003-0811-9. [DOI] [PubMed] [Google Scholar]
- 5.Liu CF, Lin CH, Chen CF, Huang TC, Lin SC. Antioxidative effects of tetramethylpyrazine on acute ethanol-induced lipid peroxidation. Am J Chin Med. 2005;33(6 ):981–8. doi: 10.1142/S0192415X05003570. [DOI] [PubMed] [Google Scholar]
- 6.Choi SH, Choi DH, Lee JJ, Park MS, Chun BG. Imidazoline drugs stabilize lysosomes and inhibit oxidative cytotoxicity in astrocytes. Free Radic Biol Med. 2002;32(5):394–405. doi: 10.1016/s0891-5849(01)00819-x. [DOI] [PubMed] [Google Scholar]
- 7.Choi SH, Kim DH, Park YG, Chun BG, Choi SH. Protective effects of rilmenidine and AGN 192403 on oxidative cytotoxicity and mitochondrial inhibitor-induced cytotoxicity in astrocytes. Free Radic Biol Med. 2002;15, 33(10):1321–33. doi: 10.1016/s0891-5849(02)01041-9. [DOI] [PubMed] [Google Scholar]
- 8.Gringnolo A, Orzalesi N, Calabria GA. Studies on the fine structure and the rhodopsin cycle of the rabbit retina in experimental degeneration induced by sodium iodate. Exp Eye Res. 1966;5(1):86–97. doi: 10.1016/s0014-4835(66)80024-6. [DOI] [PubMed] [Google Scholar]
- 9.Jiang W, Chiou GC. Effects of hydralazine on NaIO3-induced rat retinal pigment epithelium degeneration. Int J Ophthalmol. 2008;8:1504–10. [Google Scholar]
- 10.Hughes PM, Olejnik O, Chang-Lin JE, Wilson CG. Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev. 2005;57(14):2010–32. doi: 10.1016/j.addr.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Peachey NS, Stanton JB, Marmorstein AD. Noninvasive recording and response characteristics of the rat dc-electroretinogram. Vis Neurosci. 2002;19(6):693–701. doi: 10.1017/s0952523802196015. [DOI] [PubMed] [Google Scholar]
- 12.Zou Y, Xu X, Chiou GC. Effect of interleukin-1 blockers, CK112, and CK116 on rat experimental choroidal neovascularization in vivo and endothelial cell cultures in vitro. J Ocul Pharmacol Ther. 2006;22(1):19–25. doi: 10.1089/jop.2006.22.19. [DOI] [PubMed] [Google Scholar]
- 13.Chiou GC, Chen YJ. Effects of D- and L-isomers of timolol on retinal and choroidal blood flow in ocular hypertensive rabbit eyes. J Ocul Pharmacol. 1992;8(3):183–90. doi: 10.1089/jop.1992.8.183. [DOI] [PubMed] [Google Scholar]
- 14.Coleman HR, Chan CC, Ferris FL 3rd, Chew EY. Age-related macular degeneration. Lancet. 2008;22, 372(9652):835–45. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiuchi K, Yoshizawa K, Shikata N, Moriquchi K, Tsubura A. Morphologic characteristics of retinal degeneration induced by sodium iodate in mice. Curr Eye Res. 2002;25(6 ):373–9. doi: 10.1076/ceyr.25.6.373.14227. [DOI] [PubMed] [Google Scholar]
- 16.Baich A, Ziegler M. The effect of sodium iodate and melanin on the formation of glyoxylate. Pigment Cell Res. 1992;5(6 ):394–5. doi: 10.1111/j.1600-0749.1992.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 17.Sorsby A, Reading HW. Experimental degeneration of the retina. XI. The effect of sodium iodate on retinal -SH levels. Vision Res. 1964;4(10 ):511–4. doi: 10.1016/0042-6989(64)90057-4. [DOI] [PubMed] [Google Scholar]
- 18.Flage T, Ringvold A. The retinal pigment epithelium diffusion barrier in the rabbit eye after sodium iodate injection. A light and electron microscopic study using horseradish peroxidase as a tracer. Exp Eye Res. 1982;34(6 ):933–40. doi: 10.1016/0014-4835(82)90072-0. [DOI] [PubMed] [Google Scholar]
- 19.Sen HA, Berkowitz BA, Ando N, de Juan E Jr. In vivo imaging of breakdown of the inner and outer blood-retinal barriers. Invest Ophthalmol Vis Sci. 1992;33(13 ):3507–12. [PubMed] [Google Scholar]
- 20.Ashburn FS Jr, Pilkerton AR, Rao NA, Marak GE. The effects of iodate and iodoacetate on the retinal adhesion. Invest Ophthalmol Vis Sci. 1980;19(12 ):1427–32. [PubMed] [Google Scholar]
- 21.Stern WH, Ernest JT, Steinberq RH, Miller SS. Interrelationships between the retinal pigment epithelium and the neurosensory retina. Aust J Ophthalmol. 1980;8(4 ):281–8. doi: 10.1111/j.1442-9071.1980.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 22.Enzmann V, Row BW, Yamauchi Y, et al. Behavioral and anatomical abnormalities in a sodium iodate-induced model of retinal pigment epithelium degeneration. Exp Eye Res. 2006;82(3 ):441–8. doi: 10.1016/j.exer.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the ageing eye. Am J Ophthalmol. 2002;134:411–31. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 24.Xuan B, Xu XR, Chiou GCY, Zhang YH, Peng SX. Relationship between nitric oxide production and choroidal blood flow. J Ocul Pharmacol Ther. 2003;19:247–53. doi: 10.1089/108076803321908365. [DOI] [PubMed] [Google Scholar]