Abstract
The polarization and migration of eukaryotic cells are fundamental processes for the development and maintenance of a tissue. These aspects gain especial interest when it comes to stem and progenitor cells in the way that their manipulation might open new avenues in regenerative therapy. In recent years, novel biological facets of migrating hematopoietic stem cells were revealed by several groups, including ours. Among these features, the polarization of their membranous (proteins and lipids) and cytoplasmic constituents, which leads to the formation of a specialized sub-cellular structure located at the rear pole—the uropod—has gained increasing interest. In a new study we have demonstrated that such phenomena involve a coordinated mechanism between Rho GTPase signaling and the microtubule network. Specifically, our results based on the use of synthetic inhibitors and RNA interference suggest that the activity of RhoA and its effector ROCK I is indispensable for cell polarization and the active reorganization of microtubules that are required for migration.
Key words: CD133, cell migration, hematopoietic stem cell, microtubule, RhoA
Understanding the cellular and molecular trafficking mechanisms that regulate the migration of hematopoietic stem and progenitor cells (HSPCs) throughout the development of an organism and later on its homeostasis are important not only from a biological standpoint, but also with regard to therapeutic purposes. For instance, bone marrow transplantation is one recognized procedure for treating hematological diseases. However, the accurate mechanism underlying the migration and engraftment of HSPCs into the bone-marrow niche is not fully characterized. In order to gain novel insights we have developed an ex vivo co-culture system consisting of human HSPCs from healthy donors growing on primary human multipotent mesenchymal stromal cells (MSCs) as feeder cell layer (for cell isolation and culture conditions see literatures).1–3 Such cellular system reproduces numerous characteristics found within bone marrow cavities4 including adhesive interactions5 and the essential chemotactic axis6 based on the G-protein-coupled receptor CXCR4, which is expressed by HSPCs and its chemokine ligand CXCL12 (alias stromal cell-derived factor-1α; SDF-1α) secreted by MSCs.1,7
Under these conditions, HSPCs display various morphologically identifiable types of plasma membrane protrusions.1 Interestingly, in migrating HSPCs a noteworthy protrusion called uropod is formed at the rear pole (Fig. 1A).1,8 Like in leukocytes (e.g., T cells), the uropod might play a role in intercellular adhesion, communication and motility.9,10 Numerous proteins with adhesive properties are found therein including P-selectin glycoprotein ligand-1 (PSGL-1; Fig. 1A).11,12 The presence of the stem cell marker CD133 (prominin-1),13–15 a 5-span transmembrane glycoprotein that binds plasma membrane cholesterol and associates with a specific membrane microdomain (lipid raft),16 was instructive with regard to its membrane organization. Indeed, a membrane microdomain enriched in ganglioside GM1 is concentrated in the uropod,10 and its spatiotemporal regulation might engage molecules such as flotillins.17 From the cytoplasmic side, certain proteins of the ezrin/radixin/moesin (ERM) family might link membrane proteins (e.g., PSGL-1) via their juxta-membrane domains to the underlying actin cytoskeleton.18 The microtubule-organizing centre is found at the base of the uropod.12 At the front pole, a migrating HSPC exhibits a lamellipodium, which concentrates CXCR4 at its tips in agreement with a chemotactic role.11 As reported for T cells, a distinct membrane microdomain based on ganglioside GM3 instead of GM1 is found therein (Fig. 1A).11,19 From both morphological and phenotypical angles, the migrating HSPC develops thus a highly polarized structure that underlies coordinated but opposite actions at both cell sides. While retracting the uropod, the cell extends its lamellipodium at the leading edge. As a result, a net cell movement can be achieved by continuous attachment to and de-adhesion from the substratum at the front and rear pole, respectively (Fig. 1B and green arrow).
Figure 1.
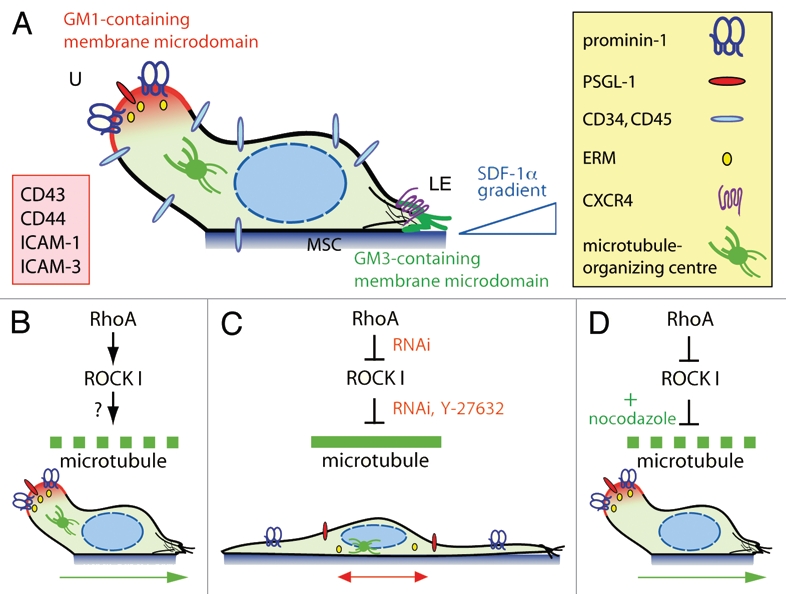
RhoA/ROCK I pathway and remodeling of microtubule network underlie the polarization and migration of HSPCs. (A) A migrating HSPC growing on MSC displays a polarized morphology with the formation of a uropod (U) at the rear pole and a leading edge (LE) at the front. Both types of plasma membrane protrusions contain a specific ganglioside-based membrane microdomain—the uropod being enriched in GM1 (red) whereas the leading edge in GM3 (green). In addition to prominin-1, a plethora of cell adhesion molecules (inset) including PSGL-1 are concentrated in the uropod whereas the chemokine receptor CXCR4 is found at the leading edge consistent with its sensory role towards an SDF-1α gradient.6 ERM proteins seem to be actively involved in the subcellular localization of some uropod-associated membrane proteins.18 Other molecules such as CD34 and CD45 are evenly distributed. The microtubule-organizing centre is found between the nucleus and the uropod. (B) The activity (↓) of RhoA and its downstream effector ROCK I contributes to the formation of the uropod, and hence polarization and migration of HSPCs. The downstream target(s) remain to be identified (?), but it might engage a protein involved in microtubule destabilization (dashed green line). (C) Inhibition (⊥) of ROCKs using Y-27632 or the specific knockdown of ROCK I or its upstream regulator RhoA by means of RNAi results in an elongated morphology where the uropod is lost. Both membrane (prominin-1, PSGL-1) and cytoplasmic (ezrin) proteins are redistributed. These cells display an impairment of migration caused by microtubule stability (solid green line). (D) In RhoA/ROCKI-deficient HSPCs, the addition (+) of nocodazole restores their proper polarization and migration highlighting the implication of unidentified microtubule-destabilizing proteins. Green and red arrows indicate the direction of migration.
From a mechanistical and/or biochemical perspective, our recent study has focused on the implication of Rho GTPase signaling pathway in these orchestrated processes (Fig. 1B).12 It is known that Rho GTPases such RhoA, Rac and Cdc42 are key players in cell polarity and migration by modulating cytoskeletal dynamics.20 As the most important downstream effectors of RhoA, Rho-associated coiled-coil protein kinases (ROCK) are implicated in various cellular functions such as actin organization and transformation. Using Rho kinase inhibitor Y-27632 and RNA interference (RNAi) directed against either RhoA or ROCK I we demonstrated that both proteins are indispensable for the polarization of HSPCs, and hence their migration. For instance, the use of the synthetic drug resulted in the complete loss of the uropod and the formation of two to three long and thin plasma membrane protrusions (Fig. 1C). Narrowed lamellipodia were formed at the tip of those protrusions rather than close to the cell body, as in untreated cells (Fig. 1C).12 Such a drastic morphological alteration was followed at the molecular level by a redistribution of plasma membrane (prominin-1 and PSGL-1) and cytoskeleton (ezrin, an ERM protein) constituents of the uropod. The asymmetric distribution of microtubule-organizing centre was also lost (Fig. 1C).12 As a functional consequence, Y-27632-treated cells displayed a net impairment of migration as evaluated by time-lapse video microscopy and Transwell-filter assay.12 Specifically, Y-27632-treated cells showed a defect in retracting the long plasma membrane protrusion located at the rear pole and frequently changed their directional movement by 180°, suggesting a perturbation in the front-rear orientation mediated by CXCR4/SDF-1α axis (Fig. 1C and red double-headed arrow). All the characteristics described above for Y-27632-treated cells were reproduced upon the use of RNAi-mediated knockdown of RhoA and ROCK I, thus confirming the direct implication of Rho GTPase signaling pathway in the polarization and migration of HSPCs (Fig. 1C). These outcomes appeared highly specific since the knockdown of ROCK II did not provide such radical effects.
Surprisingly, our study also revealed that the defect in cell polarization including the formation of the uropod could be fully rescued by the nocodazole-mediated depolymerization of the microtubule network (Fig. 1D). Not only was the polarized morphology of RhoA/ROCK I-deficient HSPCs restored, but also their locomotion. The actin depolymerization triggered by latrunculin B did not produce such reversible effects.12 The precise way that RhoA/ROCK I signaling contributes to the microtubule instability at the uropod cortex is currently unknown. Nevertheless our experiments with nocodazole/RNAi seem to exclude a feedback loop involving microtubule-associated guanine nucleotide exchange factor (GEF)-H1, which activates RhoA21 upon its release from microtubules after the disruption of the latter. However such phenomenon might occur in a natural context. The potential direct or indirect targets of ROCK remain to be identified, but they might engage enzymes that mediate tubulin detyrosination and acetylation, e.g., histone deacetylase 6, the activity of which is modulated by RhoA/ROCK.22,23 Active crosstalk between players at the leading edge and the uropod as well as a dynamic balance of the actomyosin and microtubule systems also need to be considered.24 The lack of the front-rear orientation of RhoA/ROCK I-deficient HSPCs and altered lamellipodia are consistent with it.12 Microtubule-destabilizing protein stathmin/OP18 might participate in these biochemical reactions via Rac/Cdc42,25 and the regulation of Rac by ROCK via the filamin A-binding RhoGTPase-activating protein reveals the complexity of the system.26 Similarly, members of the ERM protein family such as ezrin or moesin might be involved as well in the integrity of the uropod, and it might be more than a coincidence that these adaptor proteins, which are also potential substrates of ROCK, are playing an active role in membrane microdomain dynamics.27
Lastly, it is noteworthy that comparable data showing the implication of RhoA and microtubule network in the migration of T cells were recently reported independently28,29 indicating that our current observations might extend to cells of hematopoietic origin in general. Further studies based on a quantitative proteomic approach should lead to an exhaustive list of ROCK I substrates involved in these processes, which might represent potential therapeutic targets in the development of new strategies to improve the efficiency of bone marrow transplantation.
Acknowledgements
We thank C.A. Fargeas for critically reading the manuscript. This work was supported by grant from Deutsche Forschungsgemeinschaft (SFB 655 B3, SFB/TRR83 No.6).
Abbreviations
- ERM
ezrin/radixin/moesin
- HSPCs
hematopoietic stem and progenitor cells
- MSCs
multipotent mesenchymal stromal cells
- PSGL-1
P-selectin glycoprotein ligand-1
- ROCK
Rho-associated coiled-coil protein kinase
- RNAi
RNA interference
- SDF-1α
stromal cell-derived factor-1α
References
- 1.Freund D, Bauer N, Boxberger S, Feldmann S, Streller U, Ehninger G, et al. Polarization of human hematopoietic progenitors during contact with multipotent mesenchymal stromal cells—effects on proliferation and clonogenicity. Stem Cells Dev. 2006;15:815–829. doi: 10.1089/scd.2006.15.815. [DOI] [PubMed] [Google Scholar]
- 2.Freund D, Oswald J, Feldmann S, Ehninger G, Corbeil D, Bornhäuser M. Comparative analysis of proliferative potential and clonogenicity of MACS-immunomagnetic isolated CD34+ and CD133+ blood stem cells derived from a single donor. Cell Prolif. 2006;39:325–332. doi: 10.1111/j.1365-2184.2006.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freund D, Fonseca AV, Janich P, Bornhäuser M, Corbeil D. Differential expression of biofunctional GM1 and GM3 gangliosides within the plastic-adherent multipotent mesenchymal stromal cell population. Cytotherapy. 2010;12:131–142. doi: 10.3109/14653240903476438. [DOI] [PubMed] [Google Scholar]
- 4.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci USA. 1998;95:14423–14428. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4 and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- 7.Jing D, Fonseca AV, Alakel N, Fierro FA, Muller K, Bornhäuser M, et al. Hematopoietic stem cells in co-culture with mesenchymal stromal cells-modeling the niche compartments in vitro. Haematologica. 2010;95:542–550. doi: 10.3324/haematol.2009.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer N, Fonseca AV, Florek M, Freund D, Jászai J, Bornhäuser M, et al. New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133) Cells Tissue Organs. 2008;188:127–138. doi: 10.1159/000112847. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Madrid F, Serrador JM. Bringing up the rear: defining the roles of the uropod. Nat Rev Mol Cell Biol. 2009;10:353–359. doi: 10.1038/nrm2680. [DOI] [PubMed] [Google Scholar]
- 10.Gillette JM, Larochelle A, Dunbar CE, Lippincott-Schwartz J. Intercellular transfer to signalling endosomes regulates an ex vivo bone marrow niche. Nat Cell Biol. 2009;11:303–305. doi: 10.1038/ncb1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giebel B, Corbeil D, Beckmann J, Höhn J, Freund D, Giesen K, et al. Segregation of lipid raft markers including CD133 in polarized human hematopoietic stem and progenitor cells. Blood. 2004;104:2332–2338. doi: 10.1182/blood-2004-02-0511. [DOI] [PubMed] [Google Scholar]
- 12.Fonseca AV, Freund D, Bornhäuser M, Corbeil D. Polarization and migration of hematopoietic stem and progenitor cells rely on the RhoA/ROCK I pathway and an active reorganization of the microtubule network. J Biol Chem. 2010;285:31661–31671. doi: 10.1074/jbc.M110.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fargeas CA, Fonseca AV, Huttner WB, Corbeil D. Prominin-1 (CD133): from progenitor cells to human diseases. Future Lipidology. 2006;1:213–225. [Google Scholar]
- 14.Corbeil D, Fargeas CA, Huttner WB. Rat prominin, like its mouse and human orthologues, is a pentaspan membrane glycoprotein. Biochem Biophys Res Commun. 2001;285:939–944. doi: 10.1006/bbrc.2001.5271. [DOI] [PubMed] [Google Scholar]
- 15.Kania G, Corbeil D, Fuchs J, Tarasov KV, Blyszczuk P, Huttner WB, et al. The somatic stem cell marker prominin-1/CD133 is expressed in embryonic stem cell-derived progenitors. Stem Cells. 2005;23:791–804. doi: 10.1634/stemcells.2004-0232. [DOI] [PubMed] [Google Scholar]
- 16.Janich P, Corbeil D. GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS Lett. 2007;581:1783–1787. doi: 10.1016/j.febslet.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 17.Ludwig A, Otto GP, Riento K, Hams E, Fallon PG, Nichols BJ. Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J Cell Biol. 2010;191:771–781. doi: 10.1083/jcb.201005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serrador JM, Urzainqui A, Alonso-Lebrero JL, Cabrero JR, Montoya MC, Vicente-Manzanares M, et al. A juxta-membrane amino acid sequence of P-selectin glycoprotein ligand-1 is involved in moesin binding and ezrin/radixin/moesin-directed targeting at the trailing edge of migrating lymphocytes. Eur J Immunol. 2002;32:1560–1566. doi: 10.1002/1521-4141(200206)32:6<1560::AID-IMMU1560>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 19.Gómez-Móuton sC, Abad J, Mira E, Lacalle R, Gallardo E, Jimenez-Baranda S, et al. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc Natl Acad Sci USA. 2001;98:9642–9647. doi: 10.1073/pnas.171160298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 21.Chang YC, Nalbant P, Birkenfeld J, Chang ZF, Bokoch GM. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol Biol Cell. 2008;19:2147–2153. doi: 10.1091/mbc.E07-12-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002;21:6820–6831. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling L, Lobie PE. RhoA/ROCK activation by growth hormone abrogates p300/histone deacetylase 6 repression of Stat5-mediated transcription. J Biol Chem. 2004;279:32737–32750. doi: 10.1074/jbc.M400601200. [DOI] [PubMed] [Google Scholar]
- 24.Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol. 2007;9:299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- 25.Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J Biol Chem. 2001;276:1677–1680. doi: 10.1074/jbc.C000635200. [DOI] [PubMed] [Google Scholar]
- 26.Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8:803–814. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 27.Gupta N, Wollscheid B, Watts JD, Scheer B, Aebersold R, DeFranco AL. Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor-mediated lipid raft dynamics. Nat Immunol. 2006;6:625–633. doi: 10.1038/ni1337. [DOI] [PubMed] [Google Scholar]
- 28.Takesono A, Heasman SJ, Wojciak-Stothard B, Garg R, Ridley AJ. Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells. PLoS ONE. 2010;5:8774. doi: 10.1371/journal.pone.0008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heasman SJ, Carlin LM, Cox S, Ng T, Ridley AJ. Coordinated RhoA signaling at the leading edge and uropod is required for T cell transendothelial migration. J Cell Biol. 2010;190:553–563. doi: 10.1083/jcb.201002067. [DOI] [PMC free article] [PubMed] [Google Scholar]


