Abstract
Pancreas cancer is one of the most lethal malignancies and is characterized by activating mutations of Kras, present in 95% of patients. More than 60% of pancreatic cancers also display increased c-Src activity, which is associated with poor prognosis. Although loss of tumor suppressor function (for example, p16, p53, Smad4) combined with oncogenic Kras signaling has been shown to accelerate pancreatic duct carcinogenesis, it is unclear whether elevated Src activity contributes to Kras-dependent tumorigenesis or is simply a biomarker of disease progression. Here, we demonstrate that in the context of oncogenic Kras, activation of c-Src through deletion of C-terminal Src kinase (CSK) results in the development of invasive pancreatic ductal adenocarcinoma (PDA) by 5–8 weeks. In contrast, deletion of CSK alone fails to induce neoplasia, while oncogenic Kras expression yields PDA at low frequency after a latency of 12 months. Analysis of cell lines derived from Ras/Src-induced PDA’s indicates that oncogenic Ras/Src cooperativity may lead to genomic instability, yet Ras/Src-driven tumor cells remain dependent on Src signaling and as such, Src inhibition suppresses growth of Ras/Src-driven tumors. These findings demonstrate that oncogenic Ras/Src cooperate to accelerate PDA onset and support further studies of Src-directed therapies in pancreatic cancer.
Keywords: pancreatic cancer, Src, Ras, oncogenic cooperativity
Introduction
Almost a century ago, Peyton Rous reported the discovery of a ‘transmissible tumor-causing particle’ that produced sarcomas when injected into chickens (Rous, 1911). This early description of the Rous sarcoma virus set the stage for the identification of the first oncogene, v-Src, some 60 years later. Studies by Varmus, Bishop, Vogt and colleagues in the 1970’s established that expression of the Src retroviral oncoprotein was sufficient to transform avian cells and also promote sarcoma formation in chickens (Martin, 2001). Subsequently, the cellular counterpart, c-Src was characterized as the founding member of the Src family of protein tyrosine kinases (SFKs) (Stehelin et al., 1976; Hunter and Sefton, 1980). Since that time, Src kinase has been intrinsically linked with cellular transformation and changes in its activity impact proliferation, invasion and migration (Bromann et al., 2004). More recently, a number of studies utilizing in vivo orthotopic transplantation models have not only underscored the importance of Src activity during tumorigenesis, but also provided compelling support for Src as a therapeutic target (Yezhelyev et al., 2004; Rucci et al., 2006; Trevino et al., 2006; Koreckij et al., 2009; Vitali et al., 2009). In addition to its direct effects on the properties of a tumor cell, Src can also indirectly modulate neoplasia through its role in tumor-associated processes such as angiogenesis and vascular permeability, yet the precise role of Src during the development of malignancy remains unclear (Eliceiri et al., 1999).
Overexpression or activation of Src kinase has been described in many human cancers including those of the colon, breast, lung, liver, head and neck, and brain and pancreas (Irby and Yeatman, 2000; Ishizawar and Parsons, 2004). Furthermore, Src kinase activity has been shown to increase as a function of tumor progression, implicating Src in the transition to malignancy (Talamonti et al., 1993). As Src activity is tightly controlled posttranslationally, deregulation at this level can lead to an increase in oncogenic potential. Src activation can occur following a loss of regulation at its inhibitory C-terminal tyrosine residue (Tyr-529 in mouse, Tyr-530 in humans) due to reduced C-terminal Src kinase (CSK) abundance, elevated phosphatase activity or altered CSK-binding protein function (Irby and Yeatman, 2000). Loss of Tyr-529 phosphorylation leads to autophosphorylation at Tyr-418 (mouse)/Tyr-419 (human) and activation of Src. Alternatively, Src can be activated as a consequence of elevated signaling from the upstream receptor tyrosine kinases, epidermal growth factor receptor, RON, IGF-1R and c-Met, as well as the chemokine receptor, CXCR4 and integrins such as αvβ3, each of which have been implicated in carcinogenesis (Barton et al., 1991; Billadeau et al., 2006; Thomas et al., 2007; Desgrosellier et al., 2009; Ricono et al., 2009).
Pancreatic ductal adenocarcinoma (PDA) is one of the most lethal malignancies, causing more than 200 000 deaths worldwide annually. Despite advances in our understanding of the genetic basis of PDA, the 5-year survival rate for patients diagnosed with the disease in 2009 remains less than 5% (Warshaw and Fernandezdel Castillo, 1992; Jemal et al., 2009). Elevated Src levels have been reported in more than 70% of patients with ductal adenocarcinoma of the pancreas and more than 60% of pancreatic tumors show increased Src activity (Lutz et al., 1998; Morton et al., 2010). Furthermore, increases in Src protein and activity are associated with vascular invasion, lymph node positivity and diminished survival (Morton et al., 2010). To test the hypothesis that heightened Src activity may contribute to PDA progression, we used floxed CSK mice as a tool to enable deletion of the inhibitory kinase, CSK, resulting in concomitant activation of the Src family kinases in the embryonic pancreas. We were particularly interested in the consequences of Src activation in the context of oncogenic Kras signaling as ~95% of PDA patients harbor Kras mutations at diagnosis (Almoguera et al., 1988). Previous studies have documented that loss of tumor suppressors such as p16, p53, Smad4 or TGF-beta receptor 2, dramatically accelerates the development of Kras-dependent PDA in mice (Aguirre et al., 2003; Hingorani et al., 2005; Bardeesy et al., 2006; Ijichi et al., 2006; Izeradjene et al., 2007). Here, we show that CSK deletion and activation of c-Src dramatically accelerates both the onset of precursor lesions initiated by oncogenic Kras signaling as well as the progression to invasive adenocarcinoma. These findings support a role for Src kinase activity in KrasG12D-dependent pancreatic neoplasia, and provide a model system to study the role of Src in pancreatic tumorigenesis.
Results
Activation of Src kinase is an early event in pancreas cancer
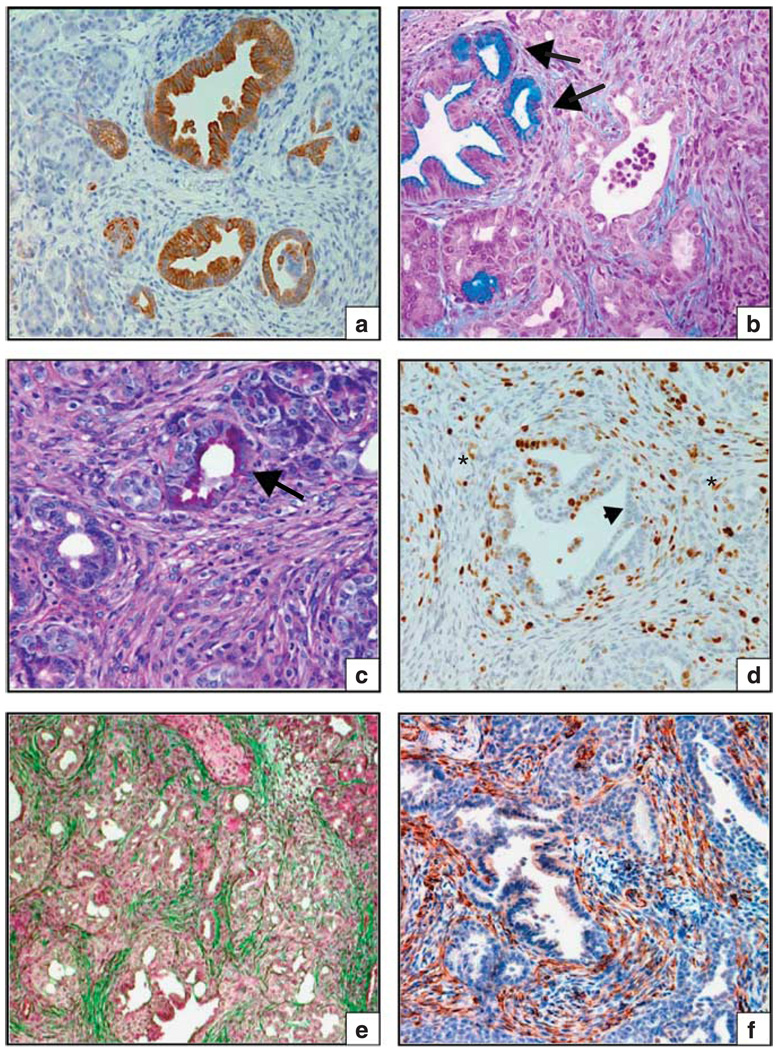
To ascertain the activation status of the SFKs during the development of PDA, we performed immunohistochemistry analysis on primary tumor specimens from PDA patients using an antibody that recognizes the phosphorylated (Y419 in human, Y418 in mouse), active form of each member of the Src-family kinases. Strong phospho-SFK (Y419) staining, denoting an active SFK enzyme, was evident in the neoplastic ducts of more than 60% (14/21) of the patient tumor specimens (Figure 1), but undetectable or present at low levels in the adjacent non-neoplastic ductal epithelium (Figures 1a and b). We noted strong pSFK(Y419) staining not only in the primary tumor (Figures 1e and f), but also in adjacent pancreatic intraepithelial neoplasia (Figures 1a and d), which suggests that SFK activation may be an early event in pancreatic tumorigenesis. These data corroborate a recent study describing elevated Src activity in human pancreas cancer (Morton et al., 2010). Given that Kras mutations are the earliest identifiable genetic alterations in PDA, we became particularly interested in the question of whether elevated SFK signaling might cooperate with oncogenic Kras to promote pancreatic neoplasia.
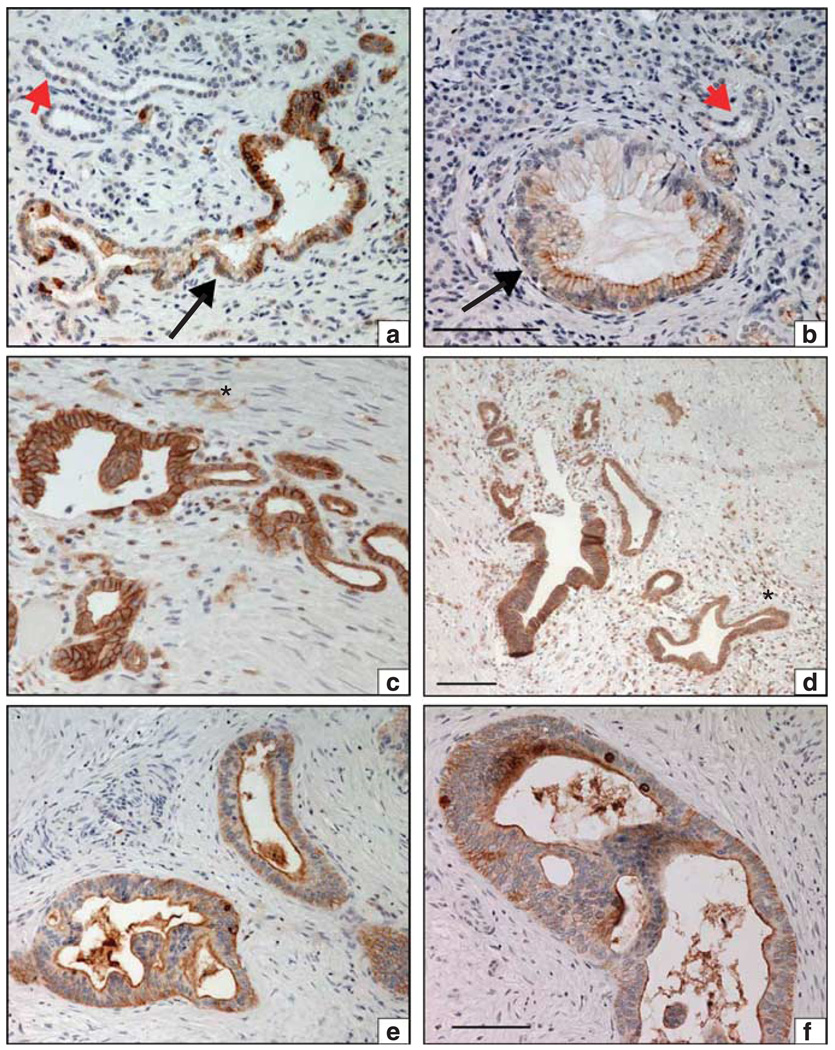
Figure 1.
SFK activation is evident at the earliest stages of pancreatic neoplasia. Immunohistochemical staining of activated SFK (pY419) in primary human pancreatic tumor specimens. (a) Large atypical ductal structure with mucinous epithelium (large arrow) surrounded by fibrosis. Ductal cells display heterogeneous pSFK(419) staining. An adjacent small atrophic non-neoplastic duct is indicated (red arrowhead). (b) Early pancreatic intraepithelial neoplasia (PanIN-1, (large arrow) with mucinous change and an adjacent non-neoplastic duct (red arrowhead) are shown. Strong pSFK staining is evident on the apical surface of some of the mucinfilled ductal epithelium. (c) Atypical ductal structure with strong junctional staining of pSFK, surrounded by cellular stromal tissue (asterisk). Note, distorted glandular shapes, intra-luminal papilla and variable height of epithelium. (d) Pancreatic intraepithelial neoplasia-2/3 with strong junctional pSFK staining surrounded by cellular stroma (asterisk), which contains numerous pSFK-positive cells (possibly inflammatory cells). (e, f) Pancreatic ductal adenocarcinoma exhibiting marked pSFK staining on the luminal surface and to a lesser extent in the basal cytoplasm. Scale bar represents 100 µm, all images ×200.
Dual Ras/SFK activation cooperate to promote PDA
To determine the role of activated SFK signaling in the onset and progression of KrasG12D initiated neoplasia, we targeted oncogenic KrasG12D expression and homozygous deletion of the SFK inhibitor, CSK to the embryonic pancreas using Pdx1-Cre-driven recombination (Figure 2a). Allele-specific genotyping confirmed the genetic composition of the compound LSL-KrasG12D;CSKf/f;Pdx1-Cre mice (Supplementary Figure S1). Activation of the Src family kinases concurrent with pancreatic KrasG12D expression led to visible changes in the pancreas by 3 weeks and precursor lesions were detectable by histological examination, even at this early age, with a penetrance of ~40% (n = 63 compound mutant mice) (Figures 2b, 3b and d). Disease progression was rapid with mice developing PDA by 5–8 weeks of age (Figures 3e and f). In the 40% of compound mice that developed tumors, median survival was 6.0 weeks (n = 26) demonstrating that activation of SFKs not only accelerates KrasG12D-driven tumor initiation but also promotes rapid progression to lethal invasive adenocarcinoma (Figures 3e and f, Supplementary Figure S2). The remaining 60% of tumors did not develop tumors or present with precursor lesions by 18 months of age. The reasons for incomplete penetrance of the phenotype have not been determined at this time. By comparison, mice that express KrasG12D only, in the context of wild-type levels of Src activity, do not develop pancreatic intraepithelial neoplasia until 2–5 months of age and only 10% ultimately develop PDA by 12 months (Hingorani et al., 2003). Deletion of pancreatic CSK alone did not impair pancreatic development indicating that CSK is not required for normal development of this organ. Most significantly, none of the CSKf/f;Pdx1-Cre animals harbored neoplastic lesions at the time of necropsy (up to 60 weeks of age, n = 25 mice; Supplementary Figure S3). These findings demonstrate that CSK deletion alone is insufficient to initiate neoplasia and that oncogenic Kras and SFK activation can cooperate to accelerate the development of PDA in mice.
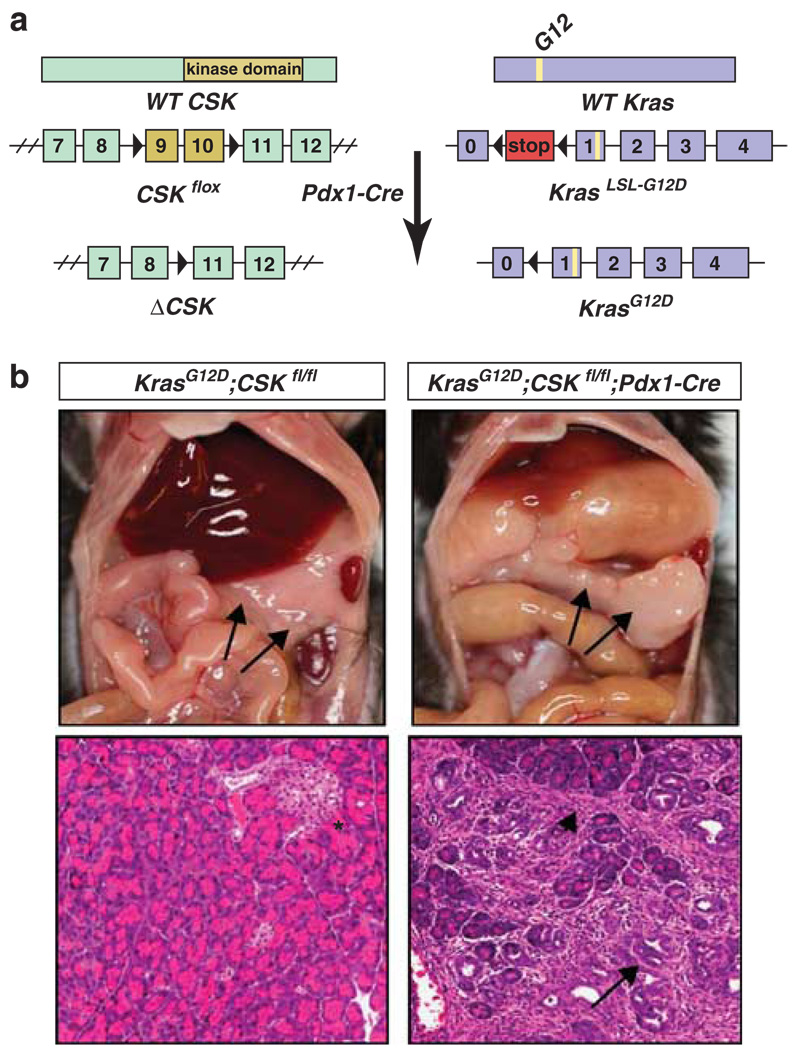
Figure 2.
Targeted CSK deletion and endogenous KrasG12D expression in the mouse pancreas. (a) Both CSK alleles are conditionally deleted and the endogenous KrasG12D allele is activated in the pancreas of mice expressing Cre recombinase under the control of the Pdx1 promoter in KrasG12D; CSKf/f; Pdx1-Cre mice. (b) Upper: gross pathological images of a non-neoplastic pancreas from a control littermate (LSL-KrasG12D;CSKf/f) (left); and enlarged tumor-bearing pancreas in a 7-week-old compound mouse (right). Lower: corresponding hematoxylin and eosin stained sections showing normal pancreatic acini and an islet (as denoted by asterisk) (left). Dual Src/Ras activation leads to a significant reduction in acinar cell content and increased numbers of disorderly proliferating ducts (large arrow) surrounded by rich cellular stroma (arrowhead) (middle).
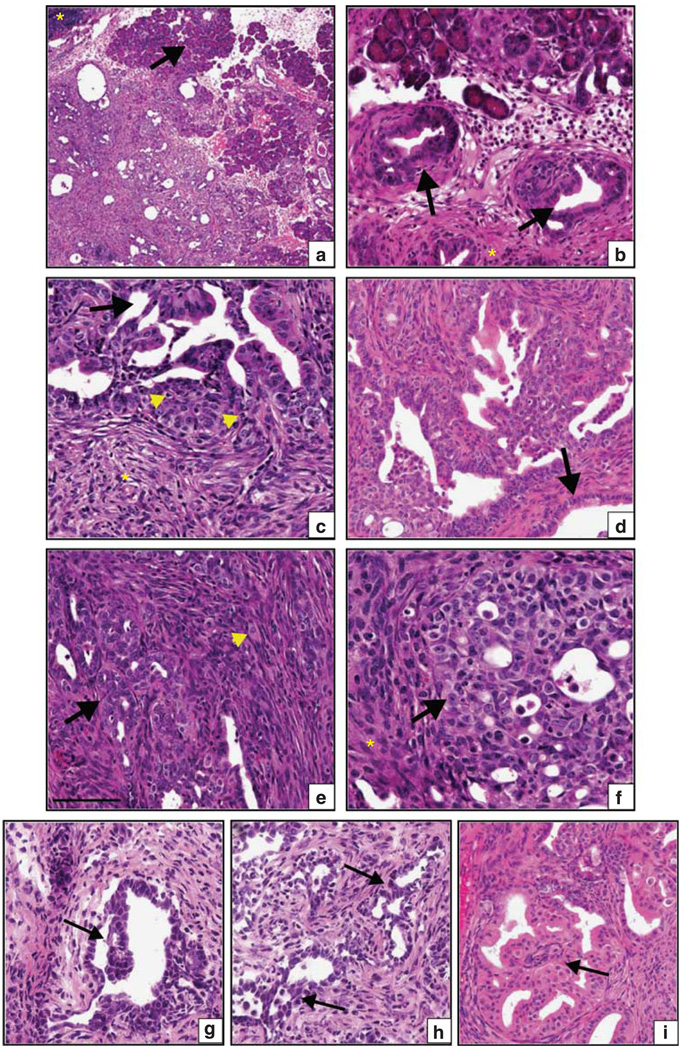
Figure 3.
Dual Ras/Src activation causes rapid development of invasive pancreatic ductal adenocarcinoma. (a–f) Histological features in LSL-KrasG12D;CSKf/f;Pdx1-Cre mice: (a) low power image of adenocarcinoma infiltrating and replacing islands of acinar cells (arrow). Residual uninvolved intra-pancreatic lymph node is indicated with asterisk. (b) Pancreatic intraepithelial neoplasia (arrow) are detectable in the pancreata at the suckling-weaning transition (3 weeks). Fibrosis is evident in the surrounding stroma (asterisk). (c) Adenocarcinoma (arrow) including papillary structures and epithelial bridging between regions of in situ carcinoma. Mitotic figures are indicated (arrowhead). (d) Moderately differentiated infiltrating ductal carcinoma adjacent to an entrapped non-neoplastic duct (arrow). (e) Invading distorted glandular structures (arrow) surrounded by cell-rich stroma. Individual invading tumor cells can be seen in the stroma (arrowhead). (f) Clump of invading pleiomorphic, poorly differentiated adenocarcinoma cells (arrow) surrounded by fibrotic stroma (asterisk). The spontaneous tumors contain histological features commonly found in the human disease including (as denoted by arrows) papillary structures (g) epithelial bridging (h) and cribriform structures (i).
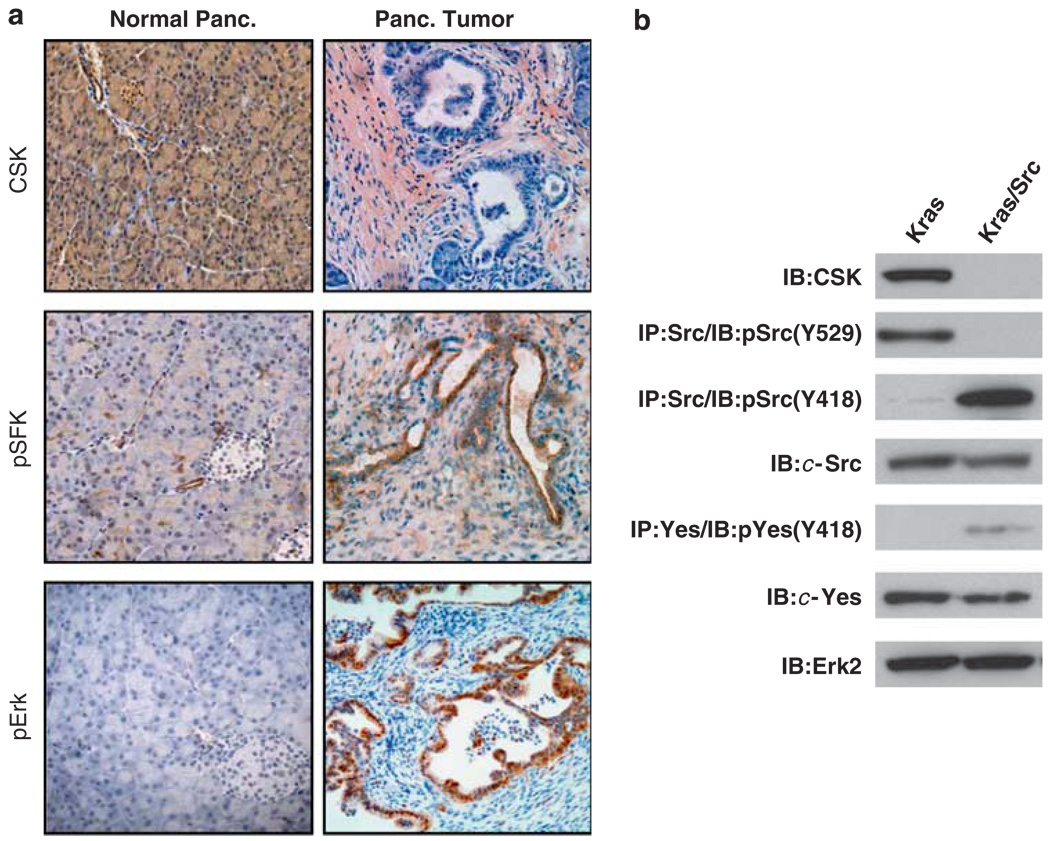
Immunohistochemical analysis of tumors from LSL-KrasG12D;CSKf/f;Pdx1-Cre mice confirmed the absence of CSK and the activation of the Src family kinases, as measured by phospho-SFK (Y418) staining in the neoplastic ducts (Figure 4a). Activation of the MAP kinase pathway was demonstrated by strong phospho-Erk staining in the ductal epithelium (Figure 4a). By contrast, Src and Erk are not activated in the non-neoplastic pancreas (Figure 4a). Src, Yes, Fyn and Lyn have each been implicated in neoplastic transformation of sites such as the pancreas, prostate and breast. To define the SFKs that are activated in this model, we developed cell lines from the murine PDA’s (Figure 4b). Src was the predominant active SFK in the lysates of tumor-derived cell lines, with Yes displaying minimal levels of activity (Figure 4b). Lyn and Fyn activity were undetectable (data not shown).
Figure 4.
Conditional CSK deletion promotes activation of specific Src-family kinases. (a) Pdx1-Cre mediated recombination of the floxed CSK alleles was evidenced by the lack of CSK immunohistochemical staining in the neoplastic ducts. Strong pSFK (Y418) and pErk staining demonstrated activation of the Src- and Ras-driven signaling cascades in the ductal epithelium. In contrast, activation of Src and Erk was not observed in normal pancreata. (b) Characterization of cell lines derived from pancreatic intraepithelial neoplasia and spontaneous tumors in LSL-KrasG12D/Pdx1-Cre (Kras) and LSL-KrasG12D/CSKf/f (Kras/Src) mice, respectively. Immunoblot analysis confirms CSK deletion, concurrent loss of CSK-mediated inhibitory phosphorylation (Y529) on the C-terminal tail of c-Src and activation of Src (pY418).
Ras/Src derived PDA displays features of the human disease
To further characterize the oncogenic cooperativity between KrasG12D and activated c-Src, we investigated the kinetics of tumor formation and the stromal/epithelial organization in the pancreata of compound mutant mice. Ductal hyper-proliferation was evident throughout the pancreas as early as the suckling-weaning transition (3 weeks) (Figure 3c). By 5 weeks, the pancreata were characterized by multiple foci of carcinoma, most of which were poorly differentiated, but which also included some moderately to well differentiated neoplastic ducts (Figures 3c and f).
Neoplastic cells in the Ras/Src-driven tumors expressed the ductal marker, cytokeratin 19 (Figure 5a), and scattered areas of tumor cells were found that secrete acidic (Alcian blue positive, Figure 5b) and neutral (periodic acid Schiff positive, Figure 5c) mucins, indicating aberrant differentiation in the spontaneous tumors. Papillary structures were regularly found in the ductal lesions as was epithelial bridging between residual islands of in situ carcinoma, two frequently observed histological features in the human disease (Hruban et al., 2004) (Figures 3g–i). At necropsy, mice typically presented with locally advanced pancreatic tumors that impinged on the stomach, spleen, liver and intestines. We did not detect visceral metastases to the liver or lungs, which could be due to a requirement for additional genetic lesions and/or the locally aggressive nature of the disease denying time for the formation of distant disease.
Figure 5.
The epithelial and stromal sub-compartments of LSL-KrasG12D;CSKf/f ;Pdx1-Cre tumors share many features in common with human pancreatic carcinoma. Both infiltrating carcinoma and precursor lesions display strong expression of the ductal marker, CK-19 (a). Acidic and neutral mucin content of infiltrating neoplastic ducts (large arrows) was demonstrated by alcian blue (b) and periodic acid Schiff (c) staining, respectively. Proliferative cells were evident in both the stroma (asterisk) and neoplastic epithelium (arrow) of invasive carcinomas as demonstrated by Ki-67 staining (d). Collagen deposition (green) can be detected by multispectral birefringence imaging (e), whereas strong desmin staining also identified the abundant fibroblast content (f).
Marked stromal hyperplasia and fibroblast infiltration was observed in the Ras/Src-driven tumors, a known feature of human tumors (Hernandez-Munoz et al., 2008) (Figure 3) and in fact, the surrounding stroma commonly featured a higher number of proliferating cells than the neoplastic ductal epithelium (Figure 5d). This led to highly fibrous tumors that were frequently palpable through the abdominal wall and had a firm consistency at the time of necropsy. This could be attributed, at least in part, to the significant levels of collagen deposition throughout the tumor, as visualized by multispectral, birefringence imaging (Figure 5e). The surrounding stroma was also characterized by high levels of desmin expression, distinguishing it clearly from the invading carcinomatous glands (Figure 5f).
Oncogenic Ras/Src cooperativity and genomic instability
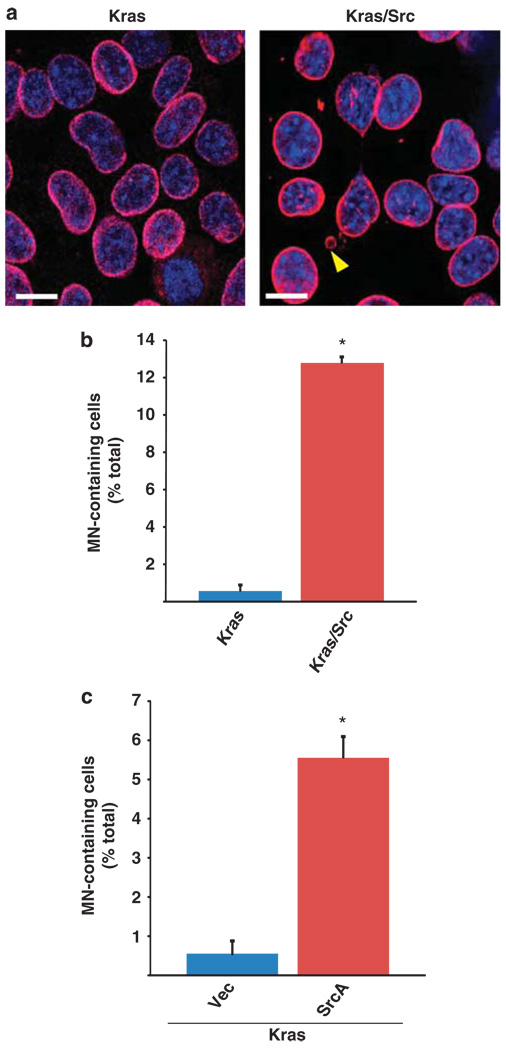
Next, we sought to characterize the underlying mechanisms of Src/Ras-driven tumorigenesis in this model. One of the hallmarks of PDA is genomic instability (Hezel et al., 2006), and in our analysis of the tumor-derived Kras/Src cells, we noted multiple examples of abnormal mitotic spindles and centrosomal amplification, as observed by immunofluorescence analysis of α-tubulin and γ-tubulin, respectively (Supplementary Figure S4A–B). Furthermore, LaminB staining of the nuclear envelope in these cells enabled the identification of discrete micronuclei (MN) (Figure 6a), which are established markers of genomic instability resulting from chromosomal malsegregation (Fenech, 2000). To determine if dual Ras/Src activation correlated with MN incidence, we quantified the numbers of MN’s in Kras/Src cells (activated Kras and Src), and Kras cells (activated Kras only) derived from the LSL-KrasG12D/Pdx1-Cre spontaneous model of PDA. Kras/Src cells contained significantly higher numbers of MN’s than Kras cells (12.6% vs 0.5% respectively, P < 0.05) (Figures 6a and b). To determine if combinatorial Src/Ras signaling is sufficient to promote MN formation, we transiently expressed activated Src kinase (SrcA) in Kras cells. Expression of activated Src in Kras cells caused a significant increase in the appearance of MN’s as compared with cells transfected with vector control (5.5 vs 0.5% respectively, P < 0.05) (Figure 6c). These findings suggest that in combination with oncogenic Kras, Src activation can promote the onset of genomic instability, a hallmark of malignant disease.
Figure 6.
Cooperative Ras/Src signaling promotes MN formation in PDA cells. (a) Immunofluorescence of LaminB staining to denote the nuclear envelope in Kras cells (left) and Kras/Src cells (right). Discrete MN (indicated by yellow arrowhead) that form during chromosomal malsegregation are visualized as extranuclear DNA-containing structures that are demarcated by a LaminB-positive membrane. (b) Quantification of the incidence of MN-containing cells in the Kras and Kras/Src PDA cell lines. Data are expressed as a percentage of the total cells that were assessed (mean of three separate experiments ± s.e.m). Kras/Src cells had a significantly higher incidence of MN than Kras cells. The * symbol indicates P < 0.05 compared with MN incidence in Kras cells; unpaired t-test. (c) Quantification of the incidence of MN-containing Kras cells that were transiently transfected with an activated Src (SrcA) or control plasmid. Cells were stained and MN incidence was quantified 48 h post transfection. Transient expression of activated Src kinase caused a significant increase in the incidence of MN in Kras cells. The symbol * indicates P < 0.05 compared with Kras cells transfected with control vector; unpaired t-test.
Tumor cells exhibit signs of Src dependency
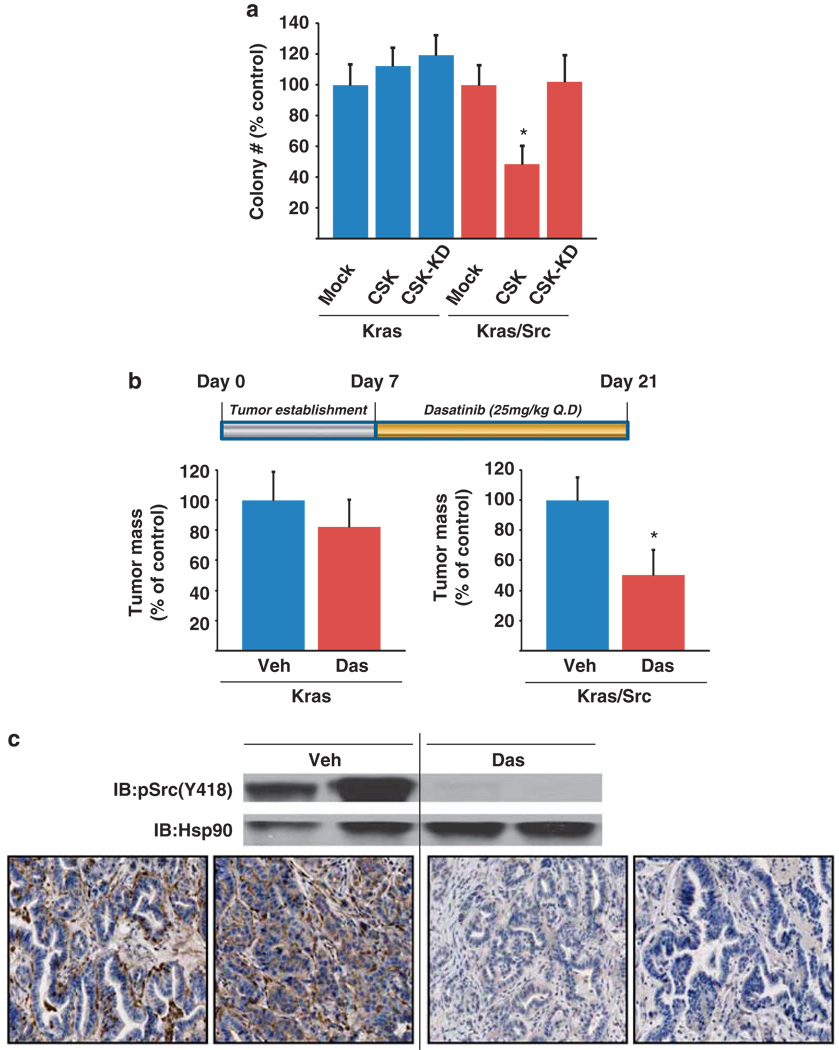
As combined Src/Ras activation may promote genetic instability, which in turn may lead to differential tumor suppressor loss, the question arises as to whether secondary genetic lesions become the drivers of tumorigenicity in the Kras/Src cells, or if the cells remain dependent on activated Src signaling. To address this question, we reintroduced CSK to Kras/Src cells and performed anchorage-independent growth assays. Importantly, reconstitution with CSK significantly reduced colony formation in soft agar whereas a kinase-dead version of CSK had no effect on colony formation in this assay (Figure 7a). In contrast, expression of either wild-type CSK or the kinase-dead derivative did not impair the colony-forming ability of Kras cells (Figure 7a), which express oncogenic Kras in the context of wild-type (non-activated) Src. These findings indicate that the Kras/Src cells exhibit a dependence on Src and might be sensitive to pharmacological inhibitors of Src.
Figure 7.
Kras/Src tumor cells exhibit Src dependency. (a) Effect of Src inhibition on anchorage-independent growth. Kras and Kras/Src cells were infected with adenovirus expressing wild-type CSK or the kinase dead derivative, or empty adenovirus. CSK, but not CSK-KD expression caused a significant reduction in colony growth of the Kras/Src, but not Kras cells on soft agar. A representative experiment is shown. In independent experiments n = 3. The symbol * indicates P < 0.05, as compared with Kras/Src cells infected with CSK or empty virus; analysis of variance with post-hoc test. (b) Effect of Src inhibition on tumor burden in an orthotopic model of PDA. Cells (5 × 105) derived from LSL-KrasG12D;Pdx1-Cre tumors (activated Kras, wild-type Src; ‘Kras’) and LSL-KrasG12D;CSKf/f; Pdx1-Cre tumors (activated Kras and activated c-Src; ‘Kras/Src’) were implanted into the tail of the pancreas in 6–8-week-old male nu/nu mice. Tumors were allowed to establish for 7 days and then mice were administered vehicle or dasatinib (25 mg/kg, p.o., qd × 14 d). At 21d, tumors were resected and weighed. Dasatinib treatment led to a significant reduction in burden of the Kras/Src, but not the Kras tumors; the symbol * indicates P = 0.0019 compared with vehicle-treated mice; paired t-test (n = 9 mice/treatment group). (c) Immunoblot analysis of activated Src levels in Kras/Src tumors following dasatinib treatment. Immunohistochemical analysis of p(Y418) Src (activated enzyme) demonstrated a reduction in Src activity in both the neoplastic ducts and surrounding stroma. Tumor-bearing mice were dosed as in b above and the tumors were resected on day 14 at 1 h post dose for analysis by immunoblot and immunohistochemistry.
Dasatinib is a small molecule Src/Abl inhibitor that was originally approved to treat imatinib (Gleevec)-resistant chronic myeloid leukemia patients (Lombardo et al., 2004) and is currently in trials against a plethora of solid tumors. To assess the sensitivity of the Kras/Src tumor-derived cell lines to Src blockade, we initially exposed the cells to varying doses of dasatinib in vitro. Dasatinib impaired tumor cellular proliferation in vitro (Supplementary Figure S5, IC50 = 47.6 nm), at a dose required to achieve inhibition of Src kinase signaling in these cells (Supplementary Figure S6). Next, to evaluate the consequences of Src kinase inhibition on tumorigenesis in vivo, Kras/Src cells were orthotopically injected into the pancreas of 8-week-old nu/nu mice. After 7 days of tumor growth, animals were systemically treated with dasatinib (25 mg/kg, p.o., qd × 14) and tumors were resected and analyzed on day 21. Dasatinib treatment caused a significant reduction (50%) in tumor burden (P = 0.0019, n = 9 mice/treatment group) relative to tumors in vehicle-treated mice (Figure 7b). Significantly, dasatinib had no effect on the growth of tumors derived from Kras cells, which express KrasG12D in the context of significantly lower levels of activated Src kinase than Kras/Src cells (Figures 4b and 7b). Dasatinib treatment caused a significant reduction in Src activation in the Kras/Src tumors (Figure 7c, upper), and while immunohistochemical analysis of the orthotopic tumors demonstrated that Src activity was most markedly reduced in the neoplastic ducts, a reduction in Src activity in the surrounding stroma was also observed (Figure 7c, lower). These studies suggest that Src activity, in concert with oncogenic Kras, has a key role in the growth of these tumors, and provide support for the inherent Src dependency of these cells.
Discussion
A novel role for Src activation in pancreatic neoplasia
Accumulating evidence from xenograft and orthotopic studies has strongly implicated Src in tumorigenesis and metastasis. However, data from genetically engineered mouse models had indicated that Src activation alone may be insufficient for tumor initiation. For example, overexpression of oncogenic v-Src in astrocytes leads to the development of astrocytomas, but the latency is long (65 weeks) and the penetrance is low as only 14% of mice develop tumors (Maddalena et al., 1999). Similarly, overexpression of SrcA(Y530F), wild-type c-Src or deletion of CSK in keratinocytes each promotes epidermal hyperplasia, but a secondary insult such as wounding, or exposure to a combination of carcinogens 7,12-dimethylbenz(a)anthracene (DMBA) and promoting agents 12-O-tetradecanoylphorbol-13-acetate (TPA) is required for carcinoma development (Matsumoto et al., 2004). In a more recent study, global over-expression of the human c-Src gene yielded neoplastic lesions after 20 months, but again, only 15% of the mice were affected (Kline et al., 2008). Intercrossing with p21 null mice increased the incidence to 26% at 14 months (Kline et al., 2008), suggesting that additional genetic events may enable the oncogenic potential of Src kinase.
Cooperative Kras/Src signaling in the development of PDA
Here, we show that cooperative signaling between KrasG12D and activated Src promotes rapid development of aggressive pancreatic cancer. Although oncogenic cooperativity has been documented previously between proteins such as c-Myc and Bcl-2 in lymphoid tumors (Fanidi et al., 1992), as well as between mutant Kras and c-Myc in mammary carcinogenesis (Podsypanina et al., 2008), this is, to our knowledge, the first demonstration that oncogenic cooperation between activated Kras/Src can promote neoplasia in the mouse. Importantly, oncogenic Ras/Src cross-talk had previously been demonstrated in Drosophila (Vidal et al., 2006), suggesting that cooperative signaling between these two classical oncoproteins may not be restricted to pancreatic carcinomas.
Deletion of CSK alone was insufficient to initiate carcinogenesis, but combined Ras activation and CSK deletion leading to activation of Src led to the development of PDA by 5–8 weeks of age, as compared with 12–14 months for Kras alone, establishing that in the context of oncogenic Kras, activated Src kinase signaling can act as a promoter of tumor progression. Loss of CSK function can lead to the activation of each of the Src family kinase members but in this model, c-Src was the only SFK member activated in tumors with oncogenic Kras expression and CSK loss. However, this does not rule out a possible role for other as yet unidentified CSK substrates in the progression of Kras-dependent PDA. Similarly, CSK loss may impact the function of key interactants, such as the p53 binding protein ASPP. Additional studies will be necessary to define the precise role of CSK and Src in pancreas cancer progression.
In recent years, a number of other spontaneous mouse models of pancreatic cancer have been generated based on the known genetic lesions in PDA patients. Although mutational activation of oncogenic Kras has been established as a key initiating event, additional driver events that contribute to disease progression include the silencing or deletion of Ink4a/Arf (80–85% of clinical PDA specimens), mutational inactivation of p53 (65%) and the homozygous deletion of Dpc4/Smad4 (55%) (Hezel et al., 2006). So, how might changes in Src activity interplay with these other key genetic lesions in pancreatic neoplasia? v-Src was the first identified oncogene, but activating mutations of c-Src are extremely rare in human cancers and none have been detected in PDA. Despite this, Src activity is increased in 60% of PDA’s and this is likely due to increased Src protein abundance or elevated signaling from activated RTK’s such as ErbB-2 (Her2-Neu) and RON (Thomas et al., 2007; Ricono et al., 2009). In fact, members of the epidermal growth factor receptor family are over-expressed in up to 70% of PDA’s (Hall et al., 1990), and upregulation of ErbB-2 is believed to be one of the earliest events in neoplastic conversion of the pancreas (Hruban et al., 2000). Genetic ablation of Ink4α/Arf in the context of oncogenic Kras signaling leads to a murine model of metastatic PDA that recapitulates features of the human disease (Aguirre et al., 2003). Intriguingly, this combination of mutations also leads to a significant upregulation of epidermal growth factor receptor and Her2 expression in the neoplastic ducts, each of which can activate Src kinase. More recently, analysis of tumors from the LSL-KrasG12D; LSLp53R172H; Pdx1-Cre mouse model, which closely recapitulates many clinical features of the disease, demonstrated that Src activity is elevated in the pancreatic intraepithelial neoplasia lesions and continues to increase as a function of disease progression in this model (Morton et al., 2010). Furthermore, progression to advanced metastatic disease was attenuated by systemic Src inhibition (Morton et al., 2010).
A model of Src dependency
Conversely, data from our model suggest that Ras activation combined with CSK deletion may actually promote genetic instability, which, in turn, could lead to loss of key tumor suppressors such as p16, p53 and Smad4. These findings are consistent with previous reports showing that Src/MAPK signaling promotes genomic instability through activation of Aurora B kinase (Kabil et al., 2008), and that SFKs have been proposed to regulate cell proliferation through abrogation of p53 function (Broome and Courtneidge, 2000). Interestingly, combined Src/MAPK signaling is also required for abscission during cytokinesis and cell proliferation (Kasahara et al., 2007). Irrespective of the impact of dual Ras/Src signaling on suppressors of Kras-mediated tumorigenesis, these tumors exhibit an ongoing dependence on the hyperactivated Src signaling cascade. Accordingly, genetic or pharmacological Src inhibition attenuates the tumorigenicity of neoplastic cells from Ras/Src derived PDA’s. In contrast, Morton et al. recently demonstrated that Src inhibition attenuated metastatic disease but not primary burden in the Ras/p53 mouse model (Morton et al., 2010), suggesting that Src may have different roles in tumorigenesis or metastasis, depending on the underlying mutational context. Elucidation of the molecular basis for the differences in Src dependency could enable the identification of patient subsets that respond to therapeutic intervention with agents targeting the Src signaling pathways.
Materials and methods
Mouse strains
CSKf/f (floxed), Lox-Stop-Lox (LSL)-KrasG12D and Pdx1-Cre mouse strains have been described (Schmedt et al., 1998; Jackson et al., 2001; Hingorani et al., 2003). These strains were interbred to generate the compound triple mutant animals; KrasLSL-G12D/+; CSKf/f; Pdx1-Cre (KCC). All animal procedures were conducted in accordance with the appropriate regulatory standards under protocol S05018 as approved by the UCSD IACUC.
Histological and immunohistochemical analysis
All histological processing of fixed tumor specimens and staining (hematoxylin and eosin, periodic acid Schiff, alcian blue) of paraffin embedded sections was performed at the Histology Core in Moores UCSD Cancer Center. Histological analysis was performed by Dr David Tarin (Moores UCSD Cancer Center) and Dr Greg Boivin (Wright State University, Dayton, OH, USA). Immunostaining was performed according to the manufacturer’s recommendations (Vector Labs, Burlingame, CA, USA), on 5 µm sections of paraffin-embedded tumors from the spontaneous mouse model or from human patients diagnosed with PDA (as approved by the Institutional Review Board at University of California, San Diego—Project#090407X). For pSFK(Y418/Y419) immunohistochemistry, antigen retrieval was performed in EDTA buffer pH 8.0. Antigen retrieval for all other antibodies was performed in citrate buffer, pH 6.0, at 95 °C for 20 min. Sections were treated with 0.3% H2O2 for 30 min, blocked in normal goat serum, phosphate-buffered saline Tween 20 (PBS-T)- for 30 min followed by Avidin-D and then incubated overnight at 4 °C with primary antibody diluted in blocking solution as follows: CSK (AbCam, Cambridge, MA, USA, 1/100), pErk (Cell Signaling, Danvers, MA, USA, 1/500), CK19/TromaIII (Hybridoma bank, Iowa City, IA, USA, 1/1000), Ki67 (AbCam, 1/1000), Desmin (Neomarkers, Fremont, CA, USA, 1/500), Insulin (Invitrogen, Carlsbad, CA, USA, 1/10 000), Glucagon (Santa Cruz, Santa Cruz, CA, USA, 1/1000), Amylase (Sigma, St Louis, MO, USA, 1/100). Tissue sections were washed and then incubated with biotinylated secondary antibody (1:500, Jackson ImmunoResearch, West Grove, PA, USA) in blocking solution for 1 h. Sections were washed and incubated with Vectastain ABC (Vector Labs) for 30 min. Staining was developed using a Nickel-enhanced diamino-benzidine reaction (Vector Labs) and sections were counter-stained with hematoxylin.
Immunofluorescence analysis
Cells were transfected with activated Src (SrcA) or control plasmids using Lipofectamine, and seeded directly on 22 mm coverslips in 6-well plates at a density of 1 × 105 per well. Following incubation at 37 °C for 48 h, cells were fixed in cold methanol at −20 °C for 10 mins (α-tubulin, β-tubulin) or 4% paraformaldehyde/PBS, pH 7 at room temperature for 10 min. Following permeabilization with 0.2% Triton X-100/PBS for 2 min, cells were blocked in 3% bovine serum albumin/PBS for 1 h at room temperature, and then incubated with the primary antibodies (α-tubulin, γ-tubulin, both AbCam; LaminB, Santa Cruz; all 1/300 dilution in 3% bovine serum albumin/PBS) at room temperature for 2 h. Cells were subsequently incubated for 1 h at room temperature with the appropriate species Alexa Fluor secondary antibodies (488 or 568). Nuclei were visualized following DraQ5 staining. Coverslips were mounted onto slides with Vectashield and images were acquired using laser scanning confocal microscopy under × 60/1.4 NA oil objective (Nikon C1si, Nikon Instruments Inc., Melville, NY, USA). Quantification of MN was achieved by capturing images of multiple high-power fields and scoring the number of cells that contained discrete MN with a LaminB demarcated boundary. A minimum of 1000 cells were quantified per condition in each experiment and all experiments were performed three times. All images presented are single sections in the Z plane.
Imaging of tumor collagen content
Images of collagen content in hematoxylin and eosin-stained primary Kras/Src tumor sections were acquired with Cri’s prototype ‘Nubrio’ imaging system, which enables the multispectral detection of birefingent structures such as collagen in tumor specimens. After setting the image acquisition parameters and taking appropriate background images for correct retardance and flat-fielding purposes, subsequent multimodal, multilayer images were acquired.
Immunoprecipitation and immunoblot analysis
Tumors/pancreata were homogenized by mechanical disruption. Both tissues and tumor cell lines were lysed in a modified radioimmuno precipitation assay buffer containing 50 mm Tris pH 7.5 at 4°C, 150 mm NaCl, 1 mm EDTA, 50 mm NaF, 5 mm sodium pyrophosphate, 10 mm β-glycerophosphate, 1% NP-40, 1 mm Na3VO4, 0.25% sodium deoxycholate, 0.1% SDS, phosphatase and protease inhibitors (Roche, Indianapolis, IN, USA). Lysates were cleared by centrifugation and the protein concentration of the cleared lysate was determined by the bicinchoninic acid method. Immunoblots were performed using the following antibodies: pSFK-Y418 (Cell Signaling), Erk2, Fyn (Santa Cruz), Yes (BD Biosciences, San Diego, CA, USA), Src, pY-4G10 (Millipore, Billerica, MA, USA). Immunoprecipitations were performed with protein A/G beads and the Src, Yes and Fyn antibodies listed above.
Pancreatic tumor cell lines
PDA cell lines were isolated as described (Aguirre et al., 2003) from primary tumors in the Kras/Src model. PDA lines used in this study from other spontaneous mouse models of pancreatic cancer have been described previously (Murphy et al., 2008; Thomas et al., 2008). The PDA lines were maintained under standard culture conditions in RPMI medium 1640 supplemented with penicillin and streptomycin, 2 mm glutamine and 10% fetal bovine serum. Proliferation rate of cell lines was determined by XTT assay (Promega, Madison, WI, USA), following 48 h incubation in complete media with vehicle (dimethyl sulfoxide) or the indicated dasatinib concentration.
Anchorage independent growth assays
After 48 h of infection with adenovirus (10 multiplicity of infection) expressing wild-type or kinase-dead CSK, or control virus, cells were suspended in 0.3% agar/complete media and grown on top of a bottom layer of 1% agar/complete media in 48 or 24-well plates. Additional media was overlayed and cells were cultured for 7–10 days before counting colonies consisting of at least five cells from fields or whole wells.
Orthotopic pancreatic carcinoma model
The orthotopic pancreatic carcinoma model has been described (Grimm et al., 2003). Briefly, 6–8-week-old nu/nu mice were injected with 5 × 105 cells derived from tumors in LSL-KrasG12D; Pdx1-Cre mice (Kras cells) or LSL-KrasG12D;CSKf/f;Pdx1-Cre mice (Kras/Src cells) in the tail of the pancreas. Tumors were allowed to establish for 7 days and then animals were administered vehicle or dasatinib (25 mg/kg, p.o., qd × 14). On day 21, mice were harvested, primary tumors were resected and tumor burden was assessed by weight.
Statistical analysis
Data presented are means ± s.e.m. Statistical analyses were performed with Prism (GraphPad, La Jolla, CA, USA). Statistical differences for one factor between two groups or more than two groups were determined with an unpaired Student’s t-test or an analysis of variance (ANOVA) with a post-hoc test, respectively. Statistical significance for the orthotopic animal studies was determined by a paired Student’s t test. Statistical significance was defined as P < 0.05.
Supplementary Material
Acknowledgements
We thank Charles Yi and Dana Wu for excellent technical assistance. We acknowledge Richard Aspinall, Lisette Acevedo, Josh Greenberg and David Tuveson for valuable discussions, Greg Boivin for histological analysis, as well as Richard Levenson and Kristin Lane for NuBrio imaging. This work was supported by NIH grants R21CA104898, P01-CA078045 (DAC) and Collaborative Translational Research grants from Moores UCSD Cancer Center (DAC, DJS and AML).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton CM, Hall PA, Hughes CM, Gullick WJ, Lemoine NR. Transforming growth factor alpha and epidermal growth factor in human pancreatic cancer. J Pathol. 1991;163:111–116. doi: 10.1002/path.1711630206. [DOI] [PubMed] [Google Scholar]
- Billadeau DD, Chatterjee S, Bramati P, Sreekumar R, Shah V, Hedin K, et al. Characterization of the CXCR4 signaling in pancreatic cancer cells. Int J Gastrointest Cancer. 2006;37:110–119. doi: 10.1007/s12029-007-0011-7. [DOI] [PubMed] [Google Scholar]
- Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–7968. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- Broome MA, Courtneidge SA. No requirement for src family kinases for PDGF signaling in fibroblasts expressing SV40 large T antigen. Oncogene. 2000;19:2867–2869. doi: 10.1038/sj.onc.1203608. [DOI] [PubMed] [Google Scholar]
- Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prevost N, et al. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med. 2009;15:1163–1169. doi: 10.1038/nm.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- Fanidi A, Harrington EA, Evan GI. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992;359:554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Grimm J, Potthast A, Wunder A, Moore A. Magnetic resonance imaging of the pancreas and pancreatic tumors in a mouse orthotopic model of human cancer. Int J Cancer. 2003;106:806–811. doi: 10.1002/ijc.11281. [DOI] [PubMed] [Google Scholar]
- Hall PA, Hughes CM, Staddon SL, Richman PI, Gullick WJ, Lemoine NR. The c-erb B-2 proto-oncogene in human pancreatic cancer. J Pathol. 1990;161:195–200. doi: 10.1002/path.1711610305. [DOI] [PubMed] [Google Scholar]
- Hernandez-Munoz I, Skoudy A, Real FX, Navarro P. Pancreatic ductal adenocarcinoma: cellular origin, signaling pathways and stroma contribution. Pancreatology. 2008;8:462–469. doi: 10.1159/000151537. [DOI] [PubMed] [Google Scholar]
- Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Wilentz RE, Kern SE. Genetic progression in the pancreatic ducts. Am J Pathol. 2000;156:1821–1825. doi: 10.1016/S0002-9440(10)65054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Sefton BM. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci USA. 1980;77:1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, et al. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20:3147–3160. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell. 2007;11:229–243. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Kabil A, Silva E, Kortenkamp A. Estrogens and genomic instability in human breast cancer cells—involvement of Src/Raf/Erk signaling in micronucleus formation by estrogenic chemicals. Carcinogenesis. 2008;29:1862–1868. doi: 10.1093/carcin/bgn138. [DOI] [PubMed] [Google Scholar]
- Kasahara K, Nakayama Y, Nakazato Y, Ikeda K, Kuga T, Yamaguchi N. Src signaling regulates completion of abscission in cytokinesis through ERK/MAPK activation at the midbody. J Biol Chem. 2007;282:5327–5339. doi: 10.1074/jbc.M608396200. [DOI] [PubMed] [Google Scholar]
- Kline CL, Jackson R, Engelman R, Pledger WJ, Yeatman TJ, Irby RB. Src kinase induces tumor formation in the c-SRC C57BL/6 mouse. Int J Cancer. 2008;122:2665–2673. doi: 10.1002/ijc.23445. [DOI] [PubMed] [Google Scholar]
- Koreckij T, Nguyen H, Brown LG, Yu EY, Vessella RL, Corey E. Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis. Br J Cancer. 2009;101:263–268. doi: 10.1038/sj.bjc.6605178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- Lutz MP, Esser IB, Flossmann-Kast BB, Vogelmann R, Luhrs H, Friess H, et al. Overexpression and activation of the tyrosine kinase src in human pancreatic carcinoma. Biochem Biophys Res Commun. 1998;243:503–508. doi: 10.1006/bbrc.1997.8043. [DOI] [PubMed] [Google Scholar]
- Maddalena AS, Hainfellner JA, Hegi ME, Glatzel M, Aguzzi A. No complementation between TP53 or RB-1 and v-src in astrocytomas of GFAP-v-src transgenic mice. Brain Pathol. 1999;9:627–637. doi: 10.1111/j.1750-3639.1999.tb00544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GS. The hunting of the src. Nat Rev Mol Cell Biol. 2001;2:467–475. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kiguchi K, Jiang J, Carbajal S, Ruffino L, Beltran L, et al. Development of transgenic mice that inducibly express an active form of c-src in the epidermis. Mol Carcinog. 2004;40:189–200. doi: 10.1002/mc.20027. [DOI] [PubMed] [Google Scholar]
- Morton JP, Karim SA, Graham K, Timpson P, Jamieson N, Athineos D, et al. Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology. 2010;139:292–303. doi: 10.1053/j.gastro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, et al. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci USA. 2008;105:9343–9348. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsypanina K, Politi K, Beverly LJ, Varmus HE. Oncogene cooperation in tumor maintenance and tumor recurrence in mouse mammary tumors induced by Myc and mutant Kras. Proc Natl Acad Sci USA. 2008;105:5242–5247. doi: 10.1073/pnas.0801197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricono JM, Huang M, Barnes LA, Lau SK, Weis SM, Schlaepfer DD, et al. Specific cross-talk between epidermal growth factor receptor and integrin alphavbeta5 promotes carcinoma cell invasion and metastasis. Cancer Res. 2009;69:1383–1391. doi: 10.1158/0008-5472.CAN-08-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rous P. A sarcoma of the fowl transmissable by an agent separable from the tumor cells. J Exp Med. 1911;13:397–411. doi: 10.1084/jem.13.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucci N, Recchia I, Angelucci A, Alamanou M, Del Fattore A, Fortunati D, et al. Inhibition of protein kinase c-Src reduces the incidence of breast cancer metastases and increases survival in mice: implications for therapy. J Pharmacol Exp Ther. 2006;318:161–172. doi: 10.1124/jpet.106.102004. [DOI] [PubMed] [Google Scholar]
- Schmedt C, Saijo K, Niidome T, Kuhn R, Aizawa S, Tarakhovsky A. Csk controls antigen receptor-mediated development and selection of T-lineage cells. Nature. 1998;394:901–904. doi: 10.1038/29802. [DOI] [PubMed] [Google Scholar]
- Stehelin D, Varmus HE, Bishop JM, Vogt PK. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976;260:170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Talamonti MS, Roh MS, Curley SA, Gallick GE. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest. 1993;91:53–60. doi: 10.1172/JCI116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RM, Kim J, Revelo-Penafiel MP, Angel R, Dawson DW, Lowy AM. The chemokine receptor CXCR4 is expressed in pancreatic intraepithelial neoplasia. Gut. 2008;57:1555–1560. doi: 10.1136/gut.2007.143941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RM, Toney K, Fenoglio-Preiser C, Revelo-Penafiel MP, Hingorani SR, Tuveson DA, et al. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res. 2007;67:6075–6082. doi: 10.1158/0008-5472.CAN-06-4128. [DOI] [PubMed] [Google Scholar]
- Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY, et al. Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol. 2006;168:962–972. doi: 10.2353/ajpath.2006.050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M, Larson DE, Cagan RL. Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev Cell. 2006;10:33–44. doi: 10.1016/j.devcel.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Vitali R, Mancini C, Cesi V, Tanno B, Piscitelli M, Mancuso M, et al. Activity of tyrosine kinase inhibitor Dasatinib in neuroblastoma cells in vitro and in orthotopic mouse model. Int J Cancer. 2009;125:2547–2555. doi: 10.1002/ijc.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- Yezhelyev MV, Koehl G, Guba M, Brabletz T, Jauch KW, Ryan A, et al. Inhibition of SRC tyrosine kinase as treatment for human pancreatic cancer growing orthotopically in nude mice. Clin Cancer Res. 2004;10:8028–8036. doi: 10.1158/1078-0432.CCR-04-0621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.