Abstract
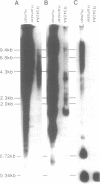
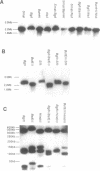
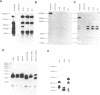
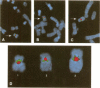
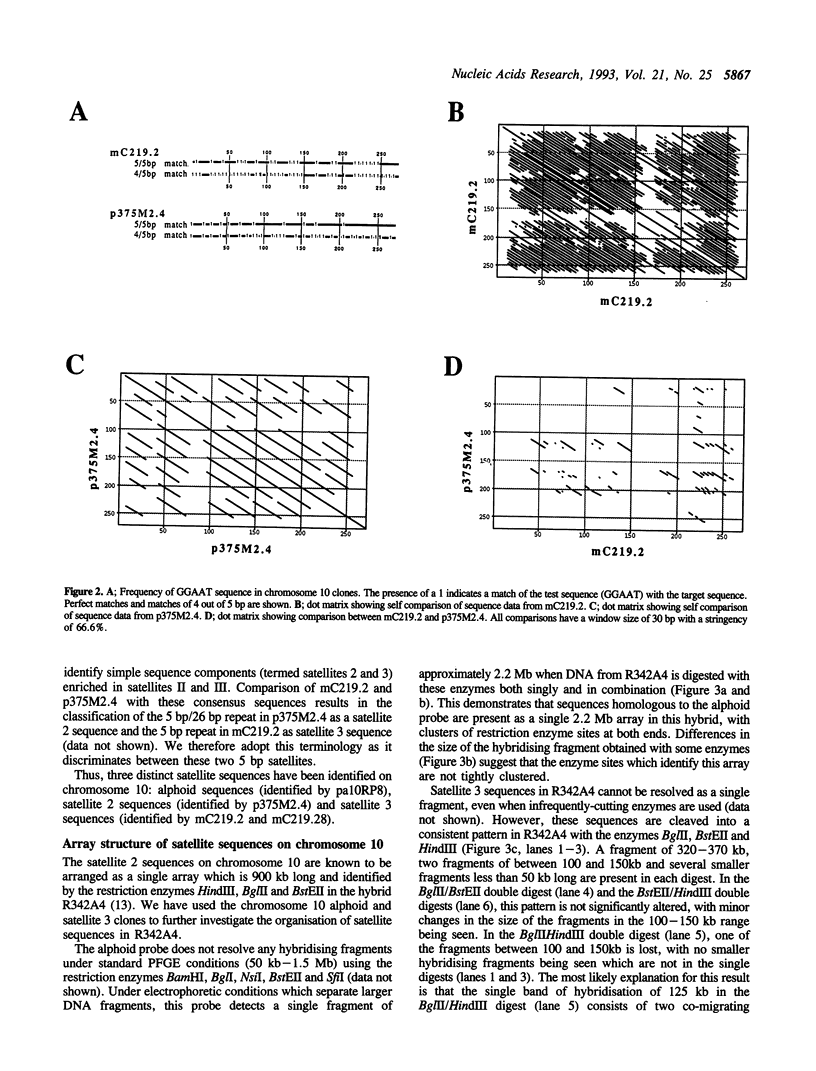
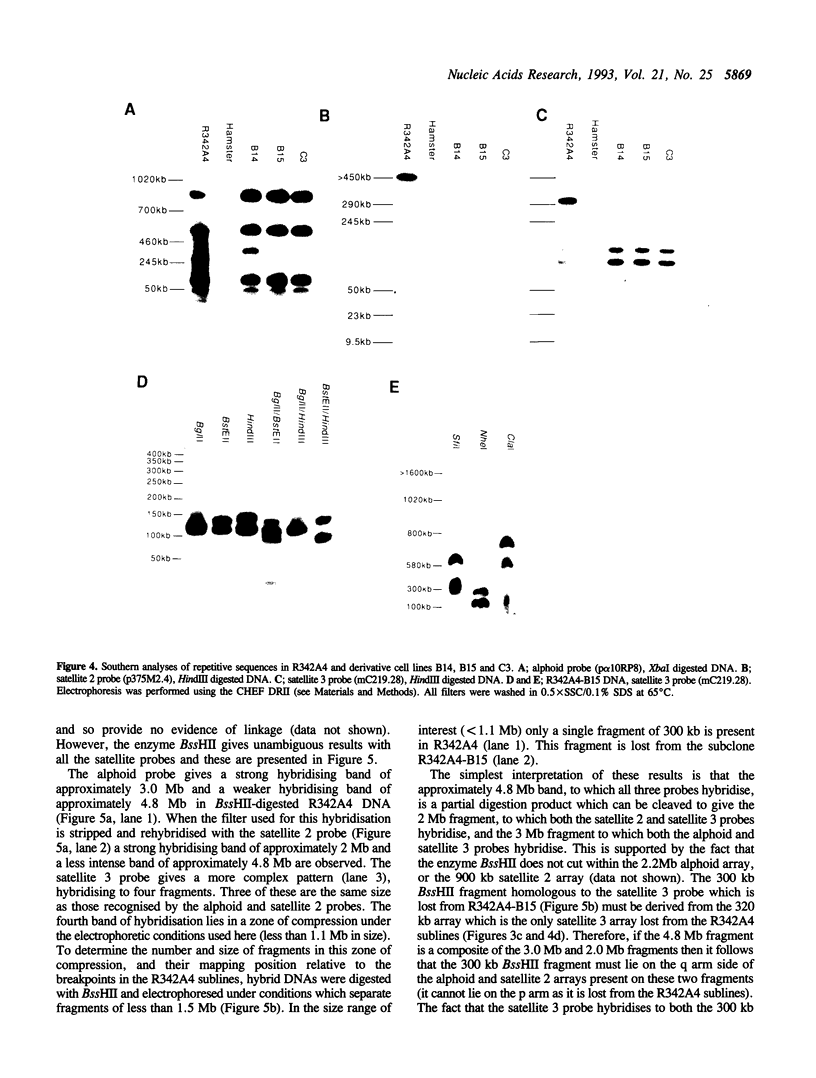
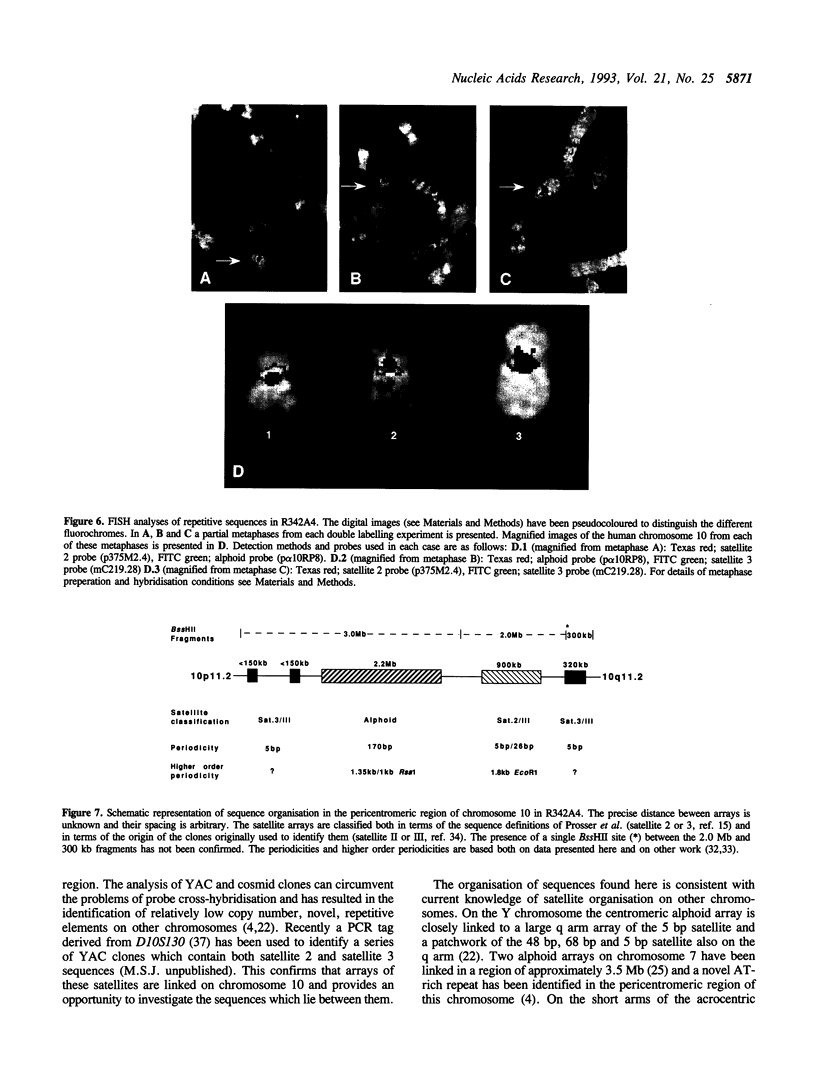
Three satellite DNA families are present in the pericentromeric region of chromosome 10; the alpha satellite and two 5 bp satellite families defined here as satellites 2 and 3. Pulsed field gel electrophoresis (PFGE) demonstrates that these sequences are organised into five discrete arrays which are linked within a region of approximately 5.3 Megabases (Mb) of DNA. The alpha satellite is largely confined to a 2.2 Mb array which is flanked on its p arm side by two 100-150 kb satellite 3 arrays and on its q arm side by a 900 kb satellite 2 array and a further 320 kb satellite 3 array. This linear order is corroborated by fluorescent in situ hybridisation analyses. In total, these arrays account for 3.6 Mb of DNA in the pericentromeric region of chromosome 10. These data provide both physical information on sequences which may be involved in centromere function and a map across the centromere which has the potential to link yeast artificial chromosome (YAC) contigs currently being developed on both arms of this chromosome.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agresti A., Rainaldi G., Lobbiani A., Magnani I., Di Lernia R., Meneveri R., Siccardi A. G., Ginelli E. Chromosomal location by in situ hybridization of the human Sau3A family of DNA repeats. Hum Genet. 1987 Apr;75(4):326–332. doi: 10.1007/BF00284102. [DOI] [PubMed] [Google Scholar]
- Alexandrov I. A., Medvedev L. I., Mashkova T. D., Kisselev L. L., Romanova L. Y., Yurov Y. B. Definition of a new alpha satellite suprachromosomal family characterized by monomeric organization. Nucleic Acids Res. 1993 May 11;21(9):2209–2215. doi: 10.1093/nar/21.9.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Beauchamp R. S., Mitchell A. R., Buckland R. A., Bostock C. J. Specific arrangements of human satellite III DNA sequences in human chromosomes. Chromosoma. 1979 Feb 21;71(2):153–166. doi: 10.1007/BF00292820. [DOI] [PubMed] [Google Scholar]
- Choo K. H., Earle E., Vissel B., Kalitsis P. A chromosome 14-specific human satellite III DNA subfamily that shows variable presence on different chromosomes 14. Am J Hum Genet. 1992 Apr;50(4):706–716. [PMC free article] [PubMed] [Google Scholar]
- Choo K. H., Vissel B., Nagy A., Earle E., Kalitsis P. A survey of the genomic distribution of alpha satellite DNA on all the human chromosomes, and derivation of a new consensus sequence. Nucleic Acids Res. 1991 Mar 25;19(6):1179–1182. doi: 10.1093/nar/19.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L. Centromeres of budding and fission yeasts. Trends Genet. 1990 May;6(5):150–154. doi: 10.1016/0168-9525(90)90149-z. [DOI] [PubMed] [Google Scholar]
- Cooke H. J., Hindley J. Cloning of human satellite III DNA: different components are on different chromosomes. Nucleic Acids Res. 1979 Jul 25;6(10):3177–3197. doi: 10.1093/nar/6.10.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K. F., Fisher R. B., Tyler-Smith C. Structure of the pericentric long arm region of the human Y chromosome. J Mol Biol. 1992 Nov 20;228(2):421–432. doi: 10.1016/0022-2836(92)90831-4. [DOI] [PubMed] [Google Scholar]
- Devilee P., Kievits T., Waye J. S., Pearson P. L., Willard H. F. Chromosome-specific alpha satellite DNA: isolation and mapping of a polymorphic alphoid repeat from human chromosome 10. Genomics. 1988 Jul;3(1):1–7. doi: 10.1016/0888-7543(88)90151-6. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Dou S., Chi D., Carlson K. M., Toshima K., Lairmore T. C., Howe J. R., Moley J. F., Goodfellow P., Wells S. A., Jr Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet. 1993 Jul;2(7):851–856. doi: 10.1093/hmg/2.7.851. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Ratrie H., 3rd, Stetten G. Visualization of centromere proteins CENP-B and CENP-C on a stable dicentric chromosome in cytological spreads. Chromosoma. 1989 Jun;98(1):1–12. doi: 10.1007/BF00293329. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gosden J. R., Mitchell A. R., Buckland R. A., Clayton R. P., Evans H. J. The location of four human satellite DNAs on human chromosomes. Exp Cell Res. 1975 Apr;92(1):148–158. doi: 10.1016/0014-4827(75)90648-5. [DOI] [PubMed] [Google Scholar]
- Grady D. L., Ratliff R. L., Robinson D. L., McCanlies E. C., Meyne J., Moyzis R. K. Highly conserved repetitive DNA sequences are present at human centromeres. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1695–1699. doi: 10.1073/pnas.89.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaf T., Warburton P. E., Willard H. F. Integration of human alpha-satellite DNA into simian chromosomes: centromere protein binding and disruption of normal chromosome segregation. Cell. 1992 Aug 21;70(4):681–696. doi: 10.1016/0092-8674(92)90436-g. [DOI] [PubMed] [Google Scholar]
- Higgins M. J., Wang H. S., Shtromas I., Haliotis T., Roder J. C., Holden J. J., White B. N. Organization of a repetitive human 1.8 kb KpnI sequence localized in the heterochromatin of chromosome 15. Chromosoma. 1985;93(1):77–86. doi: 10.1007/BF01259449. [DOI] [PubMed] [Google Scholar]
- Jackson M. S., Mole S. E., Ponder B. A. Characterisation of a boundary between satellite III and alphoid sequences on human chromosome 10. Nucleic Acids Res. 1992 Sep 25;20(18):4781–4787. doi: 10.1093/nar/20.18.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanpierre M., Turleau C., Aurias A., Prieur M., Ledeist F., Fischer A., Viegas-Pequignot E. An embryonic-like methylation pattern of classical satellite DNA is observed in ICF syndrome. Hum Mol Genet. 1993 Jun;2(6):731–735. doi: 10.1093/hmg/2.6.731. [DOI] [PubMed] [Google Scholar]
- Johnson D. H., Kroisel P. M., Klapper H. J., Rosenkranz W. Microdissection of a human marker chromosome reveals its origin and a new family of centromeric repetitive DNA. Hum Mol Genet. 1992 Dec;1(9):741–747. doi: 10.1093/hmg/1.9.741. [DOI] [PubMed] [Google Scholar]
- Kalitsis P., Earle E., Vissel B., Shaffer L. G., Choo K. H. A chromosome 13-specific human satellite I DNA subfamily with minor presence on chromosome 21: further studies on Robertsonian translocations. Genomics. 1993 Apr;16(1):104–112. doi: 10.1006/geno.1993.1147. [DOI] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Lairmore T. C., Dou S., Howe J. R., Chi D., Carlson K., Veile R., Mishra S. K., Wells S. A., Jr, Donis-Keller H. A 1.5-megabase yeast artificial chromosome contig from human chromosome 10q11.2 connecting three genetic loci (RET, D10S94, and D10S102) closely linked to the MEN2A locus. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):492–496. doi: 10.1073/pnas.90.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. C., Sasi R., Lee C., Fan Y. S., Court D. Isolation and identification of a novel tandemly repeated DNA sequence in the centromeric region of human chromosome 8. Chromosoma. 1993 May;102(5):333–339. doi: 10.1007/BF00661276. [DOI] [PubMed] [Google Scholar]
- Looijenga L. H., Oosterhuis J. W., Smit V. T., Wessels J. W., Mollevanger P., Devilee P. Alpha satellite DNAs on chromosomes 10 and 12 are both members of the dimeric suprachromosomal subfamily, but display little identity at the nucleotide sequence level. Genomics. 1992 Aug;13(4):1125–1132. doi: 10.1016/0888-7543(92)90027-p. [DOI] [PubMed] [Google Scholar]
- Masumoto H., Masukata H., Muro Y., Nozaki N., Okazaki T. A human centromere antigen (CENP-B) interacts with a short specific sequence in alphoid DNA, a human centromeric satellite. J Cell Biol. 1989 Nov;109(5):1963–1973. doi: 10.1083/jcb.109.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzdorf R., Göttert E., Blin N. A novel centromeric repetitive DNA from human chromosome 22. Chromosoma. 1988;97(2):154–158. doi: 10.1007/BF00327372. [DOI] [PubMed] [Google Scholar]
- Mitchell A. R., Gosden J. R., Miller D. A. A cloned sequence, p82H, of the alphoid repeated DNA family found at the centromeres of all human chromosomes. Chromosoma. 1985;92(5):369–377. doi: 10.1007/BF00327469. [DOI] [PubMed] [Google Scholar]
- Mitchell A., Jeppesen P., Hanratty D., Gosden J. The organisation of repetitive DNA sequences on human chromosomes with respect to the kinetochore analysed using a combination of oligonucleotide primers and CREST anticentromere serum. Chromosoma. 1992 Mar;101(5-6):333–341. doi: 10.1007/BF00346012. [DOI] [PubMed] [Google Scholar]
- Mole S. E., Jackson M. S., Tokino T., Nakamura Y., Ponder B. A. Assignment of fifty-four cosmid clones to five regions of chromosome 10. Genomics. 1993 Feb;15(2):457–458. doi: 10.1006/geno.1993.1090. [DOI] [PubMed] [Google Scholar]
- Mole S. E., Mulligan L. M., Healey C. S., Ponder B. A., Tunnacliffe A. Localisation of the gene for multiple endocrine neoplasia type 2A to a 480 kb region in chromosome band 10q11.2. Hum Mol Genet. 1993 Mar;2(3):247–252. doi: 10.1093/hmg/2.3.247. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Albright K. L., Bartholdi M. F., Cram L. S., Deaven L. L., Hildebrand C. E., Joste N. E., Longmire J. L., Meyne J., Schwarzacher-Robinson T. Human chromosome-specific repetitive DNA sequences: novel markers for genetic analysis. Chromosoma. 1987;95(6):375–386. doi: 10.1007/BF00333988. [DOI] [PubMed] [Google Scholar]
- Mulligan L. M., Kwok J. B., Healey C. S., Elsdon M. J., Eng C., Gardner E., Love D. R., Mole S. E., Moore J. K., Papi L. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993 Jun 3;363(6428):458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Mathew C. G., Sobol H., Easton D. F., Telenius H., Bragg T., Chin K., Clark J., Jones C., Lenoir G. M. Linked markers flanking the gene for multiple endocrine neoplasia type 2A. Genomics. 1989 Aug;5(2):199–203. doi: 10.1016/0888-7543(89)90046-3. [DOI] [PubMed] [Google Scholar]
- Neil D. L., Villasante A., Fisher R. B., Vetrie D., Cox B., Tyler-Smith C. Structural instability of human tandemly repeated DNA sequences cloned in yeast artificial chromosome vectors. Nucleic Acids Res. 1990 Mar 25;18(6):1421–1428. doi: 10.1093/nar/18.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakey R., Tyler-Smith C. Y chromosome DNA haplotyping suggests that most European and Asian men are descended from one of two males. Genomics. 1990 Jul;7(3):325–330. doi: 10.1016/0888-7543(90)90165-q. [DOI] [PubMed] [Google Scholar]
- Pfütz M., Gileadi O., Werner D. Identification of human satellite DNA sequences associated with chemically resistant nonhistone polypeptide adducts. Chromosoma. 1992 Oct;101(10):609–617. doi: 10.1007/BF00360538. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta A. F., Cooke C. A., Earnshaw W. C. Structure of the human centromere at metaphase. Trends Biochem Sci. 1990 May;15(5):181–185. doi: 10.1016/0968-0004(90)90158-8. [DOI] [PubMed] [Google Scholar]
- Prosser J., Frommer M., Paul C., Vincent P. C. Sequence relationships of three human satellite DNAs. J Mol Biol. 1986 Jan 20;187(2):145–155. doi: 10.1016/0022-2836(86)90224-x. [DOI] [PubMed] [Google Scholar]
- Rocchi M., Archidiacono N., Ward D. C., Baldini A. A human chromosome 9-specific alphoid DNA repeat spatially resolvable from satellite 3 DNA by fluorescent in situ hybridization. Genomics. 1991 Mar;9(3):517–523. doi: 10.1016/0888-7543(91)90419-f. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Trask B. J., van den Engh G., Christensen M., Massa H. F., Gray J. W., Van Dilla M. Characterization of somatic cell hybrids by bivariate flow karyotyping and fluorescence in situ hybridization. Somat Cell Mol Genet. 1991 Mar;17(2):117–136. doi: 10.1007/BF01232970. [DOI] [PubMed] [Google Scholar]
- Tunnacliffe A., Liu L., Moore J. K., Leversha M. A., Jackson M. S., Papi L., Ferguson-Smith M. A., Thiesen H. J., Ponder B. A. Duplicated KOX zinc finger gene clusters flank the centromere of human chromosome 10: evidence for a pericentric inversion during primate evolution. Nucleic Acids Res. 1993 Mar 25;21(6):1409–1417. doi: 10.1093/nar/21.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler-Smith C., Brown W. R. Structure of the major block of alphoid satellite DNA on the human Y chromosome. J Mol Biol. 1987 Jun 5;195(3):457–470. doi: 10.1016/0022-2836(87)90175-6. [DOI] [PubMed] [Google Scholar]
- Tyler-Smith C. Structure of repeated sequences in the centromeric region of the human Y chromosome. Development. 1987;101 (Suppl):93–100. [PubMed] [Google Scholar]
- Vissel B., Choo K. H. Evolutionary relationships of multiple alpha satellite subfamilies in the centromeres of human chromosomes 13, 14, and 21. J Mol Evol. 1992 Aug;35(2):137–146. doi: 10.1007/BF00183225. [DOI] [PubMed] [Google Scholar]
- Vissel B., Nagy A., Choo K. H. A satellite III sequence shared by human chromosomes 13, 14, and 21 that is contiguous with alpha satellite DNA. Cytogenet Cell Genet. 1992;61(2):81–86. doi: 10.1159/000133374. [DOI] [PubMed] [Google Scholar]
- Vogt P. Potential genetic functions of tandem repeated DNA sequence blocks in the human genome are based on a highly conserved "chromatin folding code". Hum Genet. 1990 Mar;84(4):301–336. doi: 10.1007/BF00196228. [DOI] [PubMed] [Google Scholar]
- Voullaire L. E., Slater H. R., Petrovic V., Choo K. H. A functional marker centromere with no detectable alpha-satellite, satellite III, or CENP-B protein: activation of a latent centromere? Am J Hum Genet. 1993 Jun;52(6):1153–1163. [PMC free article] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Chromosome specificity of satellite DNAs: short- and long-range organization of a diverged dimeric subset of human alpha satellite from chromosome 3. Chromosoma. 1989 May;97(6):475–480. doi: 10.1007/BF00295032. [DOI] [PubMed] [Google Scholar]
- Wevrick R., Willard H. F. Physical map of the centromeric region of human chromosome 7: relationship between two distinct alpha satellite arrays. Nucleic Acids Res. 1991 May 11;19(9):2295–2301. doi: 10.1093/nar/19.9.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevrick R., Willard V. P., Willard H. F. Structure of DNA near long tandem arrays of alpha satellite DNA at the centromere of human chromosome 7. Genomics. 1992 Dec;14(4):912–923. doi: 10.1016/s0888-7543(05)80112-0. [DOI] [PubMed] [Google Scholar]