Abstract
Aims: Changes in glutamatergic transmission affect many aspects of neuroplasticity associated with ethanol and drug addiction. For instance, ethanol- and drug-seeking behavior is promoted by increased glutamate transmission in key regions of the motive circuit. We hypothesized that because glutamate transporter 1 (GLT1) is responsible for the removal of most extracellular glutamate, up-regulation or activation of GLT1 would attenuate ethanol consumption. Methods: Alcohol-preferring (P) rats were given 24 h/day concurrent access to 15 and 30% ethanol, water and food for 7 weeks. During Week 6, P rats received either 25, 50, 100 or 200 mg/kg ceftriaxone (CEF, i.p.), a β-lactam antibiotic known to elevate GLT1 expression, or a saline vehicle for five consecutive days. Water intake, ethanol consumption and body weight were measured daily for 15 days starting on Day 1 of injections. We also tested the effects of CEF (100 and 200 mg/kg, i.p.) on daily sucrose (10%) consumption as a control for motivated behavioral drinking. Results: Statistical analyses revealed a significant reduction in daily ethanol, but not sucrose, consumption following CEF treatment. During the post treatment period, there was a recovery of ethanol intake across days. Dose-dependent increases in water intake were manifest concurrent with the CEF-induced decreases in ethanol intake. Nevertheless, CEF did not affect body weight. An examination of a subset of the CEF-treated ethanol-drinking rats, on the third day post CEF treatment, revealed increases in GTL1 expression levels within the prefrontal cortex and nucleus accumbens. Conclusions: These results indicate that CEF effectively reduces ethanol intake, possibly through activation of GLT1, and may be a potential therapeutic drug for alcohol addiction treatment.
INTRODUCTION
Emerging evidence suggests that many aspects of drug addiction involve changes in glutamate transmission. Glutamate-induced neuroadaptations play a key role in ethanol tolerance, dependence, withdrawal and relapse (Backstrom and Hyytia, 2005; Besheer et al., 2009; Bird et al., 2008; Cowen et al., 2005; Hodge et al., 2006; Kapasova and Szumlinski, 2008; Olive et al., 2005). The glutamatergic system in the prefrontal cortex (PFC) has been suggested to be involved in drug reinforcement (Goldstein and Volkow, 2002). The importance of glutamate projections from the PFC to the nucleus accumbens (NAc) and the ventral tegmental area (VTA) has been supported by clinical and animal studies of drugs of abuse (Goldstein and Volkow, 2002; Kalivas et al., 2009; McFarland and Kalivas, 2001).
Ample evidence indicates that glutamatergic neurotransmission is involved in ethanol-drinking behavior. For example, studies have demonstrated that the levels of extracellular glutamate are increased in central brain reward regions during ethanol consumption (Dahchour et al., 2000; Kapasova and Szumlinski, 2008; Melendez et al., 2005; Moghaddam and Bolinao, 1994; Quertemont et al., 1998; Roberto et al., 2004; Selim and Bradberry, 1996; Szumlinski et al., 2007). Moreover, a number of studies have reported that ethanol exposure alters glutamate transport (Othman et al., 2002; Smith, 1997; Smith and Weiss, 1999). Brain extracellular glutamate is regulated by a number of glutamate transporters (Anderson and Swanson, 2000; Gegelashvili and Schousboe, 1997; Seal and Amara, 1999). Of these, glutamate transporter 1 (GLT1), a sodium-dependent transporter found on astrocytes known as excitatory amino acid transporter 2 (Anderson and Swanson, 2000; Rothstein et al., 1994), is responsible for the removal of most extracellular glutamate (Danbolt, 2001; Robinson, 1998). If an increase in glutamate transmission plays a major role in ethanol drinking, as the above studies suggest, then activation of GLT1 should attenuate this behavior. We tested this hypothesis by treating male alcohol-preferring (P) rats with ceftriaxone (CEF), a β-lactam antibiotic known to activate GLT1 (Miller et al., 2008; Rothstein et al., 2005; Sari et al., 2009), for five consecutive days after they had had access to ethanol for 5 weeks. We report here the novel finding that P rats treated with CEF (25, 50, 100 or 200 mg/kg, i.p.) showed a significant reduction in ethanol consumption compared with P rats that received saline vehicle during the treatment period. Nonetheless, as is often seen after pharmacological disruption of ethanol drinking, there was a gradual recovery to pretreatment drinking levels during the post treatment period. Collectively, our results show that CEF treatment attenuates ethanol-drinking behavior, possibly through activation/up-regulation of GLT1, implicating this compound as a potential therapeutic drug for ethanol addiction.
MATERIALS AND METHODS
Animals
Data were obtained from sucrose- and ethanol-naïve adult (> postnatal day 90) male P rats. As an animal model of alcoholism, P rats readily consume pharmacologically relevant levels of ethanol without environmental manipulations (Bell et al., 2006a; McBride and Li, 1998; Murphy et al., 2002), which make them ideally suited for development of medications targeting this disorder. The P rats were obtained from the Indiana University School of Medicine (Indianapolis, IN, USA) breeding colonies. At the beginning of the experiment, the animals weighed an average of 379 ± 7 g (mean ± SEM). Five experimental groups were examined for ethanol-drinking behavior: (a) a saline vehicle control group (n = 13), (b) CEF-treated groups at doses of 25 mg/kg (CEF-25, n = 8), (c) 50 mg/kg (CEF-50, n = 11), (d) 100 mg/kg (CEF-100, n = 11) and (e) 200 mg/kg (CEF-200, n = 13). Three separate experimental groups were examined for sucrose-drinking behavior: (a) a saline vehicle control group (n = 5), (b) a CEF-treated group at a dose of 100 mg/kg (n = 5) and (c) a CEF-treated group at 200 mg/kg (n = 4). After habituation to the vivarium, animals were individually housed in wood-chip-bedded plastic cages in a temperature (21°C) and humidity (50%) controlled vivarium that was maintained on a 12/12 h light/dark cycle (lights off at 1900 hours). All animals had ad lib access to water and food, and all experimental procedures were approved (animal protocol # 07–085) by the Institutional Animal Care and Use Committee of the Indiana University (Bloomington, IN, USA) in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, 1996).
Ethanol-drinking procedures
All P rats were given concurrent access to two concentrations of ethanol (15 and 30%, v/v) beginning at the age of 3 months. Animals experienced continuous, free-choice access to ethanol for 7 weeks. It has been shown that exposure to ethanol for at least 5 weeks is associated with the development of behavioral tolerance in P rats (Stewart et al., 1991). Ethanol measurements were made (to the nearest 10th of a gram) by subtracting the weight of the bottle from its previous weight. During the initial 5-week chronic ethanol-drinking protocol, ethanol consumption for each animal was measured as grams of ethanol consumed per kilogram of body weight per day. At the end of the initial 5-week ethanol-drinking protocol, animals not meeting an intake criterion of >4 g/kg/day intake, averaged across the last 4 days, were excluded from the study. The 4 g/kg/day criterion was adapted from a report examining the development of ethanol dependence (Li et al., 1987). The average intake across these last 4 days served as the Day 1 value for the accompanying figures. Body weight, water consumption and ethanol consumption were recorded at least two times per week during the initial 5-week continuous ethanol-drinking protocol. During Week 6, P rats received 25, 50, 100 and 200 mg/kg CEF (i.p.) or saline vehicle once a day for five consecutive days. Ethanol consumption was measured daily for 15 days starting the first day of CEF injections. A subset of these animals was terminated on Day 8 to determine GLT1 levels in PFC and NAc.
Sucrose-drinking procedures
We also tested the effects of CEF on sucrose (10%) consumption as a control for motivated behavioral drinking. Three sucrose groups experienced continuous access to 10% sucrose for 18 days, with stable sucrose intake across the initial 10 days. Body weight, water consumption and sucrose consumption were measured daily. Starting on Day 11, P rats received saline, 100 mg/kg or 200 mg/kg CEF (i.p.) once a day for five consecutive days. Sucrose consumption is depicted for the 8 days starting with the first day of CEF injections.
Brain tissue harvesting
We assessed GLT1 expression levels in PFC and NAc in a subset of animals exposed to free-choice ethanol (15 and 30% v/v) and water for 5 weeks and then treated for five consecutive days with CEF (50, 100, 200 mg/kg or saline) during Week 6. Three days after the last CEF injection (Day 8) animals were euthanized by carbon dioxide inhalation and decapitated and the brains were removed. The PFC and NAc regions were dissected, frozen and stored at −70°C for further analysis by western blots.
Western blot for GLT1 expression
The western blot procedure for GLT1 was performed as previously described (Sari et al., 2009; Sari et al., 2010). Brain tissue was homogenized in lysis buffer, and the total protein was extracted and quantified (Bio-Rad, Hercules, CA, USA). Protein extractions and western blots were performed for the saline- and CEF-treated groups (50, 100 or 200 mg/kg). Extracted proteins were separated in a 4–20% glycine gel (Invitrogen). Proteins were then transferred onto a nitrocellulose membrane electrophoretically at 30 V for 1 h. The membranes were then blocked using 3% milk in Tris-Buffered Saline Tween-20 (50 mM Tris HCl; 150 mM NaCl, pH7.4; 0.1% Tween 20) for 30 min at room temperature. The membranes were then incubated with guinea pig anti-GLT1 antibody (Millipore Bioscience Research Reagents) at a 1:5000 dilution in blocking buffer at 4°C. After washing and blocking, the membranes were incubated with horseradish peroxidase (HRP)-labeled anti-guinea pig secondary antibody (1:5000 dilution) in blocking buffer. Protein loading was normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) immunoblotting as a loading control. Chemiluminescent detection of HRP (SuperSignal West Pico; Pierce) was followed by exposure of the membranes to Kodak BioMax MR film (Thermo Fisher Scientific). The film was developed on an SRX-101A machine. Digitized images of immunoreactive proteins were quantified using an MCID system. The data are reported as percentage ratios of GLT1/GAPDH.
Statistical analyses
Two-way mixed analyses of variance (ANOVAs) were used to analyze the daily body weight, ethanol, water and sucrose (where applicable) consumption data. After a significant interaction (Dose by Day) term was obtained, a priori Dunnett's multiple comparison t-tests followed significant simple effect (one-way ANOVA of dose differences for each day) analyses. The western blot data were analyzed by one-way ANOVA, and Newman–Keuls's test for comparison between groups. All statistical tests were based on an alpha of P < 0.05 level of significance.
RESULTS
Effects of CEF on ethanol intake
Ethanol (g/kg/day), and water (ml/kg/day), consumption was measured daily for 15 days starting on the first day of injections. Figure 1 shows the average ethanol consumption across these 15 days by the saline, CEF-25, CEF-50, CEF-100 and CEF-200 groups; with Day 1 being the baseline (the average ethanol intake for the 4 days prior to initiation of CEF treatment).
Fig. 1.
Daily ethanol intake of male P rats treated for 5 days with 25 mg/kg (n = 8), 50 mg/kg (n = 7), 100 mg/kg (n = 7), 200 mg/kg (n = 9) ceftriaxone (CEF) or saline (n = 9). Graph represents average daily ethanol (±SEM) intake during the treatment (Days 1–5) and post treatment periods (Days 6–15). *, depicts a significant (P < 0.05) one-way ANOVA across doses for the respective day. Protected Dunnett's t-tests revealed that (a, indicates) the lowest and highest doses of CEF significantly (P < 0.05) decreased ethanol intake relative to saline values; (b, indicates) all doses of CEF significantly (P < 0.05) decreased ethanol intake relative to saline; (c, indicates) the three highest doses of CEF significantly (P < 0.05) decreased ethanol intake relative to saline; (d, indicates) the two highest doses of CEF significantly (P < 0.05) decreased ethanol intake relative to saline; and (e, indicates) the highest dose of CEF significantly (P < 0.05) decreased ethanol intake relative to saline.
A 5 × 15 (Dose by Day) mixed ANOVA conducted on ethanol intake, followed by a priori Dunnett's (two-tailed) multiple comparisons t-tests, revealed a significant Dose by Day interaction [F(56, 490) = 3.05, P < 0.001], as well as significant main effects for Dose [F(4, 35) = 9.70, P < 0.001] and Day [F(14, 490) = 32.68, P < 0.001]. Simple effect analyses conducted as one-way ANOVAs for each day revealed significant (F > 3.50, P < 0.018) differences among the doses for Days 2 through 15. Protected Dunnett's t-tests revealed that the highest and lowest CEF doses, relative to saline, significantly decreased ethanol intake on Day 2; all CEF doses, relative to saline, significantly decreased ethanol intake on Days 3 through 7 and Day 11; the three highest CEF doses, relative to saline, significantly decreased ethanol intake on Days 8 and 9; the two highest CEF doses, relative to saline, significantly decreased ethanol intake on Days 10, 12, 13 and 15; and the highest CEF dose, relative to saline, significantly decreased ethanol intake on Day 14.
Effects of CEF treatment on water intake
A 5 × 15 (Dose by Day) mixed ANOVA conducted on water intake, followed by a priori Dunnett's (two-tailed) multiple comparisons t-tests, revealed a significant Dose by Day interaction [F(56, 490) = 1.47, P = 0.018], as well as significant main effects for Dose [F(4, 35) = 25.57, P < 0.001] and Day [F(14, 490) = 8.97, P < 0.001; Fig. 2). Simple effect analyses conducted as one-way ANOVAs for each day revealed significant [F > 4.41, P < 0.006] differences among the CEF doses for Days 2 through 12 and Day 14. Protected Dunnett's t-tests revealed all CEF doses, relative to saline, significantly increased water intake on Days 2 through 8; the three highest CEF doses, relative to saline, significantly increased water intake on Days 9 and 11; the two highest CEF doses, relative to saline, significantly increased water intake on Days 10 and 12; and the highest CEF dose, relative to saline, significantly increased water intake on Day 14.
Fig. 2.
Daily water intake of male P rats treated for 5 days with 25 mg/kg (n = 8), 50 mg/kg (n = 7), 100 mg/kg (n = 7), 200 mg/kg (n = 9) CEF or saline (n = 9). Graph represents average daily water (±SEM) intake during the treatment (Days 1–5) and post treatment periods (Days 6–15). *, depicts a significant (P < 0.05) one-way ANOVA across doses for the respective day. Protected Dunnett's t-tests revealed that (a, indicates) all CEF doses significantly (P < 0.05) increased water intake relative to saline values; (b, indicates) the three highest CEF doses significantly (P < 0.05) increased water intake relative to saline; (c, indicates) the two highest doses of CEF significantly (P < 0.05) increased water intake relative to saline; and (d, indicates) the highest dose of CEF significantly (P < 0.05) increased water intake relative to saline.
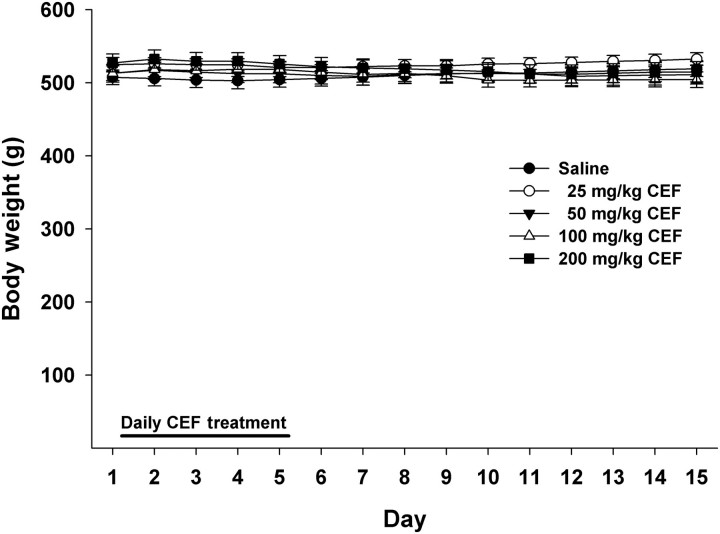
Effects of CEF treatment on body weight
A 5 × 15 (Dose by Day) mixed ANOVA conducted on body weight (Fig. 3) revealed a significant Dose by Day interaction [F(56, 490) = 5.58, P < 0.001], as well as a significant main effect of Day [F(14, 490) = 3.10, P < 0.001]. However, the main effect of Dose was not significant (F < 1.0, P > 0.79); similarly, none of the simple effect analyses for Dose within each day were significant (F < 1.1, P > 0.40). The latter results indicate that CEF did not affect body weight.
Fig. 3.
Daily body weight of male P rats treated for 5 days with 25 mg/kg (n = 8), 50 mg/kg (n = 7), 100 mg/kg (n = 7), 200 mg/kg (n = 9) CEF or saline (n = 9). Graph represents average daily body weight (±SEM) during the treatment (Days 1–5) and post treatment periods (Days 6–15). CEF did not affect body weight across the 15 days.
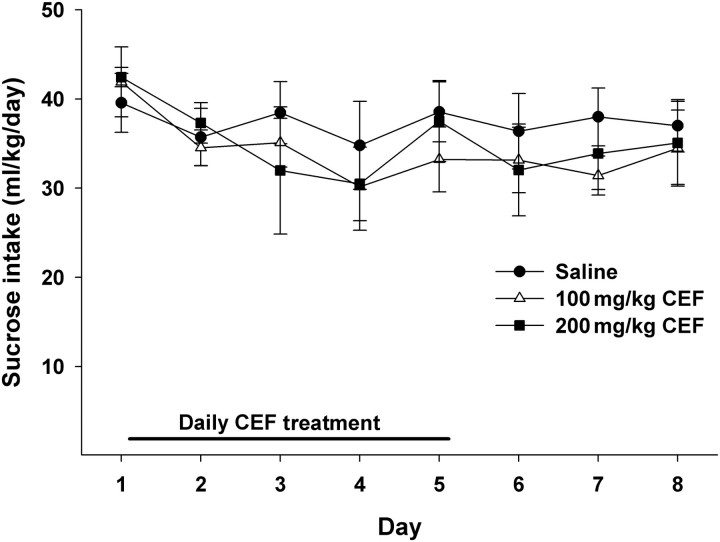
Effects of CEF treatment on sucrose intake
We also tested the effects of CEF (saline, 100 or 200 mg/kg) on sucrose consumption (Fig. 4). The two-way mixed ANOVA revealed a significant main effect of day [F(7, 77) = 3.408, P = 0.003], such that sucrose intake decreased in all three groups across days. However, neither the Dose by Day interaction [F(14, 77) = 0.598, P = 0.859] nor the Dose main effect [F(2, 11) = 0.294, P = 0.751] were significant. These results indicate that CEF did not affect sucrose intake.
Fig. 4.
Daily sucrose intake of male P rats treated for 5 days with 100 (n = 5), 200 (n = 4) mg/kg CEF or saline (n = 5). Graph represents average daily sucrose (±SEM) intake during the treatment (Days 1–5) and post treatment periods (Days 6–8). While the Day main effect was significant (P < 0.05), with a decrease in sucrose intake across days by all three groups, neither the Dose by Day interaction (P > 0.85) nor the Dose main effect (P > 0.75) were significant. Thus, CEF at the two highest doses, which had the greatest effect, in either magnitude and/or duration, on ethanol intake, did not affect intake of a palatable sucrose solution.
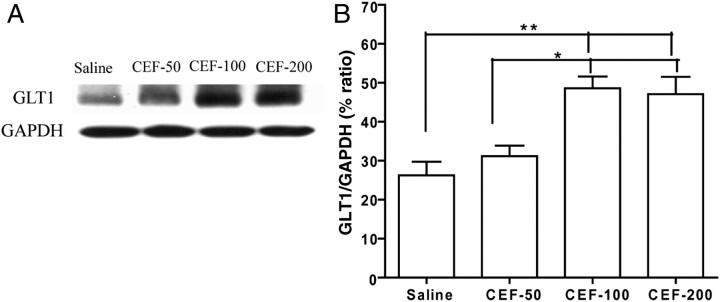
Effects of CEF treatment on GLT1 expression
Changes in the expression of GLT1 within the PFC were examined by western blot. As shown in Fig. 5, a significant up-regulation of GLT1 expression was found in both the CEF-100 and CEF-200 groups at Day 8 (3 days post CEF treatment) when compared with the saline group [F(3,15) = 10.41, P < 0.01]. CEF at a dose of 50 mg/kg did not increase GLT1 expression in the PFC. GAPDH, which was used as a loading control, did not show any differences in expression among the groups (P > 0.05).
Fig. 5.
Effects of 50 mg/kg (CEF-50, n = 4), 100 mg/kg (CEF-100, n = 4), 200 mg/kg (CEF-200, n = 4) CEF or saline treatment (n = 4) on GLT1 expression in PFC. (A) Each panel presents immunoblots for GAPDH, which was used as a control loading protein, and GLT1. (B) Quantitative analysis revealed a significant increase in the ratio of GLT1/GAPDH in the CEF-100 and CEF-200 groups when compared with the saline vehicle and CEF-50 groups. Error bars indicate SEM (*P < 0.05; **P < 0.01).
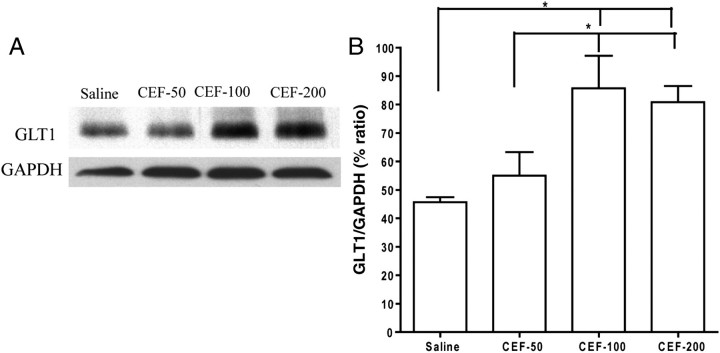
Western blot analyses were also used to examine changes in the expression of GLT1 in the NAc. As shown in Fig. 6, a significant increase in GLT1 level was found in both the CEF-100 and CEF-200 groups at Day 8 compared with the control (saline) group [F(3,15) = 6.46, P < 0.01]. There were no differences in GLT1 expression between the CEF-50 and saline groups. GAPDH did not show any differences in expression among the groups (P > 0.05).
Fig. 6.
Effects of 50 mg/kg (CEF-50, n = 4), 100 mg/kg (CEF-100, n = 4), 200 mg/kg (CEF-200, n = 4) CEF or saline treatment (n = 4) on GLT1 expression in NAc. (A) Each panel presents immunoblots for GAPDH, which was used as a control loading protein, and GLT1. (B) Quantitative analysis revealed a significant increase in the ratio of GLT1/GAPDH in the CEF-100 and CEF-200 groups when compared with the saline vehicle and CEF-50 groups. Error bars indicate SEM (*P < 0.05).
DISCUSSION
We report here that all doses of CEF tested attenuated ethanol consumption in P rats, but only the highest doses tested (100 and 200 mg/kg) were associated with an up-regulation of GLT1 expression in the PFC and NAc. Increases in the expression of GLT1 appear to be inversely associated with a post treatment attenuation of ethanol intake. It is noteworthy that the levels of ethanol intake (between 6 and 7 g/kg/day) exhibited by the saline-treated P rats result in repeated, pharmacologically relevant (at least 40–50 mg%) blood alcohol levels (Bell et al., 2006a, b; Murphy et al., 1986). Although there were significant differences in the water intake between saline- and CEF-treated groups, there were no significant differences in the body weight between all the groups. The increase in water intake could be due to the fact that decreases in ethanol intake in the CEF-treated groups were compensated, in part, by the increases in water intake.
Glutamate transmission in key brain regions of the reward circuit including PFC and NAc plays a critical role in dependence-related behaviors, including locomotor sensitization and drug-seeking behavior (Kalivas et al., 2009; Sari et al., 2009). There is a relatively high concentration of glutamate in the PFC and NAc, which is associated with addiction-related changes in cognition, emotion, sensory input and subsequent motor output (McFarland and Kalivas, 2001). The importance of glutamate projections from the PFC, particularly to the NAc and the VTA, has been confirmed by clinical neuroimaging studies during craving for commonly abused drugs such as ethanol, cocaine, methamphetamine, heroin and nicotine (Childress et al., 1999; Dom et al., 2005; Garavan et al., 2000; Goldstein and Volkow, 2002; Wexler et al., 2001; Xiao et al., 2006). We tested for changes in GLT1 protein expression levels within the PFC and NAc regions because the interactions between these two regions mediate, at least in part, drug reward (Kalivas et al., 2009). Our interest in these regions also stems from their glutamatergic input from the amygdala and hippocampus, key players in initiating drug-seeking behavior as well (Kalivas et al., 2009).
Ethanol exposure has been demonstrated to alter glutamatergic activity in the mesocorticolimbic circuit. Previous studies, using Cologne ‘ALKO’ Alcohol-Accepting (cAA) rats, investigating the effects of 20 months of ethanol exposure on glutamatergic function in the cerebral cortex (Schreiber and Freund, 2000) found that ethanol-exposed cAA rats displayed decreased glutamate transporter activity compared with naïve cAA rats (Schreiber and Freund, 2000). It is noteworthy that both the Alko Alcohol (AA) (the foundation stock for cAA rats) and P rats were selectively bred for ethanol preference, using similar criteria, and both used Wistar rats, albeit from different colonies and progenitors (Bell et al., 2005, 2006a; Sommer et al., 2006). The specific involvement of GLT1 in addiction has been tested in drug abuse models as well. For example, activation of GLT1 by MS-153 effectively attenuated morphine, methamphetamine and cocaine conditioned place preference in mice (Nakagawa et al., 2005). Additionally, our laboratory has reported that CEF attenuates cue-induced cocaine relapse in a dose-dependent manner (Sari et al., 2009). In accordance, Kalivas et al. (2009) found similar effects on cocaine relapse with CEF (Knackstedt et al., 2010). This relapse was accompanied by an increase in GLT1 expression in the PFC and NAc. Additionally, CEF was found to increase accumbal cysteine/glutamate exchanger (xCT) expression in a rat model of cocaine relapse-like behavior (Knackstedt et al., 2010). This later study demonstrated that CEF-induced increase in the xCT level was correlated with down-regulation of extracellular levels of glutamate.
In the brain, CEF is the most potent β-lactam antibiotic in inducing up-regulation or activation of GLT1 (Miller et al., 2008; Rothstein et al., 2005; Sari et al., 2010). Furthermore, single daily injections of 200 mg/kg CEF for five consecutive days in mice increased glutamate uptake in the striatum, a primary target of cortical glutamate input (Miller et al., 2008). Thus, CEF appears to have a direct central effect on glutamate transporter function.
In the present study, a lower dose of CEF (50 mg/kg) did not appear to increase GLT1 expression 3 days post treatment, but were effective in reducing ethanol intake. At the time point when GLT1 expression was determined, drinking levels in the 25 mg/kg dose group did not differ from control. Therefore, the lower doses may not have had a direct effect on GLT1 expression, at least not detectable by the methods used in the present study. This suggests that CEF may have additional pharmacological effects, or that its effect on GLT1 activity is secondary to an unknown primary effect. CEF has been shown to increase glutamate uptake in the rat hippocampus without increasing GLT1 expression (Lipski et al., 2007). In addition, a previous study using Wistar rats tested a single CEF (200 mg/kg, i.p.) injection 90 min after middle cerebral artery occlusion. Although CEF did not increase GLT1 expression, the activity of GLT1 was increased in several brain regions including the hippocampus, striatum and frontal cortex (Thone-Reineke et al., 2008).
One possible mechanism in which CEF acts indirectly on GLT1 may involve central glutathione (GSH) activity. An in vitro study has shown that ceftrixaone treatment increased GSH and xCT levels (Lewerenz et al., 2009). The CEF-induced increases in xCT and subsequent increases in GSH level may be one mechanism for reversing the glutamate transporter deficits caused by free radical oxidation. Ethanol withdrawal is associated with increases in oxygen-derived free radicals (Vallett et al., 1997), which have been shown to inhibit glutamate uptake by oxidation of thiol groups (Volterra et al., 1994). These authors reported that this effect was reversed by GSH administration. Additionally, in vitro studies have shown that GSH prevents ethanol-induced gastric mucosal damage (Loguercio et al., 1993; Mutoh et al., 1990). A number of studies implicate high alcohol intake with abnormal, relative to low ethanol-drinking rodents, levels of GSH and/or enzymes associated with GSH in high ethanol-consuming rodent lines. Naïve high alcohol-preferring mice have greater gene expression for the GSH S-transferase, mu type 1 gene than their low alcohol-preferring counterparts, suggesting that this is a candidate gene for ethanol preference (Saba et al., 2006). Naïve inbred P (iP) rats have greater GSH S-transferase, mu type 2 and GSH S-transferase gene expression in the hippocampus than their inbred alcohol non-preferring (iNP) counterparts (Edenberg et al., 2005). A subsequent study found that iP rats have lower levels of GSH S-transferase, alpha 4 gene expression in the PFC, NAc, hippocampus, amygdala and caudate-putamen as well as lower levels of GSH S-transferase omega 1, and GSH S-transferase, mu type 3 (when expression levels across all five brain regions were averaged) than iNP rats (Kimpel et al., 2007). Work with the AA and its Alko alcohol non-accepting (ANA) counterpart found that AA rats had higher GSH S-transferase alpha 4, mu 1 and mu 3, as well as GSH peroxidase 3 gene expression in the PFC than ANA rats (Sommer et al., 2006). Again, these findings suggest that this family of genes modulates ethanol preference.
Regarding ethanol exposure, five consecutive daily injections of ethanol increased GSH S-transferase-alpha protein expression in the NAc of alcohol non-preferring (NP) rats compared with naïve NP rats (McBride et al., 2009). Under operant conditions, ethanol self-administration by P rats increased GSH peroxidase 4 gene expression in the NAc relative to rats self-administering saccharin (Rodd et al., 2008). Also, chronic ethanol consumption by P rats increases hydroxyacyl glutathione hydrolase gene expression in the NAc relative to naïve P rats (Bell et al., 2009). However, it must be noted that these studies examined gene and/or protein expression levels, thus absolute levels of GSH activity were not determined. Thus, future studies addressing this important research question, ethanol-associated changes in GSH activity in vivo, are needed.
In addition, activation of protein kinase C (PKC) induces a rapid down-regulation in the cell surface expression of several neurotransmitter transporters (Beckman et al., 1999; Daniels and Amara, 1999; Melikian and Buckley, 1999; Qian et al., 1997). In particular, activation of PKC caused a rapid decrease in the cell surface expression of GLT1 (Kalandadze et al., 2002). Taken together, these findings suggest that CEF may act via a presently unidentified mechanism independent of the activation and/or up-regulation of GLT1. Further studies are warranted to investigate the full pharmacological activity of CEF in P rats.
Regarding GLT1 up-regulation, the precise cellular mechanism underlying this effect remains unknown. At least two pathways have been suggested, and they may have direct or indirect interactions with each other. First, Lee et al. (2008) demonstrated that the canonical nuclear factor kB (NF-kB) signaling pathway is necessary for the CEF-induced increase in GLT1 in human primary fetal astrocytes. While NF-kB activity itself was not measured, ethanol consumption by P rats has been shown to increase, within the NAc shell, the expression of genes associated with this signaling pathway (McBride et al., 2010). In addition, a previous study reported operant ethanol self-administration by inbred P rats reduced gene expression levels for the NF-kB-activating protein in the NAc (Rodd et al., 2008), which may or may not correspond with decreased levels of the protein itself. Secondly, it has been shown that the mammalian target of rapamycin (mTOR) pathway is also involved in regulating GLT1 expression and subsequent glutamate uptake in vitro, such that phosphorylation of mTOR by Akt appears to alter GLT1 expression levels (Wu et al., 2010). As with the NF-kB signaling pathway, McBride et al. (2010) have also reported that ethanol drinking by P rats increased gene expression for the Akt, a constituent of the Wnt/beta-catenin signaling pathway, and Akt1 proteins in the NAc shell. Again, this study was on gene expression levels. Thus, future in vivo studies examining phosphorylation of mTOR, concomitant with altered GLT1 expression levels, following ethanol self-administration are needed.
In conclusion, we report here that CEF reduced ethanol intake in an animal model of alcohol abuse. In addition, the post treatment reduction in ethanol intake was dose dependent in nature, such that higher doses had a stronger effect. However, post treatment alterations in GLT1 expression levels within the PFC and NAc occurred only in the highest dose groups. Therefore, a direct action on GLT1 levels may be limited to our high CEF doses, while lower doses may act via other mechanisms. Given previous work indicating that up-regulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats (Knackstedt et al., 2010; Sari et al., 2009), the present findings indicate that CEF, as well as possibly other manipulators of GLT1 expression, is a potential therapeutic compound targeting ethanol and drug abuse/dependence.
Funding
This work was supported in part by R21AA016115 (Y.S.), DA 02451 (G.V.R.), U01AA13522 (R.L.B.) and R24AA015512 (Lawrence Lumeng).
Acknowledgements
We would like also to thank Verity Johnson and Sonam Thadani for their help in measuring ethanol and water intake, and body weight in some cohorts of animals. The authors would like also to thank Shufan Ge for her help, in part, in the western blot test.
Conflict of interest statement. None declared.
REFERENCES
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. doi:10.1002/1098-1136(200010)32:1<1::AID-GLIA10>3.0.CO;2-W. [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–8. doi: 10.1016/j.ejphar.2005.10.051. doi:10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Beckman ML, Bernstein EM, Quick MW. Multiple G protein-coupled receptors initiate protein kinase C redistribution of GABA transporters in hippocampal neurons. J Neurosci. 1999;19:RC9. doi: 10.1523/JNEUROSCI.19-11-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RH, Rodd ZA, Murphy JM, et al. Use of selectively bred alcohol-preferring rats to study alcohol abuse, relapse and craving. In: Preedy VR, Watson RR, editors. Comprehensive Handbook of Alcohol Related Pathology. Vol. 3. New York: Academic Press; 2005. pp. 1515–33. [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, et al. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006a;11:270–88. doi: 10.1111/j.1369-1600.2005.00029.x. doi:10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, et al. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006b;83:35–46. doi: 10.1016/j.pbb.2005.12.004. doi:10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Bell RL, Kimpel MW, McClintick JN, et al. Gene expression changes in the nucleus accumbens of alcohol-preferring rats following chronic ethanol consumption. Pharmacol Biochem Behav. 2009;94:131–47. doi: 10.1016/j.pbb.2009.07.019. doi:10.1016/j.pbb.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Cannady R, et al. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2009;551:71–5. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MK, Kirchhoff J, Djouma E, et al. Metabotropic glutamate 5 receptors regulate sensitivity to ethanol in mice. Int J Neuropsychopharmacol. 2008;11:765–74. doi: 10.1017/S1461145708008572. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen MS, Djouma E, Lawrence AJ. The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther. 2005;315:590–600. doi: 10.1124/jpet.105.090449. doi:10.1124/jpet.105.090449. [DOI] [PubMed] [Google Scholar]
- Dahchour A, Hoffman A, Deitrich R, et al. Effects of ethanol on extracellular amino acid levels in high-and low-alcohol sensitive rats: a microdialysis study. Alcohol Alcohol. 2000;35:548–53. doi: 10.1093/alcalc/35.6.548. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. doi:10.1016/S0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Daniels GM, Amara SG. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J Biol Chem. 1999;274:35794–801. doi: 10.1074/jbc.274.50.35794. doi:10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, et al. Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. Br J Psychiatry. 2005;187:209–20. doi: 10.1192/bjp.187.3.209. doi:10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Strother WN, McClintick JN, et al. Gene expression in the hippocampus of inbred alcohol-preferring and -nonpreferring rats. Genes Brain Behav. 2005;4:20–30. doi: 10.1111/j.1601-183X.2004.00091.x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–98. doi: 10.1176/appi.ajp.157.11.1789. doi:10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. doi:10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, et al. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 2006;183:429–38. doi: 10.1007/s00213-005-0217-y. doi:10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalandadze A, Wu Y, Robinson MB. Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486. J Biol Chem. 2002;277:45741–50. doi: 10.1074/jbc.M203771200. doi:10.1074/jbc.M203771200. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, et al. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl. 1)):169–73. doi: 10.1016/j.neuropharm.2008.07.011. doi:10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–31. doi: 10.1111/j.1530-0277.2008.00620.x. doi:10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Kimpel MW, Strother WN, McClintick JN, et al. Functional gene expression differences between inbred alcohol-preferring and -non-preferring rats in five brain regions. Alcohol. 2007;41:95–132. doi: 10.1016/j.alcohol.2007.03.003. doi:10.1016/j.alcohol.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–4. doi: 10.1016/j.biopsych.2009.07.018. doi:10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, et al. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283:13116–23. doi: 10.1074/jbc.M707697200. doi:10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Albrecht P, Tien ML, et al. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J Neurochem. 2009;111:332–43. doi: 10.1111/j.1471-4159.2009.06347.x. doi:10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, et al. Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol Suppl. 1987;1:91–6. [PubMed] [Google Scholar]
- Lipski J, Wan CK, Bai JZ, et al. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience. 2007;146:617–29. doi: 10.1016/j.neuroscience.2007.02.003. doi:10.1016/j.neuroscience.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Loguercio C, Taranto D, Beneduce F, et al. Glutathione prevents ethanol induced gastric mucosal damage and depletion of sulfhydryl compounds in humans. Gut. 1993;34:161–5. doi: 10.1136/gut.34.2.161. doi:10.1136/gut.34.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–69. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Schultz JA, Kimpel MW, et al. Differential effects of ethanol in the nucleus accumbens shell of alcohol-preferring (P), alcohol-non-preferring (NP) and Wistar rats: a proteomics study. Pharmacol Biochem Behav. 2009;92:304–13. doi: 10.1016/j.pbb.2008.12.019. doi:10.1016/j.pbb.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, Schultz JA, et al. Changes in gene expression in regions of the extended amygdala of alcohol-preferring rats after binge-like alcohol drinking. Alcohol. 2010;44:171–83. doi: 10.1016/j.alcohol.2009.12.001. doi:10.1016/j.alcohol.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, et al. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29:326–33. doi: 10.1097/01.alc.0000156086.65665.4d. doi:10.1097/01.ALC.0000156086.65665.4D. [DOI] [PubMed] [Google Scholar]
- Melikian HE, Buckley KM. Membrane trafficking regulates the activity of the human dopamine transporter. J Neurosci. 1999;19:7699–710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, et al. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington's disease phenotype in the R6/2 mouse. Neuroscience. 2008;153:329–37. doi: 10.1016/j.neuroscience.2008.02.004. doi:10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neurosci Lett. 1994;178:99–102. doi: 10.1016/0304-3940(94)90299-2. doi:10.1016/0304-3940(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, Waller MB, et al. Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol. 1986;3:331–6. doi: 10.1016/0741-8329(86)90010-8. doi:10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–88. doi: 10.1023/a:1020266306135. doi:10.1023/A:1020266306135. [DOI] [PubMed] [Google Scholar]
- Mutoh H, Hiraishi H, Ota S, et al. Protective role of intracellular glutathione against ethanol-induced damage in cultured rat gastric mucosal cells. Gastroenterology. 1990;98:1452–9. doi: 10.1016/0016-5085(90)91075-h. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Fujio M, Ozawa T, et al. Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behav Brain Res. 2005;156:233–9. doi: 10.1016/j.bbr.2004.05.029. doi:10.1016/j.bbr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, et al. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol. 2005;67:349–55. doi: 10.1124/mol.104.003319. doi:10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- Othman T, Sinclair CJ, Haughey N, et al. Ethanol alters glutamate but not adenosine uptake in rat astrocytes: evidence for protein kinase C involvement. Neurochem Res. 2002;27:289–96. doi: 10.1023/a:1014955111742. doi:10.1023/A:1014955111742. [DOI] [PubMed] [Google Scholar]
- Qian Y, Galli A, Ramamoorthy S, et al. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quertemont E, de Neuville J, De Witte P. Changes in the amygdala amino acid microdialysate after conditioning with a cue associated with ethanol. Psychopharmacology (Berl) 1998;139:71–8. doi: 10.1007/s002130050691. doi:10.1007/s002130050691. [DOI] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, et al. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004;24:1594–603. doi: 10.1523/JNEUROSCI.5077-03.2004. doi:10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MB. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem Int. 1998;33:479–91. doi: 10.1016/s0197-0186(98)00055-2. doi:10.1016/S0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Kimpel MW, Edenberg HJ, et al. Differential gene expression in the nucleus accumbens with ethanol self-administration in inbred alcohol-preferring rats. Pharmacol Biochem Behav. 2008;89:481–98. doi: 10.1016/j.pbb.2008.01.023. doi:10.1016/j.pbb.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, et al. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–25. doi: 10.1016/0896-6273(94)90038-8. doi:10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–7. doi: 10.1038/nature03180. doi:10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Saba L, Bhave SV, Grahame N, et al. Candidate genes and their regulatory elements: alcohol preference and tolerance. Mamm Genome. 2006;17:669–88. doi: 10.1007/s00335-005-0190-0. doi:10.1007/s00335-005-0190-0. [DOI] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, et al. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–43. doi: 10.1523/JNEUROSCI.1746-09.2009. doi:10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Prieto AL, Barton SJ, et al. Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington's disease. J Biomed Sci. 2010;17:62. doi: 10.1186/1423-0127-17-62. doi:10.1186/1423-0127-17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Freund WD. Glutamate transport is downregulated in the cerebral cortex of alcohol-preferring rats. Med Sci Monit. 2000;6:649–52. [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Annu Rev Pharmacol Toxicol. 1999;39:431–56. doi: 10.1146/annurev.pharmtox.39.1.431. doi:10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- Selim M, Bradberry CW. Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex: comparison between the Lewis and Fischer 344 rat strains. Brain Res. 1996;716:157–64. doi: 10.1016/0006-8993(95)01385-7. doi:10.1016/0006-8993(95)01385-7. [DOI] [PubMed] [Google Scholar]
- Smith TL. Regulation of glutamate uptake in astrocytes continuously exposed to ethanol. Life Sci. 1997;61:2499–505. doi: 10.1016/s0024-3205(97)00985-5. doi:10.1016/S0024-3205(97)00985-5. [DOI] [PubMed] [Google Scholar]
- Smith AD, Weiss F. Ethanol exposure differentially alters central monoamine neurotransmission in alcohol-preferring versus -nonpreferring rats. J Pharmacol Exp Ther. 1999;288:1223–8. [PubMed] [Google Scholar]
- Sommer W, Hyytia P, Kiianmaa K. The alcohol-preferring AA and alcohol-avoiding ANA rats: neurobiology of the regulation of alcohol drinking. Addict Biol. 2006;11:289–309. doi: 10.1111/j.1369-1600.2006.00037.x. doi:10.1111/j.1369-1600.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- Stewart RB, McBride WJ, Lumeng L, et al. Chronic alcohol consumption in alcohol-preferring P rats attenuates subsequent conditioned taste aversion produced by ethanol injections. Psychopharmacology (Berl) 1991;105:530–4. doi: 10.1007/BF02244375. doi:10.1007/BF02244375. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Diab ME, Friedman R, et al. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology (Berl) 2007;190:415–31. doi: 10.1007/s00213-006-0641-7. doi:10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- Thone-Reineke C, Neumann C, Namsolleck P, et al. The beta-lactam antibiotic, ceftriaxone, dramatically improves survival, increases glutamate uptake and induces neurotrophins in stroke. J Hypertens. 2008;26:2426–35. doi: 10.1097/HJH.0b013e328313e403. doi:10.1097/HJH.0b013e328313e403. [DOI] [PubMed] [Google Scholar]
- Vallett M, Tabatabaie T, Briscoe RJ, et al. Free radical production during ethanol intoxication, dependence, and withdrawal. Alcohol Clin Exp Res. 1997;21:275–85. [PubMed] [Google Scholar]
- Volterra A, Trotti D, Tromba C, et al. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci. 1994;14:2924–32. doi: 10.1523/JNEUROSCI.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, et al. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. doi:10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Wu X, Kihara T, Akaike A, et al. PI3K/Akt/mTOR signaling regulates glutamate transporter 1 in astrocytes. Biochem Biophys Res Commun. 2010;393:514–8. doi: 10.1016/j.bbrc.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Lee T, Zhang JX, et al. Thirsty heroin addicts show different fMRI activations when exposed to water-related and drug-related cues. Drug Alcohol Depend. 2006;83:157–62. doi: 10.1016/j.drugalcdep.2005.11.012. doi:10.1016/j.drugalcdep.2005.11.012. [DOI] [PubMed] [Google Scholar]