Abstract
Remodeling of brain circuits, including the formation, modification and elimination of synaptic structures, occurs throughout life as animals adapt to their environment. Until very recently, known mechanisms for experience-dependent synaptic plasticity had placed neurons and their structural interactions with astrocytes in the spotlight. However microglia, the immune cells of the brain, are very active even in the absence of pathological insults and their processes periodically contact dendritic spines and axon terminals in vivo.1–3 This intriguing behavior prompted us to explore, using electron microscopy and two-photon in vivo imaging in the primary visual cortex of juvenile mice, a possible role for quiescent microglia in the modification of synaptic structures.4 Our work uncovered subtle changes in the behavior of microglia during manipulations of visual experience including regulation of perisynaptic extracellular spaces, contact with subsets of structurally dynamic and transient dendritic spines, and phagocytic engulfment of intact synapses. Based on these results, here we further discuss three means of synapse modification or elimination that could be mediated by microglia in the context of normal experience-dependent plasticity.
Key words: microglia, synaptic plasticity, dendritic spine, visual cortex, critical period, development, mouse, electron microscopy, three-dimensional reconstruction, two-photon in vivo imaging
Proteolytic Modification of the Perisynaptic Environment
Modifications of extracellular matrix (ECM) composition have emerged as key determinants of synaptic structural plasticity.5,6 Important ECM modifiers include extracellular proteases, either secreted by neurons, astrocytes or microglia, that mediate inactivation or degradation, but also activation of ECM proteins by unmasking functional epitopes.7 In particular, matrix metalloprotease (MMP)-9 has been associated in vitro with dendritic spine growth and synaptic strength increase,8,9 and tissue-plasminogen activator (tPA) has been associated in vitro with synaptic plasticity10 and in vivo with dendritic spine motility and elimination, as well as ocular-dominance plasticity.11–13 However, the cellular sources of MMP-9 and tPA during functional plasticity, as well as their ability to achieve the spatial and temporal specificity crucial for synaptic plasticity in the face of fast protease diffusion have remained obscure. Our recent study showed that microglial processes can target very specific locations and modify the extracellular space geometry locally (Fig. 1A), directly or indirectly, solely by their presence within the neuropil.4 As a consequence, this extracellular remodeling could enable compartmentalization of proteases, ions, cytokines, neuromodulators and other molecules secreted by microglia or other cell types nearby particular synapses. With our other observations that most microglial processes directly contact synaptic structures4 while being extremely motile,1–4 this suggests that quiescent microglia could regulate the dynamics of plasticity at individual synapses as they explore the local environment to pursue immune surveillance roles.
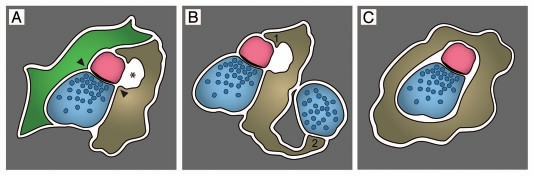
Figure 1.
Possible means of synapse modification or elimination by quiescent microglia. In (A), a synapse between an axon terminal (blue) and a dendritic spine (pink) is simultaneously contacted by an astrocytic (green) and microglial (taupe) process. Note the astrocytic and microglial contacts with the synaptic cleft (arrowheads) and the pocket of microglia-associated extracellular space (asterisk), which suggests microglial remodeling of the extracellular environment in the vicinity of synapses. Surrounding elements of neuropil are shown in grey. (B) Microglial process displaying end-feet morphological specializations. Here, these end-feet contact a dendritic spine (end-foot 1) and an axon terminal (end-foot 2), at two different synapses. Such direct cell-cell interaction between microglia and synaptic elements could contribute to local remodeling of the actin cytoskeleton during episodes of plasticity. (C) Engulfment by a microglial process of an intact synapse between an axon terminal and a dendritic spine suggesting microglial phagocytosis of synaptic elements during experience-dependent plasticity.
Remodeling of Dendritic Spine Morphology
Emerging evidence suggests that signaling pathways linking synaptic activity to changes in dendritic spine morphology locally influence the actin cytoskeleton. These changes are not limited to formation and elimination of dendritic spines but also include their subtle morphological remodeling in terms of size, shape and motility. The actin cytoskeleton additionally plays an important role in maintaining synaptic structures by facilitating the trafficking of synaptic cargos, regrouping the translation machinery, organizing the postsynaptic density and anchoring postsynaptic receptors to the membrane14 suggesting that structural remodeling is tightly linked to synapse function.15 Regulation of the actin cytoskeleton can be initiated by molecular interactions between dendritic spines and the ECM or by coordinated interactions between dendritic spines and dynamic astrocytic processes which also promote the stabilization of dendritic protrusions and their subsequent maturation into dendritic spines in vitro.16,17 Using high spatial resolution electron microscopy (EM), we have observed that microglial processes directly contact synaptic elements, either extensively or focally (Fig. 1B; see Fig. 4C in the original paper for an example of microglial process displaying an end-feet specialization where it contacts an axon terminal4). Clathrin-coated pits reminiscent of spinules18 were also observed with EM, at sites of contact between microglia and dendritic spines, axon terminals or perisynaptic astrocytic processes,4 suggesting direct communication between quiescent microglia and synapses through trans-endocytic exchange of various membranous or cytoplasmic molecules. Taken together, these findings dictate that cell-cell interactions involving extremely motile microglial processes must be taken into account when considering the mechanisms of rapid dendritic spine remodeling during experience-driven plasticity.
Phagocytic Engulfment of Dendritic Spines and Axon Terminals
Although phagocytic engulfment is in line with the macrophagic nature of microglia, their participation in synapse elimination during early postnatal development and neurodegenerative disease has remained controversial.19,20 Recent studies have demonstrated the involvement of the complement cascade in developmental pruning21,22 but whether microglia are the cell type performing the elimination of complement-tagged axon terminals remains to be confirmed.23 While EM observations of microglial processes intervening between axon terminals and neuronal cells bodies or proximal dendrites have long ago suggested microglial elimination of synapses by “synaptic stripping,” defined as the physical separation of pre- and post-synaptic elements in pathological conditions,24,25 a recent EM with three-dimensional (3D) reconstruction study failed to show any evidence of synaptic stripping in a mouse model of neurodegeneration.26 Whether synaptic stripping is linked to the elimination or degeneration of synaptic elements, and is an initial stage of phagocytosis or an advanced form of synaptic ensheathment also remains unknown. In this context, while we have shown direct contacts between microglia and axon terminals, dendritic spines, as well as synaptic clefts, with both immunocytochemical EM and serial section EM with 3D reconstruction, which could suggest synaptic stripping, we never saw instances of microglial processes penetrating into the cleft to separate pre- and post-synaptic elements.4 However, cellular inclusions resembling dendritic spines, axon terminals or entire synapses between dendritic spines and axon terminals, were frequently encountered inside microglial cell bodies and their processes, especially during adaptation to a new visual environment when plastic changes are prominent. We also reported microglial processes with phagocytic structures4 using two-photon in vivo imaging after visual manipulations. These observations add to the evidence that microglia may phagocytose synaptic elements, but in a novel context extending beyond early postnatal development and immune response to brain injury. In the future, it will be important to provide a more direct demonstration of microglial phagocytosis of synaptic elements in vivo, and examine whether microglial phagocytosis proceeds differently in health and disease.
Conclusions
Considering these means of synapse modification or elimination that could be effected by microglia during experience-dependent plasticity, the proteolytic modification of the perisynaptic environment—remodeling of dendritic spine morphology and phagocytic engulfment of dendritic spines and axon terminals, it is tempting to speculate that a quadripartite rather than tripartite view of synapses may soon emerge (see Fig. 1 for schematic representation).
Acknowledgements
This work was supported by grants from the NIH (EY019277), Whitehall Foundation, the Alfred P. Sloan Foundation, and a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund to A.K.M., as well as a core grant from the NIH (#P30 EY001319) to the Center for Visual Science. M.È.T. is funded by a Fonds de la recherche en santé du Québec (FRSQ) postdoctoral training award.
References
- 1.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 2.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 3.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dityatev A, Fellin T. Extracellular matrix in plasticity and epileptogenesis. Neuron Glia Biol. 2008;4:235–247. doi: 10.1017/S1740925X09000118. [DOI] [PubMed] [Google Scholar]
- 6.Berardi N, Pizzorusso T, Maffei L. Extracellular matrix and visual cortical plasticity: freeing the synapse. Neuron. 2004;44:905–908. doi: 10.1016/j.neuron.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 2010;11:735–746. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- 8.Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci USA. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozdagi O, Nagy V, Kwei KT, Huntley GW. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J Neurophysiol. 2007;98:334–344. doi: 10.1152/jn.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- 11.Mataga N, Mizuguchi Y, Hensch TK. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron. 2004;44:1031–1041. doi: 10.1016/j.neuron.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 12.Oray S, Majewska A, Sur M. Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron. 2004;44:1021–1030. doi: 10.1016/j.neuron.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Muller CM, Griesinger CB. Tissue plasminogen activator mediates reverse occlusion plasticity in visual cortex. Nat Neurosci. 1998;1:47–53. doi: 10.1038/248. [DOI] [PubMed] [Google Scholar]
- 14.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majewska AK, Sur M. Plasticity and specificity of cortical processing networks. Trends Neurosci. 2006;29:323–329. doi: 10.1016/j.tins.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Haber M, Zhou L, Murai KK. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci. 2006;26:8881–8891. doi: 10.1523/JNEUROSCI.1302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishida H, Okabe S. Direct astrocytic contacts regulate local maturation of dendritic spines. J Neurosci. 2007;27:331–340. doi: 10.1523/JNEUROSCI.4466-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spacek J, Harris KM. Trans-endocytosis via spinules in adult rat hippocampus. J Neurosci. 2004;24:4233–4241. doi: 10.1523/JNEUROSCI.0287-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hugh Perry V, O'Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN Neuro. 2010;2 doi: 10.1042/AN20100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graeber MB. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- 21.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 22.Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci USA. 2010;107:7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry VH, O'Connor V. C1q: the perfect complement for a synaptic feast? Nat Rev Neurosci. 2008;9:807–811. doi: 10.1038/nrn2394. [DOI] [PubMed] [Google Scholar]
- 24.Trapp BD, Wujek JR, Criste GA, Jalabi W, Yin X, Kidd GJ, et al. Evidence for synaptic stripping by cortical microglia. Glia. 2007;55:360–368. doi: 10.1002/glia.20462. [DOI] [PubMed] [Google Scholar]
- 25.Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85:145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- 26.Sisková Z, Mahad DJ, Pudney C, Campbell G, Cadogan M, Asuni A, et al. Morphological and functional abnormalities in mitochondria associated with synaptic degeneration in prion disease. Am J Pathol. 2010;177:411–21. doi: 10.2353/ajpath.2010.091037. [DOI] [PMC free article] [PubMed] [Google Scholar]