Abstract
Objectives
To study prescribing trends for antidepressants in Hawai‘i following the FDA black box warning regarding the possible risk of suicide in children and adolescents. We also explored relationships between changes in prescribing trends and patient and provider characteristics.
Study Design
Analysis of an existing insurance data set of prescriptions to children and adolescents within the State of Hawai‘i.
Study Population
Children and adolescents under 18 years old insured through the largest (>60%) third- party insurance company in the state.
Results
Our results showed variations in changes in prescribing trends for different selective serotonin reuptake inhibitors (SSRIs) following the FDA black box warning. SSRIs with more evidence-based research supporting their safety and efficacy were least affected as were those that were less implicated by the FDA analysis of the possible link between SSRIs and Suicidality. Trends were apparent for all age groups examined and for both females and males.
Conclusions
Changes in prescribing patterns of psychiatric medications for children and adolescents in Hawai‘i were identified. Differing patterns have evolved since 2003 following the series of concerns raised regarding SSRIs and suicidality in children and adolescents.
Introduction
In early 2004, the Food and Drug Administration (FDA) asked manufacturers of several antidepressants to include a label warning of possible increased risk of suicidal ideations or behavior, particularly at times of treatment initiation or dosage change.1 Later in October 2004, the FDA directed manufacturers of all antidepressants to include a warning that antidepressants increased the risk of suicidal ideations and behavior in children and adolescents.2,3 The FDA also decided that all patients receiving these medications be given a Medication Guide so that patients would be aware of possible risks and necessary precautions. Medication Guides were to be distributed by the pharmacist with each new prescription or refill of antidepressant medications.4
The FDA warning has triggered a significant number of concerns from the scientific and medical community.5–7 The American College of Neuropsychopharmacology (ACNP) Task Force on SSRIs and Suicidal Behavior in Youth reported that SSRI benefits in treating adolescent depression exceeded the risks of suicidal thoughts or attempts.8 ACNP's position was supported by the American Academy of Child and Adolescent Psychiatry (AACAP). AACAP maintained that child and adolescent psychiatrists should continue to treat depression with all available effective means including the use of antidepressants alone, or antidepressants combined with Cognitive Behavioral Therapy (CBT) or CBT alone. The combined treatment approach was thought of as the most efficacious.9–12 AACAP reached out to the parents of depressed children and adolescents to provide education and information to reduce the risk of undesirable treatment outcomes.13
This study was undertaken to explore the possible relationship between the FDA warning in Hawai‘i and treatment approaches specifically with focus on SSRIs since MAO inhibitors and other antidepressants see little use in this population. The study's goals are:
To explore changes in SSRIs prescribing trends in children and adolescents in Hawai‘i, as evidenced by prescriptions being filled
To compare changes in prescribing patterns of SSRIs by specific SSRI categories.
To examine variations in prescribing behavior of SSRIs by provider characteristics
To examine variations in provider's prescribing trends by patient age and gender.
Methods
The study is an analysis of existing data on prescriptions for SSRIs among children & adolescents obtained from the largest third-party insurance company in Hawai‘i. Children and adolescents were eligible if under 18 years old and on psychiatric medication during the time period 2002–2005. Study variables included:
Psychiatric medication: The number of antidepressant prescriptions that are filled by patients;
Patient characteristics: age and gender;
Provider characteristics: psychiatrist provider vs. non-psychiatrist provider;
The category of psychiatric medications the patient was on
The study hypothesis was that the FDA directives in 2004 regarding use of antidepressants would alter prescribing trends for psychiatric medications for depression and anxiety among child & adolescent psychiatric patients.
Statistical Analysis: Data were initially analyzed by descriptive techniques examining frequencies and cross-tabulations of variables of interest. Further analyses included logistic regression to identify significant trends.
Protection of Privacy: Patient data were identified by a coded number to maintain subject confidentiality. The study was conducted in strict compliance with HIPPA rules & regulations. Because the analyses used existing data without patient identification the study was granted an exemption from Institutional Review Board (IRB) review by the University of Hawai‘i.
Results
Prescribing patterns were compared by calendar year (2002–2005) and within three different age groups: 0–12, 13–15 & 16–18. These analyses were followed by evaluating SSRIs prescriptions by gender then by type of provider (psychiatrist/ non-psychiatrist provider).
Total SSRI prescriptions decreased slightly from 2724 in 2002 to 2238 in 2005 with variations by specific brand (Figure 1). Citalopram (Celexa) and paroxetine (Paxil) had statistically significant decreases (p-values < 0.001 and odds ratios per year (and 95% confidence intervals) of 0.6 (0.5, 0.6) and 0.5 (0.4, 0.6), respectively); whereas fluoxetine (Prozac) and escitalopram (Lexparo) had statistically significant increases (p-values < 0.001 and odds ratios per year (and 95% confidence intervals of 1.3 (1.2, 1.4) and 1.5 (1.3, 1.6), respectively). Citalopram use decreased from 21.2% in 2002 to 17.85% in 2003 to 10.6% in 2004 to 5.9% in 2005. Paroxetine prescriptions dropped from 21.4% in 2002 to 9.0% in 2003 to 5.0% in 2004 to 4.2% in 2005. On the other hand, fluoxetine prescriptions increased gradually as follows: 10.2% in 2002 to 8.9% in 2003 to 17.0% in 2004 to 19.4% in 2005. Escitalopram prescriptions increased from 1.1% in 2002 to 12.2% in 2003 to 16.4% in 2004 to 17.2% in 2005. The number of Zoloft prescriptions also increased slightly (38.4% in 2002 and 48.0% in 2005).
Figure 1.
Percent of serotonin reuptake inhibitor prescriptions by specific brand and by calendar year.
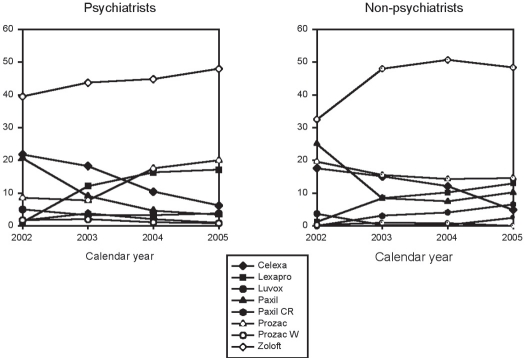
The percentage of SSRI prescriptions decreased by 35.0% in the 0–12 age group between 2002 and 2005 as compared to 3.1% for the 13–15 age group and 19.4% in the 16–19 age group (Table 1). The decreases in citalopram and paroxetine use and the increase in fluoxetine and escitalopram use were observed with all SSRI prescriptions and were present within all three age groups. When prescriptions by year were stratified by gender both genders also showed the same changes observed overall and within age groups (Table 2). Differences were also apparent when psychiatrist and non-psychiatrist prescribers were compared (Figure 2). The steady climb in fluoxetine prescriptions by psychiatrists (from 8.5% in 2002 to 20.0% in 2005) was not observed among non-psychiatrists (19.6% of prescriptions in 2002 and 14.6% of prescriptions in 2005). A consistent increase in long-acting paroxetine prescriptions by non-psychiatrists was observed from 0.0% in 2002 to 3.1% in 2003 to 4.1% in 2004 to 6.5% in 2005. This was not seen among psychiatrists (1.6% of prescriptions in 2002 and 0.9% in 2005)
Table 1.
Number and percentage of selective serotonin reuptake inhibitor (SSRI) prescriptions by prescription year and by age group.
| Age Group: 0–12 | Age Group: 13–15 | Age Group: 16–18 | ||||||||||
| SSRI | 2002 | 2003 | 2004 | 2005 | 2002 | 2003 | 2004 | 2005 | 2002 | 2003 | 2004 | 2005 |
| Celexa | 124 | 125 | 61 | 16 | 215 | 235 | 115 | 60 | 239 | 166 | 109 | 56 |
| 17.4% | 18.5% | 10.6% | 3.5% | 22.8% | 21.0% | 10.8% | 6.6% | 22.3% | 14.3% | 10.5% | 6.5% | |
| Lexapro | 1 | 59 | 106 | 92 | 14 | 131 | 155 | 142 | 15 | 154 | 155 | 140 |
| 0.1% | 8.7% | 18.4% | 19.9% | 1.5% | 11.7% | 14.6% | 15.5% | 1.4% | 13.3% | 14.9% | 16.2% | |
| Luvox | 51 | 24 | 12 | 10 | 42 | 36 | 42 | 45 | 38 | 22 | 21 | 26 |
| 7.2% | 3.6% | 2.1% | 2.2% | 4.5% | 3.2% | 3.9% | 4.9% | 3.6% | 1.9% | 2.0% | 3.0% | |
| Paxil | 158 | 58 | 23 | 3 | 194 | 99 | 43 | 46 | 230 | 109 | 69 | 44 |
| 22.2% | 8.6% | 4.0% | 0.7% | 20.6% | 8.9% | 4.0% | 5.0% | 21.5% | 9.4% | 6.6% | 5.1% | |
| Paxil | 2 | 22 | 4 | 0 | 18 | 48 | 23 | 22 | 16 | 39 | 34 | 11 |
| CR | 0.3% | 3.3% | 0.7% | 0.0% | 1.9% | 4.3% | 2.2% | 2.4% | 1.5% | 3.4% | 3.3% | 1.3% |
| Prozac | 56 | 48 | 74 | 57 | 110 | 91 | 217 | 203 | 111 | 124 | 167 | 175 |
| 7.9% | 7.1% | 12.9% | 12.3% | 11.7% | 8.1% | 20.4% | 22.2% | 10.4% | 10.7% | 16.1% | 20.3% | |
| Prozac | 1 | 0 | 0 | 0 | 34 | 36 | 5 | 5 | 6 | 18 | 23 | 11 |
| Weekly | 0.1% | 0.0% | 0.0% | 0.0% | 3.6% | 3.2% | 0.5% | 0.6% | 0.6% | 1.6% | 2.2% | 1.3% |
| Zoloft | 318 | 341 | 295 | 284 | 316 | 442 | 465 | 391 | 414 | 527 | 462 | 399 |
| 44.7% | 50.4% | 51.3% | 61.5% | 33.5% | 39.5% | 43.7% | 42.8% | 38.7% | 45.4% | 44.4% | 46.3% | |
| Total | 711 | 677 | 575 | 462 | 943 | 1,118 | 1,065 | 914 | 1,070 | 1,160 | 1,040 | 862 |
Table 2.
Number and percentage of selective serotonin reuptake inhibitor (SSRI) prescriptions by gender.
| Females | Males | |||||||
| SSRI | 2002 | 2003 | 2004 | 2005 | 2002 | 2003 | 2004 | 2005 |
| Celexa | 279 | 256 | 160 | 64 | 507 | 462 | 241 | 120 |
| 22.2% | 17.8% | 11.7% | 5.2% | 21.8% | 18.3% | 10.4% | 6.0% | |
| Lexapro | 17 | 172 | 184 | 200 | 25 | 308 | 379 | 342 |
| 1.4% | 12.0% | 13.4% | 16.2% | 1.1% | 12.2% | 16.4% | 17.2% | |
| Luvox | 30 | 11 | 16 | 15 | 116 | 81 | 74 | 75 |
| 2.4% | 0.8% | 1.2% | 1.2% | 5.0% | 3.2% | 3.2% | 3.8% | |
| Paxil | 305 | 139 | 71 | 59 | 481 | 230 | 108 | 68 |
| 24.3% | 9.7% | 5.2% | 4.8% | 20.7% | 9.1% | 4.7% | 3.4% | |
| Paxil CR | 16 | 57 | 43 | 24 | 36 | 96 | 46 | 17 |
| 1.3% | 4.0% | 3.1% | 1.9% | 1.6% | 3.8% | 2.0% | 0.9% | |
| Prozac | 137 | 118 | 236 | 259 | 198 | 197 | 406 | 399 |
| 10.9% | 8.2% | 17.2% | 21.0% | 8.5% | 7.8% | 17.5% | 20.0% | |
| Prozac | 21 | 28 | 17 | 12 | 40 | 50 | 25 | 16 |
| Weekly | 1.7% | 2.0% | 1.2% | 1.0% | 1.7% | 2.0% | 1.0% | 0.8% |
| Zoloft | 447 | 655 | 644 | 603 | 917 | 1106 | 1038 | 955 |
| 35.7% | 45.6% | 47.0% | 48.8% | 39.5% | 43.7% | 44.8% | 47.9% | |
| Total | 1,253 | 1,437 | 1,371 | 1,236 | 2,321 | 2,530 | 2,317 | 1,992 |
Figure 2.
Percent of serotonin reuptake inhibitor prescriptions by specific brand and by calendar year and by psychiatrist or non-psychiatrist prescriber.
Discussion & Conclusion
Since 2003, a series of concerns have been raised regarding SSRI use and suicidality in children and adolescents. These concerns have resulted in an FDA black box warning which, given the trends in our results, may have impacted providers' prescribing patterns of SSRIs in Hawai‘i. To our knowledge, there has not been any previously published data on the changes in prescribing patterns to SSRIs in children and adolescents in Hawai‘i. Total patients on SSRIs prescriptions decreased from 2003 to 2005. Changes in use of specific brands were observed in both males and females and across different age groups. There were, however, variations in the extent of changes in prescribing trends of different SSRIs following the FDA black box warning. SSRIs with more evidence based research supporting their safety and efficacy were least affected, as well as those that were less implicated by the FDA analysis on the link between SSRIs and suicidality.
Citalopram use declined over three fold between 2003 (21.2%) to 2005 (5.9%). A sharp decrease was also noted in paroxetine prescriptions, which decreased from 21.37% in 2002 to 5.04% in 2005. Fluoxetine, an SSRI with more evidence based clinical research supporting its efficacy and safety in children and adolescents, showed an overall increase in use from 10.17% to 19.44%. Sertraline (Zoloft), having been approved by the FDA for children and adolescents with obsessive-compulsive disorder, maintained a high percentage of overall prescriptions and showed an increase in use from 38.47% in 2002, to 47.9% in 2005. Escitalopram use also increased gradually to 16.71% in 2005. Longer acting preparations carry a higher risk of complications due to decreased ability to get the medication out of the patient's system, should a decision be made to discontinue the medication. Longer acting preparations were prescribed less. Fluoxetine weekly use decreased from 1.51% in 2002 to 0.71% in 2005. Changes in SSRIs prescribing were apparent in both genders and in different age groups.
Limitations
The insurance data utilized for this study is a fair representation of the population in the Hawaiian Islands since it relies on the largest insurer with coverage of about half the population in the state. While analysis of insurance data has been helpful in demonstrating variations in prescribing patterns with psychiatric medications in children and adolescents, one can only note the parallel association observed between such changes in prescribing patterns and the timing of the FDA black box warning. A direct causal relationship cannot be inferred from these analyses. The results, however, suggest a need for further studies of SSRIs, specifically for a prospective survey of providers and patients regarding effects of the FDA warning. Additionally, it would be interesting to evaluate changes in noninsured population and compare the differences.
Recommendations
Variations in prescribing patterns were noted over time, with the use of several SSRIs decreasing significantly. The data suggest that, following the FDA black box warning, the use of SSRIs with more evidence based research supporting their safety and efficacy as well as SSRIs less implicated by the FDA in association with suicidality changed the least. However, SSRIs continued to be frequently prescribed to manage depression in children and adolescents. Possibilities to explore in future research include prospective studies of these associations over a longer period of time and in different populations as well as changes in type of non-SSRIs antidepressants [bupropion (Wellbutrin), mirtazapine (Remeron), venlafaxine (Effexor), and duloxetine (Cymbalta)] prescribed and psychotherapy/non-prescription approaches. In addition, other issues that deserve further exploration are relationships between type of medication used and economic factors including listings in insurance company formularies. Outcomes of treatment in association with type of medication used are also needed. Further investigation of the consequences of the FDA warning should include studies to increase the evidence related to the efficacy of treatment approaches to this vulnerable population. It is very important to conduct more clinical research studies in child and adolescent psychiatry related to management of depression, both pharmacologic and psychotherapeutic, with the goal of developing evidence-based “best practices” that contribute to the well-being of this population.
| Name in the original submission Brand Name | Chemical name |
| Celexa | Citalopram |
| Lexapro | Escitalopram |
| Luvox | Fluvoxamine |
| Paxil | Paroxetine |
| Paxil CR | Paroxetine CR |
| Prozac | Fluoxetine |
| Prozac Weekly or Prozac W | Fluoxetine Weekly or Fluoxetine W |
| Zoloft | Sertraline |
Acknowledgement
The author would like to express appreciation for the following institutions:Tripler Army Medical Center, Uniformed Services University of the Health Sciences and John A Burns School of Medicine, University of Hawai‘i.
References
- 1.U.S. FDA, author. Public Health Advisory. Worsening Depression and Suicidality in Patients being treated with Antidepressant Medications. 2004. Mar 22, Available at www.fda.gov/cder/drug/antidepressants/Antidepressants.
- 2.U.S. FDA, author. Public Health Advisory. Suicidality in children and adolescents being treated with antidepressant Medications. 2004. Oct 15, Available at www.fda.gov/cder/drug/antidepressants/SSRIPHA200410.htm.
- 3.Labeling Change Request Letter for antidepressant Medications. Available at www.fda.gov/cder/drug/antidepressants/SSRIlabelChange.htm. [Google Scholar]
- 4.FDA proposed medication guide: about using antidepressants in children or teenagers. Available at www.fda.gov/cder/drug/antidepressants/SSRIMedicationGuide.htm.
- 5.Gibbons RD, Hur K, Bhaumik DK, Mann JJ. The relationship between antidepressant medication use and rate of suicide. Arch Gen Psychiatry. 2005;62:165. doi: 10.1001/archpsyc.62.2.165. [DOI] [PubMed] [Google Scholar]
- 6.Vitiello B, Swedo S. Antidepressant Medications in Children. N Engl J Med. 2004;350:1489. doi: 10.1056/NEJMp038248. [DOI] [PubMed] [Google Scholar]
- 7.Jick H, Kaye JA, Jick SS. Antidepressants and the risk of Suicidal Behaviors. JAMA. 2004;292:338. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- 8.American College of Neuropsychopharmacology, author. Preliminary Report of the Task Force on SSRIs and Suicidal Behavior in Youth. 2004. Jan 21, Available at www.Acnp.org/exec_summary.pdf.
- 9.American Academy of Child and Adolescent Psychiatry, author. Supplemental talking points for child and adolescent psychiatrists regarding the FDA black box warning on the use of antidepressants for pediatric patients. 2004. Nov, Available at www.aacap.org/announcements/psychiatricmeds.htm.
- 10.Practice Parameters for the assessment and treatment of Children and Adolescents with Depressive Disorders. AACAP. J Am Acad Child Adolescent Psychiatry. 1998;37:63S. doi: 10.1097/00004583-199810001-00005. [DOI] [PubMed] [Google Scholar]
- 11.Kutcher S, Gardner D, Virani A. A clinical approach to treating Major Depressive Disorder with SSRIs in Adolescents: 12 steps to careful monitoring. Child and Adolescent Psychopharmacology News. 2004;9(5):1. [Google Scholar]
- 12.March J, Silva S, Petrycki S, et al. Fluoxetine (Prozac), cognitive-behavioral therapy, and their adolescents with depression: Treatment for Adolescents with Depression Study (TADA) randomized controlled trial. JAMA. 2004;292:807. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 13.Fassler D. Children and SSRI Antidepressants: Information for Parents. Available at www.aacap.org/Announcements/ssri_Fassler.htm.