Abstract
Context
Children and adults with psychopathic traits and conduct or oppositional defiant disorder demonstrate poor decision making and are impaired in reversal learning. However, the neural basis of this impairment has not previously been investigated. Furthermore, despite high comorbidity of psychopathic traits and attention-deficit/hyperactivity disorder, to our knowledge, no research has attempted to distinguish neural correlates of childhood psychopathic traits and attention-deficit/hyperactivity disorder.
Objective
To determine the neural regions that underlie the reversal learning impairments in children with psychopathic traits plus conduct or oppositional defiant disorder.
Design
Case-control study.
Setting
Government clinical research institute.
Participants
Forty-two adolescents aged 10 to 17 years: 14 with psychopathic traits and oppositional defiant disorder or conduct disorder, 14 with attention-deficit/hyperactivity disorder only, and 14 healthy controls.
Main Outcome Measure
Blood oxygenation level–dependent signal as measured via functional magnetic resonance imaging during a probabilistic reversal task.
Results
Children with psychopathic traits showed abnormal responses within the ventromedial prefrontal cortex (Brodmann area 10) during punished reversal errors compared with children with attention-deficit/hyperactivity disorder and healthy children (P < .05 corrected for multiple comparisons).
Conclusions
To our knowledge, this study provides the first evidence of abnormal ventromedial prefrontal cortex responsiveness in children with psychopathic traits and demonstrates this dysfunction was not attributable to comorbid attention-deficit/hyperactivity disorder. These findings suggest that reversal learning impairments in patients with developmental psychopathic traits relate to abnormal processing of reinforcement information.
Children with the disruptive behavioral disorders of conduct disorder (CD), oppositional defiant disorder (ODD), or attention-deficit/hyperactivity disorder (ADHD) show increased rates of aggressive and antisocial behaviors.1,2 A subset of these children also displays striking callousness and psychopathic traits, including lack of guilt, empathy, or remorse.3,4 This subset with psychopathic traits is easily frustrated and is at highest risk for committing recurrent aggressive and antisocial behaviors.3,5-8 However, the neural basis for the increased aggression is unknown. While anxiety and emotional overactivity are associated with reactive aggression,9,10 this association is usually absent in persons with psychopathic traits.3,11,12 Children with high psychopathic traits do show increased levels of reactive aggression but this is thought to be frustration based.13-15 Reversal learning paradigms model a form of frustrating event16 and index the ability to flexibly adjust behavior to changes in reinforcement (ie, the ability to avoid frustration).17 In these paradigms, the individual initially learns to make a response to gain a reward. The reinforcement contingency then changes so that the correct response no longer results in reward and a new response must be learned to achieve the reward. Children and adults with psychopathic traits are impaired in reversal learning.18,19 This study was conducted to identify the neural basis of the reversal learning impairment in children with psychopathic traits.
Animal and human neuropsychological work has demonstrated that lesions in the orbital and medial prefrontal cortex (PFC) are associated with impairments in reversal learning.20-23 Human neuroimaging work in healthy adults has also implicated the ventromedial PFC (vmPFC) as well as the ventrolateral (vl) and dorsomedial (dm) PFC in reversal learning.24-28 During reversal learning, the vl and dmPFC show significantly increased activity in response to reversal errors (responses that were previously rewarded but now are punished).24,26-28 This is consistent with suggestions that the dmPFC is activated in situations that feature increased conflict29,30 and recruits the vlPFC to achieve response change.27 On the basis of early neuroimaging work stressing the role of the vlPFC in reversal learning,28 it was assumed that the reversal learning deficit seen in children and adults with psychopathic traits might reflect vlPFC dysfunction.16,19
However, recent neuroimaging and lesion data have highlighted the importance of the vmPFC in reversal learning.22,27 These findings have generated an alternative hypothesis that impairment in reversal learning in individuals with psychopathic traits might be due to dysfunction in the vmPFC.27 During reversal learning in healthy individuals, in contrast to the vlPFC and dmPFC, this region shows a significant reduction in activity during reversal errors (trials when a previously rewarded response is now punished).27 This pattern is consistent with its hypothesized role in prediction error signaling in which a decrease in neuronal firing is observed when an expected reward is not received.31 The pattern is also observed in imaging paradigms during unexpected punishment or when an expected reward or punishment is not received.32-38 In reversal learning, this signaling in the vmPFC may be needed for the detection of contingency change.27 Following these findings, the main goal of this study was to use functional magnetic resonance imaging (fMRI) to test the contrasting hypothesis that reversal impairments in children with psychopathic traits arise from dysfunction either within the vl/dmPFC or vmPFC.
Children with psychopathic traits frequently demonstrate comorbid ADHD.18,39 In contrast, not all children with ADHD demonstrate psychopathic traits. Behavioral and imaging work in children with ADHD suggests abnormalities in regions implicated in reversal learning, including frontostriatal circuits and the cingulate cortex.40-44 While these imaging findings suggest that children with ADHD may be impaired on reversal learning, only 1 study to date has examined and suggested this.45 However, participants were not screened for concurrent psychopathic traits,45 so it is unclear if the impairment in reversal performance is associated with ADHD or underlying comorbidities. Because of this uncertainty, we included a comparison group of children with ADHD without psychopathic traits. This enabled us to determine whether children with ADHD show reversal impairment and, if so, whether neural dysfunction associated with their impairment is similar or dissimilar to that of the children with psychopathic traits.
Based on the findings described earlier, we hypothesized that the neural basis of the reversal deficits observed in children with psychopathic traits results from dysfunction in either systems putatively signaling the unexpected reinforcement (ie, failure to reduce vmPFC activity when an expected reward is absent) or those implicated in processing response conflict and implementing alternative responses (ie, failure to increase dm and vlPFC activity when an expected reward is absent). We tested this using fMRI during performance of a probabilistic reversal paradigm adapted from one that has demonstrated behavioral impairments in children and adults with psychopathic traits and that has recently been characterized in healthy adults with fMRI.19,23,27 We compared the performance and neural response of 3 groups of children: children with psychopathic traits and ODD or CD, children with ADHD only, and healthy children during reversal learning.
METHODS
PARTICIPANTS
Forty-two children participated in this study: 14 children with psychopathic traits and CD or ODD, 14 children with ADHD only, and 14 healthy volunteers (Table 1). The children were recruited from the community through newspaper ads, fliers, and referrals from area mental health professionals. A statement of informed assent and consent was obtained from participating children and parents; this study was approved by the National Institute of Mental Health institutional review board.
Table 1.
Demographic and Clinical Characteristics of Children With ODD/CD and Psychopathic Traits, Children With ADHD Only, and Healthy Controls
| Patients |
|||
|---|---|---|---|
| Measure | Psychopathic Traits and CD/ODD (n=14) | ADHD (n=14) | Controls (n=14) |
| Age, y, mean (SD) | 13.8 (1.3) | 13.4 (2.6) | 13.6 (2.2) |
| IQ, mean (SD) | 101 (10.6) | 112 (12.3) | 103 (13.6) |
| Male, No. (%) | 9 (64) | 10 (71) | 9 (64) |
| DSM-IV diagnoses (current), No. (%) | |||
| ADHD | 10 (71) | 14 (100) | 0 |
| CD | 6 (43) | 0 | 0 |
| ODD | 8 (57) | 0 | 0 |
| Pediatric psychopathy rating scale scores | |||
| Antisocial Process | 29 (3.8) | 9 (5.5) | 7 (4.5) |
| Screening Device,3 mean (SD) [range] | [21-35] | [3-18] | [1-15] |
| Psychopathy Checklist: | 24 (3.5) | ||
| Youth Version,46 mean (SD) | |||
| Response Reversal Task | |||
| Percentage of Errors, No. (%) | |||
| Acquisition | 4 (11) | 4 (10) | 3 (8) |
| Reversal | 10 (27) | 8 (24) | 7 (23) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CD, conduct disorder; ODD, oppositional defiant disorder.
All children and parents completed Kiddie Schedule for Affective Disorders and Schizophrenia (K-SAD)47 assessments with an experienced clinician trained and supervised by expert child psychiatrists, with good interrater reliability (κ>0.75 for all diagnoses). Parents completed the K-SAD interview and the full Antisocial Process Screening Device (APSD).3 Exclusion criteria were pervasive developmental disorder; Tourette syndrome; current or lifetime history of psychosis, depression, bipolar disorder, generalized, social, or separation anxiety disorders, posttraumatic stress disorder, and neurologic disorder; history of head trauma; and IQ less than 80.
Children meeting K-SAD criteria for CD or ODD who had APSD scores of 20 or greater returned to complete the Youth Personality Inventory and Psychopathy Checklist–Youth Version (PCL-YV) assessments (described later). Children scoring 20 or greater on the PCL-YV were included in the psychopathic traits group, and those scoring less than 20 were excluded from the study. Children meeting K-SAD criteria for ADHD were included in the ADHD-only group if APSD scores were less than 20. Healthy children did not meet criteria for any K-SAD diagnosis and scored less than 20 on the APSD.
Children with ADHD (n=10) or CD or ODD (n=4) taking stimulant medications held medications for 48 hours prior to the study visits. Children with psychopathic traits and CD or ODD taking other medications were included if their qualifying behaviors and traits were present despite medication. Thus, 3 children in the psychopathic traits group (1 taking oxcarbazepine and buproprion hydrochloride, 1 taking risperidone, and 1 taking ziprasidone hydrochloride) were included in the study. Children with CD or ODD and psychopathic traits were matched for age, IQ, and sex with the healthy comparison children (Table 1). Children with ADHD without psychopathic traits were matched for age but demonstrated a significantly higher IQ than the other 2 groups (Table 1); therefore, analyses of covariance (ANCOVAs) with IQ as the covariate were used in subsequent analysis.
CLINICAL MEASURES
Antisocial Process Screening Device
The APSD3 is a 20-item parent-completed rating of callous/unemotional traits and conduct and impulsivity problems for the detection of antisocial processes in children. A 3-factor structure has been characterized comprising the following dimensions: callous/unemotional, narcissism, and impulsivity.48 There is no established cutoff score on the APSD for classification of high psychopathic traits.49-51 For research purposes, studies of adolescents have used cutoff of scores of 25,19 median splits (>11 for males, 9 for females),52 or percentile rankings (top one-third, score >18).50 A cutoff score of 20 or higher, one-half the maximum possible of 40 and consistent with the upper tertile of children screened, was chosen for this study.
Psychopathy Checklist: Youth Version
The PCL-YV46 is a 20-item rating scale for assessment of interpersonal, affective, and behavioral features related to psychopathic traits in adolescents based on semistructured interview and collateral information. Items assessed include impression management, grandiosity, stimulation seeking, pathological lying, manipulation, lack of remorse, shallow affect, parasitic orientation, poor anger control, impersonal sexual behavior, early behavior problems, lack of goals, impulsivity, irresponsibility, failure to accept responsibility, unstable interpersonal relationships, serious criminal behavior, violations of conditional release, and criminal versatility. A cutoff score of 20 or higher (one-half the maximum possible) was used for defining the high psychopathic traits group, as there are no standard cutoff scores for classifying youth on this measure to date.8 The PCL-YV interviews were conducted by 2 researchers trained in PCL-YV administration who demonstrated good interrater reliability (R=0.91).
PROBABILISTIC REVERSAL fMRI TASK
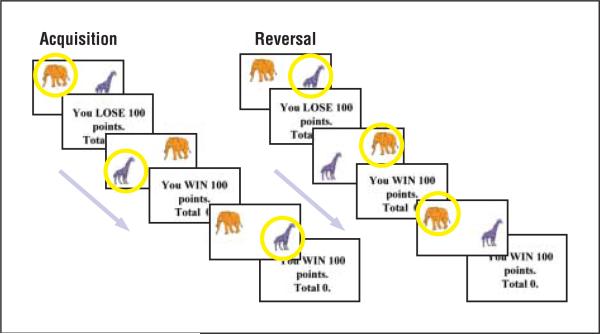
A modified version of a previously reported reversal task27,28 was developed. In this task, children were presented with pairs of images (colored Snodgrass line drawings of animals or furniture53) on a screen (Figure 1). With a button press, children selected 1 of the images and received either positive (You WIN 100 points) or negative (You LOSE 100 points) feedback depending on the accuracy of their selection and the reinforcement probability of the pair. To increase task difficulty, stimulus pairs had a reinforcement probability of either 100:0 (if the correct picture was selected, positive feedback was given for 100% of the trials; if the wrong picture was selected, negative feedback was given for 100% of the trials) or 80:20 (if the correct picture was selected, positive feedback was given for 80% of trials, while negative feedback was given for 20% of trials). After 20 or 25 acquisition trials for the 100:0 and 80:20 reinforcement pairs, respectively, the reinforcement contingency of pairs (ie, which picture is good and which is bad) was reversed for an additional 20 or 25 trials. Participants received the following instructions: Pairs of animals will appear on the screen. On each turn you have to choose 1 of these animals and the computer will tell you if your choice was correct or wrong. If it is correct, you will win 100 points. If it is wrong, you will lose 100 points. Each animal will sometimes be correct and sometimes be wrong, but 1 of the animals will be correct more often than the other one. Find out which animal is usually correct, and choose that animal every time. Stick with it even if it is sometimes wrong. At some point it may change so that the other animal is usually correct, in which case you should choose that one every time.
Figure 1.
Probabilistic reversal task. Participants made button-press responses to select one of the pair images and received positive or negative feedback. After 20 or 25 acquisition trials, the contingency was reversed for the next 20 or 25 trials so that selection of the previously “good” image now resulted in loss of points. Participants then learned to reverse their response and select the alternate image to gain points.
Children completed a brief practice run and then 6 runs of the reversal task in a 1.5-T GE scanner (GE Healthcare, Chalfont St Giles, England). Stimulus pictures were presented for 1600 milliseconds, followed by feedback for 900 milliseconds. If participants did not respond within the 1600-millisecond stimulus presentation, the feedback screen was replaced by the message “please respond faster.” Jittered fixation trials were presented for 2.5 milliseconds interspersed throughout each run to serve as the baseline (20-25 fixation trials per stimulus pair trials to equal 30% of total trials). Two stimulus pairs were presented serially in each run, so that across the 6 runs, the participants saw a total of 5 reversing pairs with 80:20 reinforcement probability, 5 reversing pairs with 100:0 reinforcement probability, one 100:0 nonreversing pair, and one 80:20 nonreversing pair, for a total of 12 pairs. Two versions of the task were developed to counterbalance stimulus pair–reinforcement associations. The order of runs and stimulus pairs within runs was randomized for each participant. Because of technical issues, for 1 child with psychopathic traits, 4 runs were available for analysis and for 1 child with ADHD and 1 healthy child, 5 runs were available for analysis.
MRI PARAMETERS
Participants were scanned during task performance using a 1.5-T GE Signa scanner. A total of 147 functional images per run were taken with a gradient echo planar imaging (EPI) sequence (repetition time=2500 milliseconds; echo time=30 milliseconds; 64×64 matrix; 90° flip angle; 24-cm field of view). Whole-brain coverage was obtained with 29 axial slices (thickness, 4 mm; in-plane resolution, 3.75×3.75 mm). A high-resolution anatomical scan (3-dimensional spoiled gradient recalled acquisition in a steady state; repetition time=8.1 milliseconds; echo time=3.2 milliseconds; 24-cm field of view; 20° flip angle; 124 axial slices; thickness, 1.0 mm; 256×256 matrix) in register with the EPI data set was obtained covering the whole brain.
IMAGING DATA PREPROCESSING
Imaging data were preprocessed and analyzed in AFNI.54 At the individual level, functional images from the first 5 trials of each run collected before equilibrium magnetization was reached were discarded. Functional images from the 6 time series were motion corrected and spatially smoothed with a 6-mm full-width half-maximum gaussian filter. The time series were normalized by dividing the signal intensity of a voxel at each point by the mean signal intensity of that voxel for each run and multiplying the result by 100. Resultant regression coefficients represented a percentage of signal change from the mean. Following this, regressors characterizing the trial and response types by phase (rewarded correct responses, punished correct responses, punished errors, rewarded errors by acquisition or reversal), fixation point trials, and trials where no response was made were created by convolving the train of stimulus events with a gamma-variate hemodynamic response function to account for the slow hemodynamic response. Linear regression modeling was performed using the 10 regressors described earlier plus regressors to model a first-order baseline drift function. This produced a β coefficient and associated t statistic for each voxel and regressor. Participants’ anatomical scans were individually registered to the Talairach and Tournoux atlas,55 following findings that normalization of brain volumes from age 7 to 8 years onward does not introduce major age-related distortions in localization or time course of the blood oxygenation level–dependent (BOLD) signal in event-related fMRI.56,57 In AFNI, individuals’ functional EPI data were registered to their Talairach anatomical scan.
fMRI DATA ANALYSIS
The group analysis of the BOLD data was then performed on regression coefficients from individual subject analyses using a 3 (diagnosis: psychopathic traits, ADHD only, healthy)×2 (response type: all correct rewarded responses or punished reversal errors) ANCOVA with IQ as the covariate. The term punished reversal errors is used to specify those trials in which the participant failed to appropriately reverse a response following the change in contingency and for which they received negative feedback. The threshold was set at P<.001 (corrected at P<.05 for multiple comparisons). In AFNI, a mixed-effects model was used with diagnosis and subject treated as random factors and response type treated as a fixed factor. Threshold correction was done using the AlphaSim program in AFNI, which applies Monte Carlo simulations to calculate the probability of false-positive detection, taking into consideration both the individual voxel probability thresholding and cluster size. Average percentage of signal change was measured within each significant cluster of 50 mm3 or greater. Post hoc analysis of significant main effects and interactions was assessed with 1-way analyses of variance (ANOVAs) and t tests (2-tailed) to further characterize the percentage of signal change.
RESULTS
PARTICIPANTS
One-way between-groups ANOVAs demonstrated no significant differences in age between the 3 diagnostic groups (children with psychopathic traits, children with ADHD only, or healthy controls [F2,39<1; P=.85]) but did demonstrate significant IQ differences (F2,390=3.3; P=.05) (Table 1). Follow-up ANOVAs demonstrated no significant differences in IQ between the psychopathic traits and healthy children groups (F2,26<1; P=.83), while children with ADHD had higher IQ scores than healthy controls (F1,26=6.9; P<.05) and a trend of higher scores than children with psychopathic traits (F1,26=3.3; P=.08).
REVERSAL LEARNING BEHAVIORAL RESULTS
A 3 (diagnosis: psychopathic traits, ADHD only, or healthy control)×2 (phase: acquisition or reversal) repeated-measures ANCOVA with IQ as a covariate was conducted on the percentage of errors in the acquisition and reversal phases (Table 1). There were no significant main effects (phase: F1,38=1.92; P=.17; diagnosis: F2,38=1.6; P=.22) and no significant interaction (F2,38<1; P=.88).
fMRI RESULTS
A 3 (diagnosis: psychopathic traits, ADHD only, or healthy control)×2 (response type: punished reversal errors vs all correct rewarded responses) ANCOVA with IQ as the covariate was conducted on the BOLD response data. This yielded regions showing significant main effects of response and diagnosis as well a significant diagnosis×response interaction.
DIAGNOSIS × RESPONSE TYPE INTERACTION
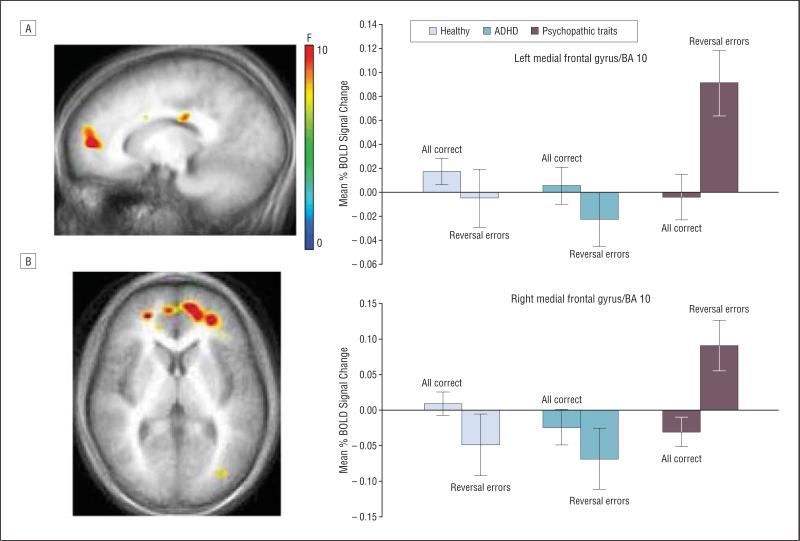
A significant diagnosis×response type interaction was observed bilaterally in the medial frontal gyri (Brodmann area [BA] 10) (Table 2)(Figure 2 and eFigure 1, available at http://archgenpsychiatry.com). Further examination of the interaction using ANCOVA with IQ as a covariate revealed that BOLD responses during rewarded correct responses were not significantly different across groups in both regions (F2,38<1; P=.62) but were significantly different during reversal errors (left medial frontal gyrus [BA 10]: F2,38=6.3; P<.005; right medial frontal gyrus [BA 10]: F2,38=6.7; P<.005). As in prior studies in healthy adults,27 healthy children and children with ADHD demonstrated decreased activity in this region during reversal errors compared with correct responses. Children with psychopathic traits did not show this pattern of suppression but instead displayed increased activity in bilateral medial frontal gyri (BA 10) during punished reversal errors (reversal errors: left medial frontal gyrus, psychopathic traits vs healthy children, t26=2.6; P<.01; psychopathic traits vs ADHD only, t26=3.2; P<.005; right medial frontal gyrus, psychopathic traits vs healthy children, t26=3.2; P<.005; psychopathic traits vs ADHD only, t26=2.3; P<.005). To exclude the possibility that medications in the children with psychopathic traits were driving this effect, we ran a follow-up ANCOVA in AFNI excluding the 3 children with psychopathic traits taking medication and 3 age- and sex-matched children with ADHD and healthy children. The diagnosis×response type interaction and BOLD response pattern described earlier was observed in the same region of the medial frontal gyri (BA 10) in the nonmedicated sample (left BA 10: F=8.62; P<.005; right BA 10: F=6.29; P<.006) (eTable, available at http://archgenpsychiatry.com).
Table 2.
Regions Demonstrating Differential BOLD Responses During Probabilistic Response Reversala
| MNI Coordinate |
|||||||
|---|---|---|---|---|---|---|---|
| Region | L/R | BA | x | y | z | F Value | Cluster Volume, mm3 |
| Group × Reversal Error Interaction | |||||||
| Medial frontal gyrus | L | 10 | –17 | 48 | 8 | 11.8 | 1944 |
| R | 10 | 23 | 47 | 12 | 10.3 | 297 | |
| Caudate | R | 20 | –25 | 27 | 10.3 | 378 | |
| | |||||||
| Main Effect of Reversal Error | |||||||
| Reversal errors>correct responses | |||||||
| Inferior frontal gyrus/anterior insula | R | 13, 45, 47 | 38 | 20 | 7 | 39.7 | 8883 |
| Medial frontal gyrus/cingulate cortex | R | 32 | 11 | 34 | 34 | 26.4 | 8046 |
| Insula/claustrum | L | 13 | –26 | 20 | 4 | 26.5 | 2619 |
| Superior frontal gyrus | L | 9 | –35 | 34 | 37 | 24.4 | 864 |
| Supramarginal gyrus | R | 40 | 53 | –53 | 36 | 16.9 | 810 |
| Reversal errors<correct responses | |||||||
| Right parahippocampal gyrus/amygdala | R | 35 | –19 | –12 | 17.1 | 1512 | |
| Superior temporal gyrus | L | 41 | –50 | –30 | 14 | 21.9 | 1431 |
| R | 22 | 56 | –5 | 6 | 22.0 | 1107 | |
| Insula | R | 13 | 38 | –15 | 18 | 16.4 | 1296 |
| Lentiform nucleus/putamen | L | –29 | –15 | 12 | 21.8 | 783 | |
| Postcentral gyrus | R | 2 | 26 | –39 | 63 | 17.1 | 837 |
| | |||||||
| Main Effect of Group (P<.001) | |||||||
| Psychopathic and ADHD>HC | |||||||
| Precuneus | L | 39 | –29 | –65 | 32 | 16.1 | 7371 |
| R | 31 | 2 | –70 | 19 | 12.6 | 2430 | |
| Superior frontal gyrus | L | 10 | –17 | 56 | 22 | 15.1 | 864 |
| R | 10 | 17 | 56 | 29 | 13.7 | 2052 | |
| Superior temporal gyrus | L | –50 | –40 | 17 | 22.9 | 2187 | |
| R | 38 | –59 | 29 | 13.8 | 2862 | ||
| Precentral gyrus | R | 47 | –19 | 37 | 17.0 | 918 | |
| Psychopathic>HC | |||||||
| Middle occipital gyrus | L | –35 | –69 | –4 | 25.5 | 4077 | |
| Psychopathic>HC and ADHD | |||||||
| Cuneus | R | 18 | 17 | –92 | 17 | 19.7 | 1161 |
| Superior parietal lobule | R | 7 | 26 | –60 | 58 | 11.3 | 810 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; BA, Brodmann area; BOLD, blood oxygen level dependent; HC, healthy controls; L, left; MNI, Montreal Neurological Institute; R, right.
Montreal Neurological Institute coordinates of peak activation. Regions and BA according to Talairach Daemon atlas. All clusters survive correction for multiple comparison at P<.05; clusters that did not survive correction are not listed.
Figure 2.
Diagnosis×response type interaction. In the left (A) and right (B) ventromedial prefrontal cortex/Brodmann area (BA) 10, healthy children and children with attention-deficit/hyperactivity disorder (ADHD) demonstrated decreased blood oxygenation level–dependent (BOLD) responses during punished reversal errors compared with correct rewarded responses. In contrast, patients with psychopathic traits showed increased BOLD responses during punished reversal errors in this region. Error bars represent standard errors.
A significant interaction was also observed in the caudate. In this region, healthy control children demonstrated decreased BOLD activity during punished reversal errors compared with rewarded correct responses (reversal errors: psychopathic vs HC, t=4.4; P<.005). In contrast, children with psychopathic traits demonstrated the opposite pattern, with increased BOLD activity during punished reversal errors compared with rewarded correct responses. Caudate responses in children with ADHD were increased for both correct trials and reversal errors and were not significantly different from those of healthy controls or children with psychopathic traits.
MAIN EFFECT OF RESPONSE TYPE
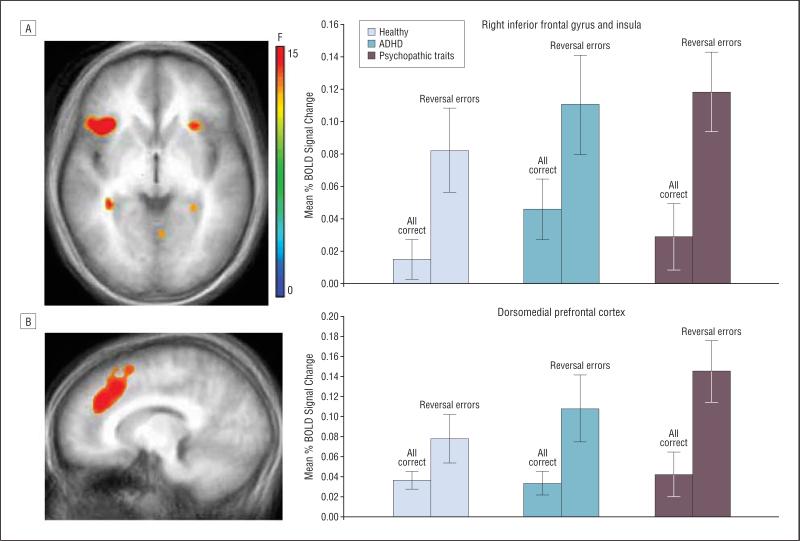
The main effect of response type identified regions showing a differential BOLD signal to punished reversal errors vs all rewarded correct responses (Table 2 and eFigure 2). Consistent with findings of reversal learning studies in adults, this contrast demonstrated regions with 2 distinct patterns of activation. Notably, in the first group of regions, including the vl and dmPFC, BOLD responses were greater in all groups of children during punished reversal errors compared with correct rewarded responses (Figure 3). In the second group of regions, BOLD responses were diminished in all 3 diagnostic groups during punished reversal errors compared with correct rewarded responses (Table 2).
Figure 3.
Main effect of response type. Blood oxygenation level–dependent (BOLD) responses in the ventrolateral prefrontal cortex (A) and dorsomedial prefrontal cortex (B) were significantly greater during punished reversal errors compared with correct rewarded responses in all 3 groups: psychopathic traits, attention-deficit/hyperactivity disorder (ADHD), and healthy controls. Error bars represent standard errors.
MAIN EFFECT OF DIAGNOSIS
The second main effect identified regions showing a differential response for diagnosis (psychopathic traits, ADHD only, or healthy controls) (Table 2). Greater BOLD responses were notable in the psychopathic traits and ADHD-only groups compared with healthy controls in the precuneus (left: psychopathic vs healthy controls, F1,25=5.4; P<.05; ADHD only vs healthy controls, F1,25=4.9; P<.05), right superior frontal gyrus (psychopathic vs healthy controls, F1,25=8.5; P<.01; ADHD vs healthy controls, F1,25=5.8; P<.05), and a trend in the right superior temporal gyrus (ADHD vs healthy controls, F1,25=10.0; P<.005; psychopathic vs healthy controls, F1,25=3.6; P=.07).
TRAIT CORRELATIONS WITH vmPFC ACTIVITY
Post hoc analysis demonstrated a significant correlation between differential activity in the vmPFC and APSD total and subscale scores. Follow-up multiple regression analysis indicated callous and unemotional traits predicted variance in vmPFC BOLD responses (eTable 2).
COMMENT
We contrasted 2 hypotheses regarding the neural basis of the reversal learning deficit typically found in children and adults with psychopathic traits. Specifically, we predicted that children with psychopathic traits would demonstrate dysfunction in either regions of the vl and dmPFC associated with processing response conflict and implementing alternative responses or, alternatively, regions of the vmPFC associated with reinforcement processing. Consistent with the latter hypothesis, we found that children with psychopathic traits displayed abnormal activity in the vmPFC (BA 10) during punished reversal errors. There were no indications of atypical activity in the vl and dmPFC in the children with psychopathic traits.
Reversal learning impairments have been repeatedly reported in children and adults with psychopathic traits.18,19,23 On the basis of the early human neuroimaging literature, which stressed the importance of the vlPFC in reversal learning,24,28,58 it was initially assumed that the reversal impairment in persons with psychopathic traits was due to dys-function in the vlPFC.16,19,23 However, the current data do not support this theory. As can be seen in Figure 3, the children with psychopathic traits showed appropriate increases in vlPFC activation during punished reversal errors.
Recent neuroimaging data highlighting the importance of the vmPFC in reversal learning suggested that dys-function in this region may account for the impairment in individuals with psychopathic traits.27 In line with this hypothesis, we found that children with psychopathic traits displayed abnormal activity in the vmPFC during punished reversal errors. To our knowledge, this is the first report of abnormal vmPFC activation in children with severe disruptive behaviors and high psychopathic traits. This finding is consistent with neuropsychological work in adults that has associated orbital and vmPFC dysfunction with individuals with developmental psychopathy.16,18,59 Furthermore, fMRI studies of adults with high psychopathic traits have demonstrated functional abnormalities in the ventromedial cortex during aversive conditioning60 and a correlation with the vmPFC (BA 10) and psychopathy scores during a prisoner's dilemma task.61 The high prevalence of substance abuse in adults with psychopathic traits has been an important caveat in prior studies,16,18 as exposure to illicit drugs, such as cocaine, can impair orbitofrontal cortex function and reversal learning.62-65 Herein, this potential confound was reduced by studying children and young adolescents who have not had significant exposure to illicit substances, further strengthening the evidence for vmPFC abnormality in individuals with psychopathic traits.
A critical role of the vmPFC in reversal learning appears to be its role in processing reinforcement expectations and information. Studies across species support the role of the orbital and vmPFC in the generation and representation of reinforcement expectations.24,31,32,35,38,64,66 Consistent with these studies and replicating the findings of this reversal task in healthy adults,27 our comparison groups demonstrated decreased BOLD signal responses in the vmPFC during punished reversal errors compared with correct rewarded responses. In contrast, children with psychopathic traits did not show a decrease in vmPFC BOLD signal during punished reversal errors but instead demonstrated increased BOLD signal compared with correct rewarded responses. The lack of the typical reduction in activity in this region during punished reversal errors indicates children with psychopathic traits may not be appropriately processing the violation of reinforcement expectations. Failure to appropriately process this violation may impair their ability to detect the contingency change.
A similar pattern was observed in the caudate. As in the vmPFC, healthy children showed decreased BOLD responses during punished reversal errors. This is in line with recent work implicating the caudate in addition to the medial PFC in prediction error signaling.67,68 In contrast, children with psychopathic traits showed an increase in caudate BOLD signal during punished reversal errors in comparison with correct control responses. This atypical caudate responding in the children with psychopathic traits may be due to abnormal input from the vmPFC or may reflect a more general impairment in prediction error signaling. The current result must be interpreted with caution, however, as activity in the caudate in children with ADHD alone was not significantly different from the control or psychopathic traits groups.
Given the high prevalence of comorbid ADHD and psychopathic traits, it is notable that we did not observe the abnormalities in the vmPFC in children with ADHD only. In the vmPFC, activity in children with ADHD only did not differ from that of healthy comparison children. This suggests that vmPFC dysfunction is specific to the group with psychopathic traits and is not likely attributable to comorbid ADHD. Many early studies implicating ADHD with increased criminality did not assess for comorbid CD,69 and fewer assessed for the presence of psychopathic traits.4,70 Interestingly, a recent prospective longitudinal study of hyperactive children that did assess for the presence of childhood conduct problems found that while adult recidivism rates for hyperactive children with early conduct problems were significantly elevated, rates for hyperactive children without childhood conduct problems were no higher than control children.69 The appropriate pattern of vmPFC activity observed in our ADHD-only group offers some of the first neural-level evidence of how children with ADHD plus psychopathic traits differ from those with ADHD only. However, the present study does not distinguish whether the vmPFC abnormality is specific to children high in psychopathic traits or whether such dysfunction would be also observed in children with severe conduct problems but low in psychopathic traits.
Additional caveats to the present study should be mentioned. The first is that the behavioral performance of children with psychopathic traits did not significantly differ from that of either comparison group. The relatively preserved reversal performance in children with psychopathic traits contrasts with data from previous studies18,19,23 and likely reflects task differences between the current fMRI study and previous behavioral work. In particular, this study used serial presentation of the stimulus pairs during the imaging runs unlike previous behavioral studies where the participants learned about several pairs simultaneously. This reduced task difficulty and thus also reduced the likelihood of confounds introduced by large performance differences. The presence of vmPFC dysfunction in this psychopathic traits cohort, despite comparable task performance, indicates that group differences in this region stem from neural abnormalities rather than performance differences. A second potential limitation is the inclusion of children with psychopathic traits and ADHD with medication histories. While simple stimulant medications were held in all children with psychopathic traits and ADHD for 48 hours prior to the scanning session, the long-term effects of these medications may have impacted the current results. While no orbitofrontal cortex dysfunction was observed in this study, this does not imply that the orbitofrontal cortex, a region previously causally related to psychopathy, is intact in children high in psychopathic traits. The absence of dysfunction may reflect a lack of involvement of this region in this form of reversal task or signal loss in the inferior orbitofrontal cortex.
Models of developmental psychopathic traits have postulated functional abnormalities in the amygdala,71,72 vmPFC,59,73 or both.23,74 The vmPFC and amygdala are reciprocally connected75 and the interaction of these 2 structures is important in stimulus-reinforcement learning.76,77 It is possible that the aberrant signal in the vmPFC in children with psychopathic traits arises from abnormal inputs from the amygdala. However, recent nonhuman primate studies demonstrate that amygdala lesions alone do not impair reversal learning.78 We therefore propose that the altered signal in the present study represents dysfunction within the vmPFC. Whether vmPFC dysfunction occurs independently, or as a developmental consequence of abnormal inputs from the amygdala, remains to be determined. In conclusion, this study provides one of the first confirmations at the neural level of dysfunction specific to the vmPFC in children with high psychopathic traits and disruptive behavioral disorders. The functional specificity indicated by the current study suggests that impairments in the processing of reinforcement information and expectations in the vmPFC may predispose these individuals to impaired decision making, leading to increased frustration and reactive aggression.
Footnotes
Additional Information: The eTables and eFigures are available at http://archgenpsychiatry.com.
Financial Disclosure: None reported.
Funding/Support: This research was supported by the Intramural Research Program of the National Institutes of Health National Institute of Mental Health.
Previous Presentations: Portions of this study were presented in abstract form at the annual meeting of the Society of Biological Psychiatry; May 17, 2007; San Diego, California, and in abstract form at the Society for the Scientific Study of Psychopathy; April 28, 2007; St Pete Beach, Florida.
REFERENCES
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 2.Turgay A. Aggression and disruptive behavior disorders in children and adolescents. Expert Rev Neurother. 2004;4(4):623–632. doi: 10.1586/14737175.4.4.623. [DOI] [PubMed] [Google Scholar]
- 3.Frick PJ, Lilienfeld SO, Ellis M, Loney B, Silverthorn P. The association between anxiety and psychopathy dimensions in children. J Abnorm Child Psychol. 1999;27(5):383–392. doi: 10.1023/a:1021928018403. [DOI] [PubMed] [Google Scholar]
- 4.Barry CT, Frick PJ, DeShazo TM, McCoy MG, Ellis M, Loney BR. The importance of callous-unemotional traits for extending the concept of psychopathy to children. J Abnorm Psychol. 2000;109(2):335–340. doi: 10.1037/0021-843X.109.2.335. [DOI] [PubMed] [Google Scholar]
- 5.Christian RE, Frick PJ, Hill NL, Tyler L, Frazer DR. Psychopathy and conduct problems in children, II. J Am Acad Child Adolesc Psychiatry. 1997;36(2):233–241. doi: 10.1097/00004583-199702000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Dadds MR, Fraser J, Frost A, Hawes DJ. Disentangling the underlying dimensions of psychopathy and conduct problems in childhood: a community study. J Consult Clin Psychol. 2005;73(3):400–410. doi: 10.1037/0022-006X.73.3.400. [DOI] [PubMed] [Google Scholar]
- 7.Brandt JR, Kennedy WA, Patrick CJ, Curtin JJ. Assessment of psychopathy in a population of incarcerated adolescent offenders. Psychol Assess. 1997;9(4):429–435. [Google Scholar]
- 8.Forth A, Kosson DS, Hare RD. Hare Psychopathy Checklist-Youth Version. Multi-Health Systems Inc; Toronto, ON, Canada: 2004. [Google Scholar]
- 9.Tómasson K, Vaglum P. Antisocial addicts: the importance of additional axis I disorders for the 28-month outcome. Eur Psychiatry. 2000;15(8):443–449. doi: 10.1016/s0924-9338(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin RD, Hamilton SP. Lifetime comorbidity of antisocial personality disorder and anxiety disorders among adults in the community. Psychiatry Res. 2003;117(2):159–166. doi: 10.1016/s0165-1781(02)00320-7. [DOI] [PubMed] [Google Scholar]
- 11.Patrick CJ. Emotion and psychopathy. Psychophysiology. 1994;31(4):319–330. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- 12.Dolan MC, Rennie CE. Is juvenile psychopathy associated with low anxiety and fear in conduct-disordered male offenders? [published online ahead of print January 10, 2007]. J Anxiety Disord. 2007;21(8):1028. doi: 10.1016/j.janxdis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Blair RJ, Mitchell DG, Blair KS. The Psychopath: Emotion and the Brain. Blackwell Publishing Limited; Oxford, England: 2005. [Google Scholar]
- 14.Dollard J, Doob CW, Miller NE, Mowrer OH, Sears RR. Frustration and Aggression. Yale University Press; New Haven, CT: 1939. [Google Scholar]
- 15.Berkowitz L. Frustration-aggression hypothesis: examination and reformulation. Psychol Bull. 1989;106(1):59–73. doi: 10.1037/0033-2909.106.1.59. [DOI] [PubMed] [Google Scholar]
- 16.Blair RJ. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. 2004;55(1):198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 17.Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57(12):1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blair RJ, Colledge E, Murray L, Mitchell DG. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. J Abnorm Child Psychol. 2001;29(6):491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- 19.Budhani S, Blair RJ. Response reversal and children with psychopathic tendencies. J Child Psychol Psychiatry. 2005;46(9):972–981. doi: 10.1111/j.1469-7610.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 20.Roberts AC, Robbins TW, Everitt BJ, Muir JL. A specific form of cognitive rigidity following excitotoxic lesions of the basal forebrain in marmosets. Neuroscience. 1992;47(2):251–264. doi: 10.1016/0306-4522(92)90241-s. [DOI] [PubMed] [Google Scholar]
- 21.Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11(4):376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 22.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans. Brain. 2003;126(pt 8):1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 23.Blair RJ, Peschardt KS, Budhani S, Mitchell DG, Pine DS. The development of psychopathy. J Child Psychol Psychiatry. 2006;47(3-4):262–276. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- 24.O'Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23(21):7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remijnse PL, Nielen MM, Uylings HB, Veltman DJ. Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage. 2005;26(2):609–618. doi: 10.1016/j.neuroimage.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Kringelbach ML, Rolls ET. Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage. 2003;20(2):1371–1383. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- 27.Budhani S, Marsh AA, Pine DS, Blair RJ. Neural correlates of response reversal: considering acquisition. Neuroimage. 2007;34(4):1754–1765. doi: 10.1016/j.neuroimage.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 28.Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22(11):4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 31.Tremblay L, Schultz W. Modifications of reward expectation-related neuronal activity during learning in primate orbitofrontal cortex. J Neurophysiol. 2000;83(4):1877–1885. doi: 10.1152/jn.2000.83.4.1877. [DOI] [PubMed] [Google Scholar]
- 32.Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55(6):594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Jensen J, Smith AJ, Willeit M, Crawley AP, Mikulis DJ, Vitcu I, Kapur S. Separate brain regions code for salience vs valence during reward prediction in humans. Hum Brain Mapp. 2007;28(4):294–302. doi: 10.1002/hbm.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yacubian J, Glascher J, Schroeder K, Sommer T, Braus DF, Buchel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26(37):9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 36.O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 37.McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38(2):339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 38.Ramnani N, Elliott R, Athwal BS, Passingham RE. Prediction error for free monetary reward in the human prefrontal cortex. Neuroimage. 2004;23(3):777–786. doi: 10.1016/j.neuroimage.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Kosson DS, Suchy Y, Mayer AR, Libby J. Facial affect recognition in criminal psychopaths. Emotion. 2002;2(4):398–411. doi: 10.1037/1528-3542.2.4.398. [DOI] [PubMed] [Google Scholar]
- 40.Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Ulug AM, Casey BJ. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53(10):871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 41.Dickstein SG, Bannon K, Xavier Castellanos F, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 42.Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J. Anterior cingulate cortex dysfunction in attention-deficit/ hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry. 1999;45(12):1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 43.Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD). J Child Psychol Psychiatry. 2005;46(1):94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 44.Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JD. Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2005;162(9):1605–1613. doi: 10.1176/appi.ajp.162.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Itami S, Uno H. Orbitofrontal cortex dysfunction in attention-deficit hyperactivity disorder revealed by reversal and extinction tasks. Neuroreport. 2002;13(18):2453–2457. doi: 10.1097/00001756-200212200-00016. [DOI] [PubMed] [Google Scholar]
- 46.Forth AE, Kosson DS, Hare RD. The Hare Psychopathy Checklist: Youth Version. Multi-Health Systems; Toronto, ON, Canada: 2003. [Google Scholar]
- 47.Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 1997;75(1):31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 48.Frick PJ, Bodin SD, Barry CT. Psychopathic traits and conduct problems in community and clinic-referred samples of children: further development of the psychopathy screening device. Psychol Assess. 2000;12(4):382–393. [PubMed] [Google Scholar]
- 49.Edens JF, Skeem JL, Cruise KR, Cauffman E. Assessment of “juvenile psychopathy” and its association with violence. Behav Sci Law. 2001;19(1):53–80. doi: 10.1002/bsl.425. [DOI] [PubMed] [Google Scholar]
- 50.Murrie DC, Cornell DG. Psychopathy screening of incarcerated juveniles: a comparison of measures. Psychol Assess. 2002;14(4):390–396. [PubMed] [Google Scholar]
- 51.Frick PJ, Hare RD. Antisocial Process Screening Device. Multi-Health Systems Inc; Toronto, ON, Canada: 2001. [Google Scholar]
- 52.Vitale JE, Newman JP, Bates JE, Goodnight J, Dodge KA, Pettit GS. Deficient behavioral inhibition and anomalous selective attention in a community sample of adolescents with psychopathic traits and low-anxiety traits. J Abnorm Child Psychol. 2005;33(4):461–470. doi: 10.1007/s10802-005-5727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- 54.Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10(4-5):171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 55.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme; Stuttgart, Germany: 1988. [Google Scholar]
- 56.Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19(1):16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 57.Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- 58.Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW. Probabilistic learning and reversal deficits in patients with Parkinson's disease or frontal or temporal lobe lesions. Neuropsychologia. 2000;38(5):596–612. doi: 10.1016/s0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 59.Lapierre D, Braun CM, Hodgins S. Ventral frontal deficits in psychopathy: neuropsychological test findings. Neuropsychologia. 1995;33(2):139–151. doi: 10.1016/0028-3932(94)00110-b. [DOI] [PubMed] [Google Scholar]
- 60.Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient fear conditioning in psychopathy. Arch Gen Psychiatry. 2005;62(7):799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- 61.Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, Lilienfeld SO. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biol Psychiatry. 2007;61(11):1260–1271. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 62.Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20(4):322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 63.Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 64.Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13(6):885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- 65.Jentsch JD, Olausson P, De La Garza R, II, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26(2):183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- 66.Hampton AN, Adolphs R, Tyszka MJ, O'Doherty JP. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55(4):545–555. doi: 10.1016/j.neuron.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 67.Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J Neurophysiol. 2006;95(2):948–959. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- 68.Haruno M, Kawato M. Heterarchical reinforcement-learning model for integration of multiple cortico-striatal loops. Neural Netw. 2006;19(8):1242–1254. doi: 10.1016/j.neunet.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 69.Satterfield JH, Faller KJ, Crinella FM, Schell AM, Swanson JM, Homer LD. A 30-year prospective follow-up study of hyperactive boys with conduct problems: adult criminality. J Am Acad Child Adolesc Psychiatry. 2007;46(5):601–610. doi: 10.1097/chi.0b013e318033ff59. [DOI] [PubMed] [Google Scholar]
- 70.Frick PJ, Cornell AH, Barry CT, Bodin SD, Dane HE. Callous-unemotional traits and conduct problems in the prediction of conduct problem severity, aggression, and self-report of delinquency. J Abnorm Child Psychol. 2003;31(4):457–470. doi: 10.1023/a:1023899703866. [DOI] [PubMed] [Google Scholar]
- 71.Flor H, Birbaumer N, Hermann C, Ziegler S, Patrick CJ. Aversive Pavlovian conditioning in psychopaths: peripheral and central correlates. Psychophysiology. 2002;39(4):505–518. doi: 10.1017.S0048577202394046. [DOI] [PubMed] [Google Scholar]
- 72.Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(pt 5):883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- 73.Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry. 2000;57(2):119–127. doi: 10.1001/archpsyc.57.2.119. discussion 128-119. [DOI] [PubMed] [Google Scholar]
- 74.Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Res. 2006;142(2-3):107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Price JL, Carmichael ST, Drevets WC. Networks related to the orbital and medial prefrontal cortex. Prog Brain Res. 1996;107:523–536. doi: 10.1016/s0079-6123(08)61885-3. [DOI] [PubMed] [Google Scholar]
- 76.Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1(2):155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 77.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 78.Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci. 2007;27(5):1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]