Abstract
Purpose:
To present a series of microsporidial keratoconjunctivitis in 24 eyes.
Methods:
Retrospective non-comparative observational case series. Medical records were retrieved and individuals evaluated based on symptoms, risk factors, visual acuity, slit lamp biomicroscopy and pathological examination of cornea epithelial scrapings. Demographic features, clinical course, predisposing factors, microbiological profile, treatment, final clinical outcome and visual acuity were recorded.
Results:
Of the 22 patients, 90.9% were men, with a mean age of 30.3 years (range 15 – 76 years). Two (9.1%) had bilateral involvement, 15 (68.2%) were non-contact lens users, 17 (77.3%) reported contamination with mud within 2 weeks (mean 6.8 days) of onset of symptoms. All patients presented with conjunctivitis and coarse, multifocal, punctate epithelial keratitis. Two out of 24 eyes (8.3%) had anterior stromal infiltrates, while 8 (33.3%) had anterior uveitis. Microsporidial spores were identified on modified trichrome staining of corneal epithelial scrapes in all eyes. All eyes were treated with epithelial debridement, topical fluoroquinolone and hexamidine diisethionate, 7 (31.8%) patients received oral albendazole, and all eyes with anterior uveitis received topical steroids. All cases resolved without visually significant sequelae.
Conclusion:
Microsporidial keratoconjunctivitis occurred mainly in males, is usually unilateral, presents as conjunctivitis and coarse, multifocal, punctate epithelial keratitis, and may incite anterior uveitis. Soil contamination is an important risk factor. Treatment with debridement, fluoroquinolones, hexamidine diisethionate with or without systemic albendazole is effective, with steroids reserved for any associated anterior uveitis.
Keywords: Microsporidia, keratitis, conjunctivitis, uveitis.
INTRODUCTION
Microsporidia are obligate, intracellular, spore-forming protozoan parasites belonging to the phylum Microspora, a species-rich group of minute, single-celled, intra-cellular parasites. Lacking normal mitochondria and with unique cytology, microsporidia have sometimes been thought to be a lineage of ancient eukaryotes. More recently (2005), phylogenetic studies have revealed many other molecules suggesting instead a relationship with fungi [1].
In human eyes, microsporidia are opportunistic pathogens initially reported to cause two distinct clinical entities: deep corneal stromal infection in immunocompetent patients and bilateral diffuse punctate epithelial keratopathy in immunocompromised patients and patients with AIDS [2,3]. Recent series have reported multifocal coarse punctate epithelial keratitis caused by microsporidia in immuno-competent individuals [4,5]. Risk factors for microsporidial keratoconjunctivitis include systemic or local immuno-suppression, ocular trauma and exposure to unclean riverwaters [4,5]. Although no specific treatment exists, empiric treatment options including debridement, topical antibiotics (fluoroquinolones, diamidines, fumagillin), anti-fungal (itraconazole), systemic anti-helminthic (albenda-zole), antiseptics (polyhexamethylene biguanide, chlorhexi-dine, hexamidine diisethionate), and topical steroids have been described [4,5].
We report a series of 24 eyes of 22 patients with microsporidial spores identified on corneal epithelial scrapings, including risk factors, clinical presentations, treatment regimes and visual outcomes.
MATERIALS AND METHODOLOGY
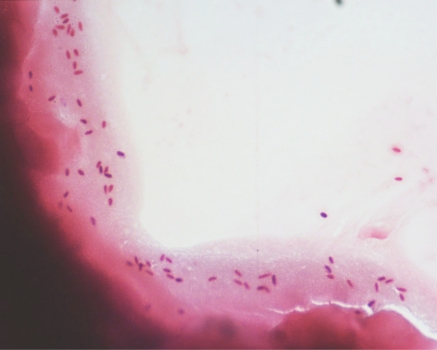
Corneal epithelial scapings were performed on patients presenting to the Ophthalmology unit at Tan Tock Seng Hospital with symptoms and signs suggestive of microsporidia keratoconjunctivitis [4]. The results of all corneal epithelial scrapings for microsporidia microscopy for a 17-month period from October 2006 to February 2008 were examined. Subtotal corneal epithelial scrapings were obtained using a 27-gauge hypodermic needle, air dried on a glass slide, and fixed in methanol for five minutes. The slides were subsequently placed in modified trichome stain for 10 minutes at 50 degrees Celsius, then rinsed in acid alcohol for 10 seconds, followed by 95% alcohol and finally in distilled water [6]. The slides were examined for 100 to 300 fields at a magnification of 100X. Microsporidial spores were identified as ovoid, refractile bodies measuring 0.9 to 1.5 micrometers in size, each with a reddish-pink cellular wall and a horizontal or diagonal stripe representing the polar tube (Fig. 1).
Fig. (1).
Low (x10) magnification light microscopy appearance of microsporidial spores.
We performed a retrospective, non-comparative review of clinical records of patients with scrapings that were positive for microsporidia. Patients were evaluated based on systemic medical history (for immunocompromised states like diabetes or HIV), symptoms, risk factors (exposure to foreign matter [including environmental agents like soil or mud], sporting activities [such as swimming], contact lens wear), steroid treatment prior to presentation, presenting visual acuity, slit-lamp biomicroscopy findings, treatment, and clinical course. Patients who defaulted follow-up were interviewed via telephone regarding symptomatic resolution. There was no standard follow-up schedule as this was a retrospective analysis. Institutional Review Board Ethics Committee approval was obtained and the study adhered to the tenets of the Declaration of Helsinski.
RESULTS
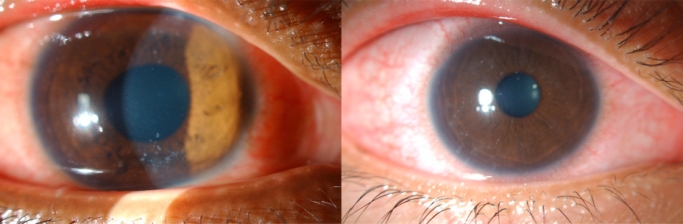
The results are summarized in Table 1. Of the 204 corneal epithelial scrapings performed for suspected microsporidial keratoconjunctivitis examined between November 2006 to February 2008, 24 (11.8%) were positive for microsporidia. Gram-stain smears and cultures of these scrapings did not reveal any other microorganisms. There was a male preponderance (male:female 10:1), with a median age of 27 years (mean age 30.3 years, range 15 – 76 years). Two patients (9.1%) had bilateral involvement, while the rest were unilateral. Seven patients (31.8%) were contact lens users and 17 (77.3%) reported foreign body contamination between 5 to 14 days (mean 6.8 days) prior to presentation. The mean duration of symptoms at presentation was 6.2 days (range 2 – 14 days). Mud or soil were the predominant ocular contaminants (88.2%), reportedly occurring during sporting activities such as soccer (80.0%) or rugby (13.3%) in muddy fields (Table 2), prior to onset of symptoms, which included redness, foreign body sensation in the eye, tearing, clear discharge and blurring of vision (Table 3). Presenting visual acuity (VA) ranged from 6/6 to 6/24 with 18/24 (75%) with VA of 6/12 or better. Diffuse, multifocal, punctate epithelial keratitis with subepithelial infiltrates (Fig. 2) was observed in all eyes, with 2 eyes (8.3%) having deeper stromal infiltrates. Stromal infiltrates were observed to be centrally located, anterior to mid stromal opacities. Mild non-granulomatous anterior uveitis with fine keratitic precipitates, and anterior chamber activity ranging from 0.5+ (5 eyes) to 1+ (3 eyes) was present in 8 eyes (33.3%).7 No patients had any symptoms or signs of systemic microsporidia infection.
Table 1.
Clinical Characteristics of 22 Patients (24 Eyes) with Microsporidia Keratoconjunctivitis
| No. | Age/Gender/Race | Risk Factors (Duration Prior to Presentation) | Symptoms (Duration) | Presenting BCVA | Signs | Treatment |
|---|---|---|---|---|---|---|
| 1 | 20/ Male/ Indian | Foreign matter entering eye (7 days prior) | Redness, foreign body sensation (5 days) | 6/6 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G levofloxacin q2H, G hexamidine q2H, PO albendazole 400mg od |
| 2 | 17/ Male/ Eurasian | Mud entering eye (5 days prior) | Redness, discharge, blurring (5 days) | 6/6 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G levofloxacin q1H, G hexamidine q3H, PO albendazole 400mg od |
| 3 | 30/ Male/ Chinese | Mud entering eye (5 days prior), contact lens wear | Redness, discharge (7 days) | 6/7.5 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G levofloxacin q2H, G hexamidine q2H |
| 4 | 21/ Male/ Chinese | Mud entering eye (5 days prior) | Redness, discharge, blurring, photophobia (5 days) | 6/18 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G ciprofloxacin q3H, G hexamidine q3H |
| 5 | 39/ Male/ Caucasian | Mud entering eye (8 days prior), contact lens wear | Redness, discharge, foreign body sensation (5 days) | 6/9 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G levofloxacin q3H, G hexamidine q3H, PO albendazole 400mg od |
| 6 | 76/ Male/ Chinese | Nil | Redness, discharge, foreign body sensation (5 days) | 6/24 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis, anterior uveitis | G levofloxacin q3H, G hexamidine q3H, PO albendazole 400mg od |
| 7 | 17/ Female/ Indian | Mud entering eye (14 days prior) | Redness, discharge, blurring of vision (6 days) | 6/6 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G levofloxacin q2H, G hexamindine q2H |
| 8 | 41/ Male/ Chinese | Nil | Redness, discomfort (5 days) | 6/6 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G levofloxacin q3H, G hexamidine qds, PO albendazole 200mg od, G fluormetholone tds |
| 9 | 15/ Male/ Malay | Mud entering eye (14 days prior) | Redness, tearing (14 days) | 6/12 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis, anterior uveitis | G levofloxacin q3H, G hexamidine qds |
| 10 | 29/ Male/ Chinese | Contact lens wear | Foreign body sensation (7 days) | 6/9 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G levofloxacin q3H, G hexamidine qds, G fluormetholone tds |
| 11 | 46/ Male/ Chinese | Nil | Redness (10 days) | 6/7.5 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G levofloxacin q3H, G hexamidine q3H, G prednisolone acetate 0.12% qds |
| 12 | 48/ Male/ Malay | Mud entering eye (7 days prior) | Redness, swelling (14 days) | 6/9 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G ciprofloxacin q3H, G hexamidine tds, |
| 13 | 16/ Female/ Malay | Mud entering eye (4 days prior) | Redness, swelling, discharge (4 days) | 6/18 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G cefazolin (50mg/ml) qds, G dexamethasone 1% qds, G gentamicin (14mg/ml) q3H, G levofloxacin qds, G hexamidine qds |
| 14 | 23/ Male/ Malay | Nil | Redness, discharge, pain (2 days) | 6/6 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G optodexine q3H, G levofloxacin q3H, G hexamidine qds |
| 15 | 19/ Male/ Malay | Contact lens wear | Redness, discomfort (5 days) | 6/6 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis, anterior uveitis | G levofloxacin q3H, G hexamidine qds, PO albendazole 200mg om, G Prednisolone acetate 0.12% bd |
| 16 | 25/ Male/ Chinese | Mud entering eye (7 days prior) | Redness, discharge (7 days) | 6/18 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis, anterior uveitis | G levofloxacin q3H, G hexamidine q3H, G prednisolone acetate 0.12% qds |
| 17 | 29/ Male/ Chinese | Mud entering eye (7 days prior), contact lens wear | Redness, discomfort (4 days) | 6/9 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G levofloxacin q3H, G hexamidine q3H, G prednisolone acetate 1% qds, PO albendazole 200mg od |
| 18 | 19/ Male/ Chinese | Mud entering eye (4 days) | Redness (4 days) | 6/6 OU | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis, anterior uveitis (bilateral) | G levofloxacin q3H, G hexamidine qds, G prednisolone acetate 1% q3H |
| 19 | 32/ Male/ Chinese | Mud entering eye (7 days) | Redness, discharge, blurring of vision (7 days) | 6/12 OD 6/6 OS | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis (bilateral) | G levofloxacin qds, G hexamidine qds, G prednisolone acetate 0.12% bd |
| 20 | 37/ Male/ Indian | Mud entering eye (7 days) | Redness, swelling (7 days) | 6/9 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis, anterior uveitis | G levofloxacin qds, G hexamidine qds, G prednisolone acetate 0.12% qds |
| 21 | 16/ Male/ Chinese | Mud entering eye (7 days) | Redness, discharge, blurring of vision (2 days) | 6/18 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G levofloxacin q3H, G hexamidine qds |
| 22 | 51/ Indian/ Male | Mud entering eye (7 days) | Redness, discomfort (7 days) | 6/15 | Diffuse coarse punctate epithelial keratitis, diffuse conjunctivitis | G levofloxacin qds, G hexamidine qds |
G – guttae; PO – by mouth; od – once daily, in the morning; bd – twice daily; tds – three times daily; qds – four times daily; q1H – every one hour; q2H – every 2 hours; q3H – every 3 hours etc.; OD – right eye; OS – left eye; Defaulted – did not attend subsequent scheduled appointment for review; BCVA – best corrected visual acuity.
Table 2.
Risk Factors Identified in Patients with Microsporidial Keratoconjunctivitis (n=24 Eyes)
| Risk factors | |
|---|---|
| Contact Lens Wear | 29.2% (7) |
| Foreign body contamination | 70.8% (17) |
| Mud/ soil exposure | 88.2% (15) |
| Soccer | 80.0% (12) |
| Rugby | 13.3% (2) |
| Other activity | 6.7% (1) |
| Other foreign body | 11.8% (2) |
Table 3.
Symptoms and Signs at Presentation (n=24 Eyes)
| Presentation | ||
|---|---|---|
| Redness | 95.8% (23) | |
| Tearing | 41.7% (10) | |
| Symptoms | Foreign body sensation | 20.8% (5) |
| Blurring of vision | 20.8% (5) | |
| Diffuse multifocal punctate epithelial keratitis | 100% (24) | |
| Signs | Stromal inflitrates | 8.3% (2) |
| Anterior uveitis | 33.3% (8) | |
Fig. (2).
Anterior segment photographs showing diffuse, multifocal, punctate epithelial keratitis.
All 24 eyes were treated with corneal epithelial debridement (performed under local anaesthetic at the slit lamp using a 27-gauge hypodermic needle), followed by topical fluorquinolone (levofloxacin 0.5% [19 eyes], ciprofloxacin 0.3% [3 eyes]), and topical hexamidine diisethionate 0.1%. Seven patients (31.8%) were given systemic anti-parasitic treatment with oral albendazole (200mg to 400mg once a day for 3 – 7 days) and 14 eyes (58.3%) with deep stromal infiltrates or anterior uveitis received concomitant topical steroids (dexamethasone 0.1% [3 eyes], fluormetholone 0.1% [2 eyes], prednisolone acetate 0.12% [7 eyes], prednisolone acetate 1% [2 eyes]).
The duration of follow-up ranged from 4 to 66 days (mean 20 days). Fifteen patients (68.2%) subsequently defaulted follow-up, but reported complete symptomatic resolution when contacted. The 7 patients who did not default achieved complete resolution between 3 – 6 weeks (mean 3.7 weeks) from initiation of treatment. Of these 7, two who had received oral albendazole achieved resolution at an average of 2.5 weeks while the 5 who did not, achieved resolution at an average of 4.2 weeks (p=0.11). Four eyes developed asymptomatic subepithelial scarring. All but one had final best-corrected visual acuities (BCVA) equal to or better than 6/7.5. One 76 year-old patient, in the absence of corneal scarring, had a final BCVA of 6/18, which was similar to the baseline visual acuity, and attributable to the presence of a significant cataract.
DISCUSSION
The aim of this retrospective study is to report a large series of microsporidial keratoconjunctivitis in healthy individuals. Initially reported to cause bilateral diffuse punctate epithelial keratopathy in immunocompromised individuals [2,3], microsporidia has been recently reported to cause multifocal coarse punctate epithelial keratitis in immunocompetent individuals [4,5]. Joseph et al. (2006) reported a series of 19 cases of microsporidial kerato-conjunctivitis in immunocompetent individuals [5]. With increasing awareness amongst ophthalmologists, this previously under-diagnosed infection is being more commonly recognized in the immunocompetent host. In our series, formal serological testing for immunocompromised states such as HIV infection was not performed. This is in recognition of the fact that microsporidial kerato-conjunctivitis does occur in healthy immunocompetent individuals. Instead, a history of common immunocompromising conditions (such as diabetes mellitus, HIV, chronic steroid use) was obtained from all cases. All patients denied having any of these conditions, and were systemically well.
More than 75% of cases reported contamination with soil or mud between 5 to 14 days prior to presentation, reaffirming previous reports of foreign body exposure as a risk factor for microsporidial infection. In their series, Joseph et al. reported predisposing factors for microsporidial keratoconjunctivitis as trauma, dust particles, insect bites, and bathing in unclean river waters [5]. Soil or mud contamination was the predominant risk factor identified in our series. Common sources of soil or mud contamination amongst our patients include sporting activities such as soccer and rugby, especially in males between 11 to 30 years of age. Majority of cases reported coming into close contact with soil or mud while playing sports in muddy fields, from a day up to 2 weeks prior to onset of symptoms. It is conceivable that the patients contracted the infection while engaging in these activities.
Most patients (86.4%) did not seek ophthalmic consults prior to presentation at our institution. Three patients received prior steroids prescribed by general practitioners, with no improvement in symptoms. The steroid therapy may have contributed to the persistence of microsporidial infection and worsening of symptoms, or created a localized immunosuppressed state, resulting in superinfection by microsporidia, similar to that postulated by Chan et al. [4].
Of 204 corneal epithelial scrapings only 24 eyes were positive for microsporidia. While the large number of negative scapings could be accounted for by fewer inflitrates in these eyes, the number and location of infiltrates were not consistently documented in the clinical records from which data was obtained in this retrospective study. We postulate that the reason for the negative cases could most likely be due to the difficulty in identification of these spores because of their small size (1 to 1.5 micrometers) and the range of stain intensity.
No definitive treatment for microsporidial keratoconjunctivitis exists. All cases in our series were treated with epithelial debridement followed by topical fluorquinolone and topical hexamidine diisethionate 0.1%. Epithelial debridement therapeutically debulks the infective organisms from the corneal epithelium, but may increase the risk of penetration of the organism into the deep stromal layers, or increase the risk of secondary infection [7]. A broad-spectrum antibiotic such as fluorquinolone was employed to prevent secondary infection. Hexamidine diisethionate, a disinfectant frequently used in the treatment of acanthamoeba keratitis, was used empirically against microsporidia, with therapeutic success. Seven cases were given a short course of oral albendazole, a broad-spectrum antihelminthic that has been shown to be effective in the treatment of systemic microsporidiosis [8], while 14 cases, who had accompanying anterior uveitis, received concomitant topical steroids, after being treated initially with a combination of fluoroquinolone and hexamidine diisethionate. This combination of treatment appears effective in controlling both the infection and inflammatory reaction that occasionally accompanies the infection. While successful anecdotal drug treatments of microsporidiosis using itraconazole, propamidine isethionate and fumagillin have been reported, our large series showed equal efficacy with a combination of epithelial debridement, topical fluoroquinolones, hexamidine diisethionate with or without systemic albendazole, and topical steroids for cases with associated anterior uveitis.
As this was a retrospective study, there was neither randomization of treatment protocols nor a standard follow-up schedule. We are thus unable to determine if the additional treatment of oral albendazole had any effect on disease resolution. Although cases that received oral albendazole appeared to have a shorter period to complete resolution compared to those that did not, this did not reach statistical significance in our study, possibly due to the small sample size of the study. Chan et al. previously reported spontaneous resolution of keratitis within 2 weeks of discontinuation of topical steroids, suggesting the ability of an immunocompetent host to counter the infection spontaneously, once the steroid induced localized immunosuppression has been discontinued [4]. It would be interesting in future studies, to compare the efficacy of various treatment modalities against a control group not receiving any treatment, to observe the natural history of this infection.
Our study had a high default rate of 68%, presumably due to rapid full symptomatic recovery. When contacted, however, all defaulters reported complete symptomatic resolution. For cases followed-up to resolution, BCVA ranged between 6/6 to 6/7.5. This reaffirms the good visual outcomes of treatment of microsporidial keratitis reported in other series [4,5]. Other limitations of this retrospective review include a relatively small sample size and lack of a control group. We are thus unable to compare our treatment regime with other medications (e.g. polyhexamethylene biguanide or chlorhexidine) previously reported to be used in the treatment of microsporidial keratitis [4,5]. Further larger scale, prospective, randomized controlled trials would be required to elucidate the effect of the various treatment modalities, and better understand the natural history of this disease.
CONCLUSION
From our series, microsporidial keratoconjunctivitis occurs mainly in males, is usually unilateral, causes a diffuse, multifocal, punctate epithelial keratitis, and may incite mild anterior uveitis. Mud exposure is a significant risk factor. Combination treatment with epithelial debridement, topical fluoroquinolones, hexamidine diisethionate with or without systemic albendazole is effective, with topical steroids used concomitantly for any associated anterior uveitis. Visually non-significant corneal subepithelial scarring occurs in some cases, but full symptomatic resolution is the norm.
ACKNOWLEDGMENTS
None.
TRIAL REGISTRATION
Not applicable.
ETHICAL APPROVAL
Institutional review board approval was obtained for this study.
CONFLICTS OF INTEREST
All authors: NONE.
REFERENCES
- 1.Fischer WM, Palmer JD. Evidence from small-subunit ribosomal RNA sequences for a fungal origin of Microsporidia. Mol Phylogenet Evol. 2005;36:606–22. doi: 10.1016/j.ympev.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 2.Weber R, Bryan RT, Schwartz DA, Owen RL. Human microsporidial infections. Clin Microbiol Rev. 1994;7:426–61. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber R, Bryan RT. Microsporidial infections in immunodeficient and immunocompetent patients. Clin Infect Dis. 1994;19:517–21. doi: 10.1093/clinids/19.3.517. [DOI] [PubMed] [Google Scholar]
- 4.Chan CM, Theng JT, Li L, Tan DT. Microsporidial keratoconjunctivitis in healthy individuals: a case series. Ophthalmology. 2003;110:1420–5. doi: 10.1016/S0161-6420(03)00448-2. [DOI] [PubMed] [Google Scholar]
- 5.Joseph J, Sridhar MS, Murthy S, Sharma S. Clinical and microbiological profile of microsporidial keratoconjunctivitis in southern India. Ophthalmology. 2006;113:531–7. doi: 10.1016/j.ophtha.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 6.Isenberg HD, editor. Clinical Microbiology Procedures Handbook. 2nd ed. vol. 2. 9.3.6.1-9.3.6.6. ASM Press; 2004. [Google Scholar]
- 7.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gritz DC, Holsclaw DS, Negar RE, et al. Ocular and sinus microsporidial infection cured with systemic albendazole. Am J Ophthalmol. 1997;124:241–3. doi: 10.1016/s0002-9394(14)70792-5. [DOI] [PubMed] [Google Scholar]