Abstract
We have examined whether epigallocatechin-3-gallate (EGCG), and extract of green tea, in combination with taxane (i.e., paclitaxel and docetaxel), exerts a synergistic activity in blocking human prostate PC-3ML tumor cell growth in vitro and in vivo. Growth assays in vitro revealed that the IC50 values were ∼30 µM, ∼3 nM, and ∼6 nM, for EGCG, paclitaxel and docetaxel, respectively. Isobolograms generated from the data clearly indicated that EGCG in combination with paclitaxel or docetaxel had an additive effect in blocking tumor cell growth. EGCG combined with taxane also had an additive effect to increase the expression of apoptotic genes, (p53, p73, p21, and caspase 3) and the percent apoptosis observed in vitro and in tumor modeling studies in severe combined immunodeficient mice. The tumor modeling studies clearly showed that EGCG plus taxane injected intraperitoneally (i.p.) induced a significant increase in apoptosis rates (TUNEL assays) and eliminated preexisting tumors generated from PC-3ML cells implanted i.p., increasing disease-free survival rates to greater than 90%. More importantly, the combination therapy (i.p. biweekly) blocked metastases after intravenous injection of PC-3ML cells through the tail vein. In mice treated with EGCG plus taxane, the disease-free survival rates increased from 0% (in untreated mice) to more than 70% to 80% in treated mice. Taken together, these data demonstrate for the first time that EGCG in combination with taxane may provide a novel therapeutic treatment of advanced prostate cancer.
Introduction
Prostate cancer is the most commonly diagnosed neoplasm in men, accounting for approximately 29% of all of new cancers. In 2000, an estimated 180,400 men will be diagnosed with prostate cancer, and approximately 32,000 will die [1]. Surgery and radiation therapy (external beam or brachytherapy seed implantation) remain the most common therapeutic modality for the treatment of clinically localized prostate cancer [2]. Once prostate cancer enters a metastatic stage, the current treatment is androgen ablation therapy, which provides relief from otherwise uncontrollable bone pain and increases life expectancy by 6 to 18 months in 70% of men [3]. For many years, it was felt that chemotherapy did not play any role in the treatment of advanced prostate cancer. However, this negative impression may be starting to change because activity is being seen with new drugs and drug combinations [4] and in particular with the use of docetaxel [5].
We have previously carried out animal model studies with purified epigallocatechin-3-gallate (EGCG) [6]. The animal studies have demonstrated that the continuous intraperitoneally (i.p.) administration of EGCG at a dose of 200 µM/animal (228 mg/kg) every 3 days can significantly reduce distant prostate tumor xenograft growths in CB17 severe combined immunodeficient (SCID) mice [6]. In the past 10 years, EGCG has entered phase 1/2 clinical trials for the treatment of leukemia and lung cancer [7,8].
Although conventional cancer therapies (surgery, chemotherapy, and radiation) are often effective at curing early stage disease, few human metastatic cancers are curable with a single modality, and the death rate from metastatic prostate cancer has not decreased significantly in the past 30 years. In an attempt to widen the curative therapeutic window of EGCG, we explored a neoadjuvant therapy that consisted of combining EGCG with chemotherapeutic agents such as doxorubicin and showed that EGCG increased the therapeutic efficacy of doxorubicin in vivo [6].
In the present study, we examined the efficacy of EGCG in combination with taxane (paclitaxel and docetaxel) against human prostate cancer cells. Synergistic efficacy was observed both in vitro and in vivo. The in vitro studies revealed that EGCG plus taxane had an additive effect in blocking tumor cell growth and inducing apoptosis of PC-3ML cells. The in vivo tumor modeling studies showed that the combination of EGCG (200 µM or 228 mg/kg) with docetaxel (i.e., at therapeutic doses effective in humans) eliminated PC-3ML xenografts within 5 to 6 weeks and increased mouse survival rates by more than 90%. In addition, EGCG plus paclitaxel blocked bone metastases by PC-3ML cells injected intravenously (i.v.) through the tail vein and increased mouse survival rates from 0% in untreated mice to more than 70% to 80% in treated mice. Taken together, these results suggest that EGCG used in combination with taxane can offer a curative therapeutic approach for eradication of primary tumors and metastatic prostate cancer.
Materials and Methods
Cell Cultures
PC-3ML cells were previously isolated as single cell clones derived from bone marrow metastases by PC-3 cells in CB17 SCID mice [9]. The PC-3ML cultures were maintained in Dulbecco modified Eagle medium (DMEM) plus 10% fetal bovine serum according to previously described protocols of the American Tissue Culture Collection (Bethesda, MD) [9].
MTT Assays of Cell Growth/Viability
Growth and cell viability assays were carried out using 3-[4,5-dimethylthiazole-2-4]-2,5-diphenyl-2H-tetrazolium bromide (MTT). PC-3ML cells were seeded at 5000 cells/well in 96-well plates (Falcon; Discovery Labware, Bedford, MA) for 24 hours before treatment with the drug. The MTT assays were carried out as described previously by our group [6]. Cells were treated either with EGCG, paclitaxel, or docetaxel or with EGCG in combination with paclitaxel or docetaxel. Cell growth (Figure 1, A and B) and cell viability (Figure 1, C–G) were measured at the times indicated by removing the medium and replacing it with DMEM containing 1 mg/ml MTT (Sigma Chemical Co, St Louis, MO) (100 µl of MTT for 3 hours at 37°C). For the growth assays, the plates were read on a Microtiter Plate Reader (A490 nm) (Molecular Dynamics, Sunnyvale, CA) [6]. For cell viability assays, the MTT solution was removed, and the crystals that remained in the wells were solubilized by the addition of 50 µl of isopropanol followed by vigorous shaking [10]. The absorbency was determined using a microplate reader (Molecular Dynamics) at 560 nm (test wavelength) and 690 nm (reference wavelength). The percentage of surviving cells was estimated by dividing the A550 nm - A650 nm of treated cells by the A550 nm - A650 nm of mock-treated cells [10]. Twelve replica samples were taken for each time point, and each experiment was repeated at least three times.
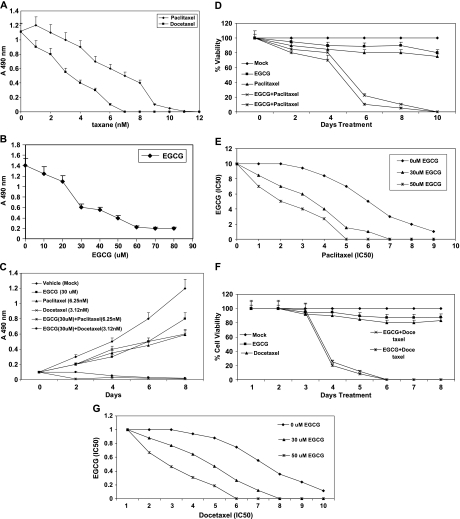
Figure 1.
(A–C) MTT growth assays with PC-3ML cells. (A) Cells were treated with 0 to 12 nM paclitaxel or docetaxel for 8 days. IC50 values were ∼3 nM docetaxel and ∼6 nM paclitaxel, respectively. (B) Cells were treated with 0 to 80 µM EGCG for 8 days. The IC50 was ∼30 µM EGCG. (C) Cells were treated with vehicle (mock), EGCG (30 µM), paclitaxel (6.25 nM), docetaxel (3.12 nM), EGCG (30 µM) plus paclitaxel (6.25 nM), and EGCG (30 µM) plus docetaxel (3.12 nM) for 8 days. (A–C) Cells were seeded at ∼25% confluence in 96-well plates overnight before beginning the drug treatments. In combination drug studies, cells were exposed to EGCG plus taxane with changes of medium every 2 days. Values represent the mean ± SD from three experiments. (D–G) MTT growth assays with PC-3ML cells treated with EGCG (30 µM), paclitaxel (6.25 nM), docetaxel (3.12 nm), or a combination of these drugs at these concentrations. (D) Shows the decrease in cell viability of PC-3ML cells treated with EGCG (x) 30 µM and (*) 50 µM plus paclitaxel (6.25 nm). (E) Isobologram analysis of the observed data for the combination of EGCG and paclitaxel shown in D. (F) Decrease in cell viability of PC-3ML cells treated with EGCG (x) 30 µM and (*) 50 µM plus docetaxel (3.12 nm). (G) Isobologram analysis of the observed data for the combination of EGCG and docetaxel in F. The concentration that produced 50% cell growth inhibition (IC50) is expressed as 1.0 in the ordinate and the abscissa of the isobologram. y axis, EGCG (IC50); x axis, paclitaxel or docetaxel (IC50).
Apoptosis Assays
Apoptosis was measured by flow cytometry using Annexin V antibodies and a kit from Guava, Inc (Redwood, CA) according to published methods by our group [11]. TUNEL assays were carried with antibodies from Sigma using established protocols [11].
Gel Electrophoresis and Western Blots
Cultured cells or cells isolated from tumors by collagenase I digestion (100 ng/ml for 30 minutes) were lysed with modified RIPA buffer [11]. Protein measurements were carried out according to Sambrook and Russell [12], and crude extracts prepared for SDS-PAGE and electroblotted onto Hybond enhanced chemiluminescence membranes (Amersham Pharmacia, Buckinghamshire, England) according to standard procedures [12]. Western blots were carried out according to standard procedures [11,12] using Super Signal West Pico chemiluminescent substrate (Pierce Biotechnology, Inc, Rockford, IL) for antibody detection. The reaction was visualized using the ChemiDoc XRS Gel Documentation system and band densities analyzed by Multi-Analyst software (Bio-Rad Laboratories, Inc, Hercules, CA). Antibodies specific for p53, p71, p21, caspase 3, and β-actin were purchased from Sigma-Aldrich.
Drug Studies
EGCG (Sigma-Aldrich) was prepared fresh by resuspension of EGCG in PBS, pH 5, containing 3% ascorbic acid, and 10mMEDTA (the solvent). The EGCG solution was filter sterilized and added to cells or injected in mice. Paclitaxel (Taxol; Bristol-Myers Squibb, Princeton, NJ) and docetaxel (Taxotere; Rhone-Poulenc Rorer Pharmaceuticals, Inc, Collegeville, PA) were solubilized in 50% dimethylsulfoxide and aliquots of the stock solutions diluted with DMEM for in vitro studies and with 0.9% NaCl for in vivo studies.
The IC50 was defined as the concentration of drug that produced 50% cell growth inhibition, that is, 50% reduction in absorbance in MTT growth assays. The dose-response curves were plotted with CurveExpert (Version 1.34) on a semilog scale as a percentage of the control, the absorbance of which was obtained from the samples not exposed to the drugs. On the basis of the dose-response curves of EGCG alone and taxane alone, isobolograms (three isoeffect curves, mode 1 and mode 2 lines) were computed. The envelope of additivity, surrounded by mode 1 and mode 2 isobologram lines, was constructed from the dose-response curves of EGCG alone and taxane alone. The observed data were compared with the predicted maximum and minimum data for the presence of synergism, additivity, or antagonism by a statistical analysis using the Stat View 4.01 software program (Abacus Concepts, Berkeley, CA). When the data points of the drug combination fall within the area surrounded by mode 1 and/or mode 2 lines (i.e., within the envelope of additivity), the combination is described as additive. A combination that gives data points to the left of the envelope of additivity can be described as supra-additive (synergism), and a combination that gives data points to the right of the envelope of additivity can be described as subadditive (antagonistic [13]).
Tumor Xenografts in CB17 SCID Mice
Methods previously described by our group were used to develop xenograph tumors i.p. [6]. In brief, single-cell suspensions of PC-3ML cells (1 x 106 cells) at less than passage 10 were injected i.p. in 5- to 6-week-old CB17 SCID mice (Taconic Labs, Pottstown, NY). When tumors reached a minimal volume of 0.15 to 0.18 mm3 (after ∼1 week), drug was injected i.p. in a volume of 0.50 ml per mouse. Each group had 5 or 10 mice, and tumor volumes were determined by measurement of tumor length (L) and width (l) with a caliper and calculated according to the formula: (L x l2) / 2.
Bone metastases studies were carried out according to published methods by our group [9]. In brief, PC-3ML cells were injected i.v. through the tail veil in 0.1 ml of DMEM. Once tumors had been allowed to establish for 1 or 2 weeks, mice were treated with drug injected i.p. according to the above approach. Mice were kept in a double-barrier facility and handled according to an approved Institutional Animal Control Use Committee protocol in accordance with our institution's policy.
Effects of Drug on Established Tumors
PC-3ML xenografts were established as described [6]. Mice that bore tumors were randomized into groups of four. The first group received 200 µM of EGCG (228 mg/kg) at day 1. EGCG was injected i.p. using a 28-gauge needle on a weekly basis. The second group was given taxane only. Paclitaxel was administered i.p. at a dose of 15 mg/kg weekly. Docetaxel was administered i.p. at a dose of 5 or 12.5 mg/kg i.p. weekly. The third group was given EGCG (i.p.) at day 1 and taxane at the same doses and schedule as the second group. As a control (vehicle), a fourth group was treated with 0.1 ml of normal saline (i.e., control) i.p.
For docetaxel, the dose was selected based on the human clinical dose (Rhone-Poulenc Rorer Pharmaceuticals, Inc) and determined by a dose range-finding study in nude mice [14]. Tumor volumes were measured as described previously [6].
Immunohistochemistry
Four groups of mice (n = 5) were treated with vehicle, EGCG (200 µM/animal or 228 mg/kg), paclitaxel (15 mg/kg), or a combination of EGCG and paclitaxel. Half of the animals were killed on day 9 and the other half on day 16. Histology methods were as described previously [6]. Apoptotic cells were detected using the TUNEL assay [6,12]. Paraffin-embedded tumor sections were heated in citric acid buffer for 15 minutes to retrieve antigen, hybridized with the TUNEL antibody, then counterstained with Harries hematoxylin (Roche Molecular Biochemicals, Mannheim, Germany). The stained sections were analyzed under a light microscope, and the numbers of apoptotic cells were counted in five fields of view from three different tumors.
Statistical Analysis
Significant changes were assessed by Student's t test. The results are presented as the mean ± SD. P < .05 was accepted as the level of significance. The dose-response interactions between taxane and EGCG at the point of IC50 were evaluated by the isobologram method of Steel and Peckham [15] as modified by Aoe et al. [16].
Results
Combination of EGCG with Paclitaxel or Docetaxel Inhibits Growth of PC-3ML Cells in a Synergistic Manner
Growth assays were carried out to assess the potential synergistic activities of EGCG in combination with paclitaxel or docetaxel on the proliferation of malignant human prostate PC-3ML cells (Figure 1, A–C). The dose-response curves indicated that the IC50 values were ∼6 nM paclitaxel, ∼3 nM docetaxel, and ∼30 µM EGCG after treatment of the cells with increased dosages of each agent for 8 days (Figure 1, A and B). Growth assays further showed that EGCG (30 µM) in combination with either paclitaxel (6.25 nM) or docetaxel (3.12 nM) exhibited a synergistic activity in reducing growth rates to near zero by ∼2 to 8 days (Figure 1C). Likewise, cell viability assays further demonstrated that the combination of EGCG (30 and 50 µM) with paclitaxel (6.25 nM) reduced cell survival to zero after ∼6 to 10 days (Figure 1D).
Isobolograms were generated from the models to determine the presence of synergy, additivity, or antagonism between EGCG and paclitaxel. Figure 1E shows isobologram representation of the statistical modeling used to analyze the drug interactions between two dosages of EGCG (0, 30, and 50 µM) and paclitaxel (6.3 nM). The combined data points fell within the envelope of additivity or were smaller than that of the predicted minimum data, which indicates that sequential exposure to EGCG for 24 hours followed by EGCG plus paclitaxel produced synergistic effects by ∼6 to 10 days (Figure 1E).
Enhanced cytotoxicity was also observed in the combination treatment of EGCG and docetaxel. Cell viability assays revealed that EGCG (30 µM) or docetaxel (3.12 nm) alone produced more than 80% cell survival of prostate carcinoma PC-3ML cells after 2 to 8 days treatment (Figure 1F). In comparison, EGCG at (x) 30 µM and (*) 50 µM combined with docetaxel (3.12 nm) reduced cell viability to less than 10% after 5 to 8 days treatment (Figure 1F).
Isobologram representation of the statistical modeling used to analyze the interaction between EGCG and docetaxel revealed that the combined data points fell to the left of the envelope of additivity, or, restated, the IC50 of EGCG in combination with docetaxel occurred at smaller doses than that predicted from the use of EGCG or docetaxel alone by 6 to 10 days (Figure 1G). Thus, exposure to EGCG combined with docetaxel produced synergistic effects in the inhibition of PC-3ML growth.
Combination of Taxane and EGCG Increases p53, p73, p21, and Caspase 3 Expression
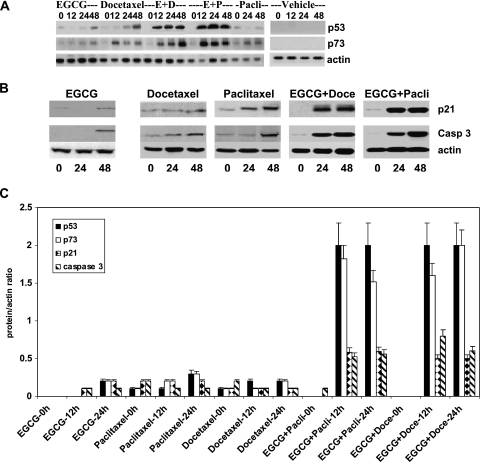
We have examined the effect of these drugs alone and in combination on the expression of apoptotic-related protein markers in attempts to address the potential mechanisms, which account for the synergistic activity of the combined drug therapy. The PC-3ML cells were treated with the individual agents and with EGCG (30 µM) plus docetaxel (3.12 nm) or paclitaxel (6.25 nm) for 0, 12, 24, and 48 hours. Western blots showed that the combination of EGCG and docetaxel or EGCG plus paclitaxel induced significant increases in p53 and p73 expression after 12, 24, and 48 hours. In comparison, EGCG and docetaxel alone had little or no effect on p53 expression after 12 and 24 hours, albeit they both induced expression after 48 hours (Figure 2A). Paclitaxel alone failed to induce expression of p53 after 12, 24, and 48 hours, however. Interestingly, EGCG, docetaxel, and paclitaxel alone induced p73 expression after 12 to 48 hours. Similarly, Western blots with p21 and caspase 3 antibodies showed that EGCG plus docetaxel or EGCG plus paclitaxel combined induced a significant increase in the expression of these two proteins after 24 and 48 hours of treatment (Figure 2B). In comparison, the individual agents had a significantly reduced effect on the levels of p21 and caspase 3 expression after 24 and 48 hours (Figure 2B). Note that the vehicle failed to induce increased expression of p53, p73, p21, or caspase 3 (Figure 2, A and B). Densitometric scans of the Western blots and calculations of the fold change in the antigen/actin ratio demonstrated that EGCG plus paclitaxel and EGCG plus docetaxel induced more than 2-fold and more than 1.5-fold increases in p53 and p73, respectively, after 12 and 24 hours (i.e., compared with cells exposed to EGCG alone). Likewise, the combined drug treatments induced more than 0.5-fold increases in p21 and caspase 3 after 12 and 24 hours of treatment (Figure 2C). In comparison, the individual agents induced only small increases in expression of these proteins. Taken together, the data suggest that EGCG plus taxane induced genes associated with apoptosis.
Figure 2.
(A) Western blots of p53 and p73 expression in PC-3ML cells subjected to EGCG (30 µM), docetaxel (3.12 nM), paclitaxel (6.25 nM), and vehicle treatments alone and with (E + D) EGCG (30 µM) plus docetaxel (3.12 nM) and (E + P) EGCG (30 µM) plus paclitaxel (6.25 nM) for 0, 12, 24, and 48 hours. Control cells were treated with vehicle. (B) Western blots with p21 and caspase 3 antibodies of crude extracts from PC-3ML cells treated with EGCG (30 µM), docetaxel (3.12 nM), paclitaxel (6.25 nM), and a combination of EGCG + docetaxel or EGCG + paclitaxel at these same dosages for 0, 24, and 48 hours. (A and B) Cells were ∼70% confluent at the time of initiation of treatments. Control blots were with β-actin antibodies. (C) Calculations of the ratio of protein/actin based on densitometric scans of Western blots for p53, p73, p21, and caspase 3. PC-3ML cells at ∼70% confluence were treated with EGCG, docetaxel, paclitaxel, EGCG + paclitaxel, EGCG + docetaxel at dosages shown in B for 0, 12, and 24 hours, respectively. Data represent the mean ± SD of measurements from three independent experiments.
EGCG in Combination with Taxane Can Increase Apoptosis Rates
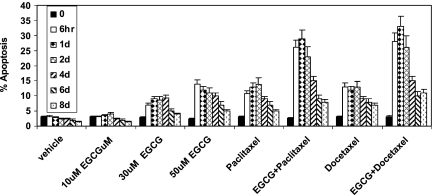
Assays were then carried out using flow cytometry with annexin V antibodies to assess whether EGCG and taxane had a synergistic activity in promoting apoptosis of PC-3ML cells. The percent apoptosis was measured after increased time intervals of 0 and 6 hours and after 1, 2, 4, 6, and 8 days, respectively (Figure 3). The data showed that treatment of PC-3ML cell with EGCG (30 µM) combined with either paclitaxel (6.25 nM) or docetaxel (3.12 nM) resulted in more than 25% apoptosis after 6 hours, 1 day, and 2 days compared with ∼6% to 10% apoptosis in the presence of the individual agents alone (Figure 3). However, the percent apoptosis observed decreased to less than 10% in the presence of EGCG and taxane or the individual agents alone after 6 to 8 days (Figure 3). Note that higher EGCG levels (50 µM) alone induced more than 10% apoptosis after 6 hours to 4 days compared with less than 8% apoptosis observed in the presence of 30 µM EGCG (Figure 3), indicating that higher dosages of EGCG may be more efficacious.
Figure 3.
Percent apoptosis by PC-3ML cells. PC-3ML cells (∼70% confluent) were treated with vehicle and increased dosages of EGCG (i.e., 10, 30, and 50 µM), paclitaxel (6.25 nm), and docetaxel (3.12 nm) alone for 0 and 6 hours and 1, 2, 4, 6, and 8 days, respectively.
Therapeutic Efficacy of EGCG Combined with Taxane In Vivo
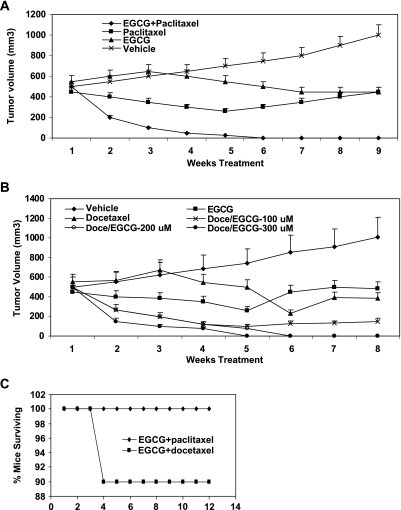
The antitumor activities of EGCG in combination with taxane was assessed in the PC-3ML mouse xenograft model. We have shown previously that PC-3ML cells injected i.p. (1 x 106 cells in 1 ml) rapidly form tumors after ∼2 week (i.e., >0.5 cm3) [6]. In mice repeatedly dosed i.p. with EGCG at 200 µM (228 mg/kg) biweekly (starting at week 1), tumor growth was reduced significantly and mouse survival rates increased dramatically from 5 to 6 weeks to more than 3 months [6]. Unfortunately, EGCG (228 mg/kg) alone only slowed growth of established tumors (i.e., 0.5 cm3). In this study, we have examined whether tumors established i.p. could be eliminated or reduced in size with treatments of EGCG and taxane combined. For these experiments, established human prostate tumors (PC-3ML cells) of ∼0.5 cm3 were treated with vehicle, EGCG (200 µM/animal), paclitaxel (20 mg/kg), or both EGCG and paclitaxel for 9 weeks by i.p. injection on a weekly basis.
Measurements of tumor volumes after 1 to 9 weeks showed that there was a significant decrease in tumor volume compared with the controls (vehicle) in mice treated with EGCG (200 µM, 228 mg/kg) plus paclitaxel (20 mg/kg) after 2, 3, 4, 5, and 6 to 9 weeks, respectively (i.e., >67%, 85%, 96%, 99%, and 100%, respectively) (Figure 4A). Likewise, similar results were observed in mice treated with EGCG plus docetaxel (12.5 mg/kg) (data not shown). In comparison, in the presence of EGCG alone, tumor growth was not reduced after 2 to 3 weeks but was reduced to near zero after prolonged intervals of 4 to 9 weeks of treatment. Paclitaxel (or docetaxel) alone also reduced tumor growth rates to near zero at all stages of the experiment (Figure 4A). However, EGCG, paclitaxel, or docetaxel did not independently reduce tumor volumes significantly or eradicated tumors (Figure 4A). Overall, comparisons of the combined therapy to EGCG or paclitaxel (or docetaxel) alone indicated that the effects of EGCG plus paclitaxel were statistically significant (P > .05).
Figure 4.
(A and B) In vivo efficacy of EGCG and taxane against established PC-3ML xenografts in CB17 SCID mice. (A) PC-3ML tumor volume in mice treated with either vehicle, EGCG (200 µM, or 228 mg/kg), paclitaxel (20 mg/kg), or 200 µM EGCG and paclitaxel (20 mg/kg) for 1 to 9 weeks. (B) PC-3ML tumor volume in mice treated with vehicle, EGCG (200 µM, 228 mg/kg), docetaxel (5 mg/kg), or increased amounts of EGCG (100, 200, 300 µM) plus docetaxel (5 mg/kg). Tumor volumes were measured by sacrificing animals each week (n = 3/time point/treatment). Drug was administered on a weekly basis i.p. starting on week 3 after inoculation of mice with PC-3ML cells (1 x 106 cells/animal i.p.), and treatment was continued for 1 to 9 weeks. Data represent the mean ± SD of tumors found i.p. in mice (n = 5/data point). (C) Mouse survival rates after treatment of mice for 8 weeks with EGCG (300 µM) plus docetaxel (12.5 mg/kg) or paclitaxel (20 mg/kg) (see methods in B). After discontinuation of treatment, mouse survival was monitored for an additional 12 weeks (n = 10 mice/treatment). Note: Control mice treated with EGCG or taxane alone failed to survive more than 1 to 2 weeks after treatment (data not shown).
The combination drug therapy studies were expanded to determine whether increased dosages of EGCG in combination with docetaxel (12.5 mg/kg) might increase efficacy. We found that with increased dosages of EGCG (i.e., in a range of 100–300 µM), the antitumor activity increased significantly at dosages of 200 to 300 µM EGCG compared with the lower dosages of 100 µM EGCG tested (P > .05) (Figure 4B). In fact, EGCG at concentrations greater than 200 to 300 µM (i.e., in combination with docetaxel) seemed to eliminate the tumors after 5 to 8 weeks (Figure 4B). Finally, mouse survival studies showed that mice normally died of tumor burden after 9 weeks treatment with EGCG or taxane alone, but less than 10% of the mice exhibited tumors or died in the groups treated with EGCG plus taxane for 8 weeks followed by discontinuation of the treatment for an additional 12 weeks (Figure 4C).
Expression of p53, p73, and p21 In Vivo
Western blots showed that freshly established PC-3ML tumors (i.e., 2 weeks after inoculation of mice i.p.) normally expressed little or no p53, p73, and p21 protein (Figure 5). However, after treatment of the mice with daily injections i.p. of EGCG, paclitaxel, or docetaxel alone, the expression of p53 and p73 was induced to more than one-fold, three-folds, and five-folds, respectively, by each of these drugs after 2 or 4 days of treatment (Figure 5) (i.e., compared with untreated cells). In addition, treatment with the individual agents had little or no effect on p21 expression, however. In comparison, EGCG plus taxane increased the levels of p53, p73. and p21 by more than 5- to 10-fold after 2 and 4 days of treatment, respectively (Figure 5). In sum, the data suggest that increased apoptotic gene expression may be associated with EGCG- and taxane-induced apoptosis.
Figure 5.
Western blots of p53, p73, and p21 expression in extracts from freshly established PC-3ML tumors (i.e., 2 weeks after inoculation of mice with PC-3ML cells). Mice were treated with EGCG, paclitaxel, docetaxel, EGCG + paclitaxel (E + P), EGCG + docetaxel (E + D) injected i.p. daily for 0, 2, and 4 days. Control blots with β-actin antibodies. Drug concentrations were 200 µM EGCG, 20 mg/kg paclitaxel, and 5 mg/kg docetaxel.
TUNEL Assays to Assess Drug Effects on Apoptosis
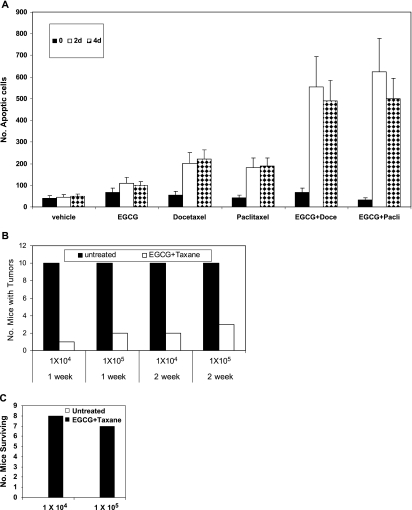
The percent apoptosis was measured on histologic sections of tumors using the TUNEL assay. Three tumors from each group of mice treated with vehicle, EGCG, docetaxel, or both EGCG and docetaxel for 0, 2, and 4 days were evaluated (Figure 6A). Relatively few apoptotic cells were detected on day 0 (untreated tumors) and in the vehicle-treated tumors on days 0, 2, and 4 (Figure 6A). A comparatively large number of apoptotic cells were present in the tumors exposed to EGCG (i.e., >90 cells/field of view), docetaxel, and paclitaxel (>200 cells/field of view) on days 2 and 4 (Figure 6A). More importantly, in tumors treated with EGCG and docetaxel or EGCG plus paclitaxel, the combined treatment produced a significant increase in the numbers of apoptotic cells (>500 cells/field of view) in tumors treated for 2 and 4 days (Figure 6A).
Figure 6.
(A) Summary of data from TUNEL assays of apoptotic cells in tumors treated with vehicle, EGCG, docetaxel, paclitaxel, EGCG plus docetaxel, and EGCG plus paclitaxel for 0, 2, and 4 days. Using a 40x objective, the numbers of apoptotic cells were counted in five fields of view in three different tumors. Data represent the mean ± SD of numbers of apoptotic cells/field of view. Note: We avoided counting apoptotic cells in areas of necrosis, but the necrotic areas were more prevalent in tumors treated with EGCG plus taxane than in tumors treated with each agent alone. (B) PC-3ML cell metastases to the bone marrow in CB17 SCID mice. Mice (male mice, 8–10 weeks of age) were injected i.v. via the tail vein with 1 x 104 and 1 x 105 PC-ML cells in 0.1 ml of DMEM. After 1 or 2 weeks, mice were then treated with EGCG (228 mg/kg) plus paclitaxel (20 mg/kg) given i.p. biweekly. Mice were treated for 2 months and killed to evaluate tumor metastases by gross dissection and histology. n = 10 mice/experiment. (C) Mouse survival rates after treatment. Mice were injected with PC-3ML cells at 1 x 104 and 1 x 105 cells/ml in 0.1 ml for 2 weeks. Mice were untreated (empty bar) or treated with EGCG + paclitaxel (solid bar) for 2 months according to methods in B. Then treatment was discontinued for 3 months before sacrifice of the mice.
Interestingly, we observed that in groups of mice treated with the combination therapy, the mice were healthier and approximately 24% heavier overall than those treated with EGCG or taxane alone (Table 1). Of particular interest is the transient weight loss caused by using docetaxel alone, from which animals are protected by the use of EGCG in combination with docetaxel (Table 1). Presumably, the improved weight gain is related to the reduced tumor burden.
Table 1.
Relative Body Weight.
| Week | Vehicle | EGCG | Docetaxel (12.5 mg/kg) | EGCG + Docetaxel |
| 0 | 100 | 100 | 100 | 100 |
| 3 | 95.22 | 91.22 | 74.32 | 94.06 |
| 6 | 99.6 | 93.41 | 79.45 | 128.33 |
| 10 | 110.05 | 103.70 | 104.00 | 134.21 |
EGCG Combined with Paclitaxel Blocks Bone Metastases by PC-3ML Cells
We have previously demonstrated that the injection of PC-3ML cells i.v. through the tail vein results in bone marrow metastases in CB17 SCID mice [9]. We have used this model to determine whether EGCG plus paclitaxel delivered i.p. can block metastatic tumor growth. Tumors were allowed to establish for 1 or 2 weeks before beginning treatment with drug i.p. biweekly for 2 months. Data in Figure 6B show that the numbers of mice with bone marrow tumors decreased from 100% in untreated mice (n = 10/10 mice) to less than 10% to 30% (i.e., 1/10, 2/10, or 3/10) mice with tumors, depending on the cell number injected or whether they were allowed 1 week versus 2 weeks to establish tumors before the onset of drug therapy (Figure 6B). Next, we examined whether mouse survival rates increased significantly after discontinuation of drug therapy. All of the untreated mice died within 6 to 7 weeks after inoculation (Figure 6C). In comparison, after treating the mice with drug for 2 months, with the discontinuation of treatment for 3 months, most mice survived (i.e., n = 8/10 and n = 7/10 mice survived) (Figure 6C). Those that died were tumor-bearing mice, and they died within ∼3 weeks of stopping the drug treatments. Dissection and histology examination of the bone marrows of the surviving mice indicated that they were free of any detectable tumor burden in the lumbar vertebrate, fibia, or soft organ tissues (i.e., muscle, lungs, liver, kidney, gut, intestine, mesenchymal, brain, and heart tissues).
Discussion
The treatment of human prostate metastatic cancer remains a formidable problem in as much as therapeutic advances have not significantly improved clinical outcome. The utilization of EGCG as a therapeutic agent has shown considerable promise in clinical trials [7,8]. In an earlier study, we found that EGCG alone was effective in preventing tumor growth from PC-3ML cells in SCID mice [6]. However, total eradication of the tumors and/or reduction in the volume of established tumors was not possible [6]. In efforts to augment the cytotoxic activity of EGCG, we have investigated a combination therapy using paclitaxel and docetaxel. In this article, we have shown that the combination therapy had a synergistic effect in decreasing cell viability and inhibiting the growth of PC-3ML cells in vitro. The combined drug treatment also increased apoptosis rates significantly from less than 3% in untreated cells to more than 25% in treated cells. More importantly, we found that the combination therapy had a synergistic additive effect in blocking growth and eradicating established tumors in mouse tumor modeling studies. A significant increase in overall, disease-free mouse survival rates was observed after treatment of mice harboring primary tumors (i.e., >90%) and metastatic lesions (>70%). The implication is that EGCG combined with taxane might be used to treat patients with advanced prostate cancer and metastatic disease.
From a clinical point of view, we also found that the combination drug therapy helped maintain healthier animals, characterized by increased body weight compared with groups treated with either agent alone. Second, toxicology studies failed to show synergistic or additive toxicity from the combined use of docetaxel and EGCG (data not shown).
Previous studies have shown that EGCG in combination with doxorubicin has a synergistic or an additive effect in preventing tumor growth in several cancer models [17]. Likewise, we have found that localized delivery of relatively high doses of EGCG (228 mg/kg or 200 µM) in combination with very low doses of doxorubicin (<0.14 mg/kg or 2 µM) can either eradicate or significantly reduce PC-3ML tumors growing i.p. in CB17 SCID mice [6]. Data showed that EGCG promoted doxorubicin retention by the tumor cells to increase efficacy. Consequently, it was feasible to treat mice frequently with relatively nontoxic dosages of doxorubicin, thereby increasing drug bioavailability and uptake by newly arising tumor cells. However, in these studies, the tumors were relatively small (<0.2 mm3) at the time treatment was initiated, and this may explain, in part, the success of the drug therapy. Other experiments in this study showed that attempts to treat large established tumors with EGCG and doxorubicin failed to eradicate the tumors [6], indicating that higher dosages of doxorubicin were required or a better drug combination was needed.
Green tea has previously been reported to have useful antioxidative effects and to inhibit carcinogenesis [17–21]. The antitumor effects of green tea are due to the components of green tea such as the catechin group, the vitamin group, caffeine, and theanine [18–24]. Specifically, green tea contains ascorbic acid (vitamin C), several B vitamins, riboflavin, niacin, folic acid, and pantothenic acid. Interestingly, there have also been several reports showing that different green tea components in combination with doxorubicin can block tumor growth [24,25]. Nagata [24] and Sadzuka et al. [24,25] found that theanine and caffeine inhibited doxorubicin efflux from Ehrlich ascites tumor cells and that theanine rendered doxorubicin resistant Ehrlich ascites tumors sensitive to doxorubicin and increased doxorubicin suppression of metastasis. They suggested that flavonoids enhanced the doxorubicin-induced antitumor activity and increase the doxorubicin concentration in tumors through the inhibition of doxorubicin efflux [26]. Green tea extracts in combination with doxorubicin also have been found to enhance the inhibitory effect of doxorubicin on Ehrlich ascites tumors in mice [26]. Likewise, EGCG was found to enhance the apoptotic effect of sulindac and tamoxifen in tumor cells [27]. These studies failed to address where EGCG in combination with a chemotherapeutic agent could eradicate established tumors.
Overall, the data suggest EGCG is a relatively nontoxic agent that can be used in combination with a chemotherapeutic drug to treat cancer. EGCG has minimal toxic adverse effects because it does not seem to accumulate in normal cells and tissue or to hamper their survival. Neither does the high dosages given to mice seem to have any toxic adverse effects (i.e., induce hair loss, neuropathy, serious weight loss, and tissue damage). More importantly, EGCG seems to readily accumulate in tumor cells and tumors, turning them brown. EGCG's primary activity is to block drug export, reduce tumor cell viability, induce apoptosis, sensitize cells to apoptotic effects of chemotherapeutic drugs, and block the expression of genes essential for invasion and metastases (i.e., MMP-2/9) [28,29].
Role of p53, p73, and p21 in Tumor Cell Apoptosis
More than 50% of all human cancers contain mutations in the p53 tumor suppressor gene [30]. In response to cellular stresses such as DNA damage and hypoxia, p53 is activated and can mediate cell cycle arrest and apoptosis through the up-regulation of numerous target genes [31]. p21WAF1, a potent inducer of cell cycle arrest [32], and Bax [33], initiators of the apoptotic cascade, are p53 target genes. Interestingly, p53 probably does not work alone and undoubtedly has partners with similar functional activities. One important clue was that p53-null mice maintain normal embryonic development [34], suggesting a family of closely related proteins with overlapping functions must exist. Recently, two new genes, TP73 and TRP63, which encode p73 and p63 proteins, have been identified which exhibit structures and functions related to p53 (for reviews, see Levrero et al. [35]). The first p53 homolog described was p73 [34], and p73 shares considerable sequence identity with p53, reaching 63% within the DNA-binding domain and including highly conserved DNA contact residues frequently mutated in tumors [36]. Mechanistic studies indicate that p73 can bind to the p53 consensus DNA-binding motif and activate a number of p53-regulated genes, including p21WAF1 [37]. p73 can also induce cell cycle arrest and apoptosis [37]. The differential regulation of p53 target genes by p73 indicates that, although the activities of p53 and p73 overlap, these two proteins also maintain separate and unique functions within a cell [37]. In any event, the data in this article clearly show that EGCG combined with taxane can promote the overexpression of both p53 and p73 proteins in PC-3ML cells and tumors. Specifically, we found that p53 levels increased several fold in response to treatment with EGCG, thereby increasing cell sensitivity to taxane-induced apoptosis. In response to the combined drug therapy, there was more than two- to eight-fold increases in p53 and p73 than those observed in cells treated with either docetaxel alone or EGCG alone. These cells also exhibited more than two-fold increases in p21 and caspase 3 levels and significant increases in the percent apoptosis in vitro and in vivo. Most likely, EGCG and taxane affect distinct yet complementary signaling pathways that promote the expression of genes involved in apoptosis (i.e., p53, p73, p21, caspase 3) and cell survival. Further investigation of the possible mechanism(s) modulating the synergistic activities of EGCG and taxane is in progress.
In summary, we have developed a novel therapeutic strategy for the treatment of prostate cancer with EGCG in combination with conventional chemotherapeutic agents. This combination therapy produced an additional therapeutic benefit over either individual modality. In particular, relatively low-dose taxane produced a synergistic effect with EGCG, which suggests an important possible neoadjuvant therapy for the treatment of prostate cancer. More importantly, with the bone metastases studies, we found that treatment of mice i.p. with the combination therapy reduced bone metastases and increased mouse survival rates by more than 70% to 80%.
In conclusion, based on the preclinical mouse tumor modeling studies presented here, we believe that EGCG might be effectively used at high dosages delivered locally to increase the efficacy of taxanes in eradicating highly aggressive and metastatic tumors in patients.
Footnotes
Supported by the National Institutes of Health/National Cancer Institute (CA076639-7) to M.E.S.
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.D'Amico AV, Whittington R, Malkowicz SB, Wu YH, Chen MH, Hurwitz M, Kantoff PW, Tomaszewski JE, Renshaw AA, Wein A, et al. Utilizing predictions of early prostate-specific antigen failure to optimize patient selection for adjuvant systemic therapy trials. J Clin Oncol. 2000;18:3240–3246. doi: 10.1200/JCO.2000.18.18.3240. [DOI] [PubMed] [Google Scholar]
- 3.Oh WK, Kantoff PW. Treatment of locally advanced prostate cancer: is chemotherapy the next step? J Clin Oncol. 1999;17:3664–3675. doi: 10.1200/JCO.1999.17.11.3664. [DOI] [PubMed] [Google Scholar]
- 4.Weitzman AL, Shelton G, Zuech N, Owen CE, Judge T, Benson M, Sawczuk I, Katz A, Olsson CA, Bafiella E, et al. Dexamethasone does not significantly contribute to the response rate of docetaxel and estramustine in androgen independent prostate cancer. J Urol. 2000;163:834–837. [PubMed] [Google Scholar]
- 5.Petrylak DP. Docetaxel (Taxotere) in hormone-refractory prostate cancer. Semin Oncol. 2000;27:24–29. [PubMed] [Google Scholar]
- 6.Stearns ME, Amatangelo MD, Varma D, Sell C, Goodyear SM. Combination therapy with epigallocatechin-3-gallate and doxorubicin in human prostate tumor modeling studies. Inhibition of metastatic tumor growth in SCID mice. Am J Pathol. 2010;177:3169–3179. doi: 10.2353/ajpath.2010.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanafelt TD, Call TG, Zent CS, LaPlant B, Bowen DA, Roos M, Secreto CR, Ghosh AK, Kabat BF, Lee MJ, et al. Phase I trial of daily oral polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J Clin Oncol. 2009;10:3808–3814. doi: 10.1200/JCO.2008.21.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu H, He J, Mei F, Zhang Q, Hara Y, Ryota S, Lubet RA, Chen R, Chen DR, You M. Lung cancer inhibitory effect of epigallocatechin-3-gallate is dependent on its presence in a complex mixture (polyphenon E) Cancer Prev Res (Phila) 2009;2:531–537. doi: 10.1158/1940-6207.CAPR-08-0185. [DOI] [PubMed] [Google Scholar]
- 9.Stearns ME, Wang M. Isolation and characterization of PC-3 human prostatic tumor sublines which preferentially metastasize to select organs in s.c.i.d. mice. Differentiation. 1991;48:115–125. doi: 10.1111/j.1432-0436.1991.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 10.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 11.Stearns ME, Jason B, Zhang H, Francis MK, Sell C. Insulin-like growth factor 1 (IGF-1) induction of vascular endothelial growth factor (VEGF) is dependent upon activated Ras. Cancer Res. 2005;65:2085–2088. doi: 10.1158/0008-5472.CAN-04-4100. [DOI] [PubMed] [Google Scholar]
- 12.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual, Biotechnology; Molecular Biology; Biochemistry; Laboratory Manuals. 3rd ed. Vol. 1. Cold Spring Harbor, NY: Cold Spring Harbor Press; 2003. pp. 2.82–2.108. [Google Scholar]
- 13.Chahinian AP, Mandell JP, Gluck H, Naim H, Teirstein AS, Holland JF. Effectiveness of cisplatin, paclitaxel, and suramin against human malignant mesothelioma xenografts in athymic nude mice. J Surg Oncol. 1998;67:104–111. doi: 10.1002/(sici)1096-9098(199802)67:2<104::aid-jso6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Riondel J, Jacrot M, Picot F, Beriel H, Mouriquand C, Potier P. Therapeutic response to taxol of six human tumors xenografted into nude mice. Cancer Chemother Pharmacol. 1986;17:137–142. doi: 10.1007/BF00306742. [DOI] [PubMed] [Google Scholar]
- 15.Steel GG, Peckham MJ. Exploitable mechanisms in combined radiotherapy-chemotherapy: the concept of additivity. Int J Radiat Oncol Biol Phys. 1993;5:85–91. doi: 10.1016/0360-3016(79)90044-0. [DOI] [PubMed] [Google Scholar]
- 16.Aoe K, Kiura K, Ueoka H, Tabata M, Matsumura T, Chikamor IM, Matsushita A, Kohara H, Harada M. Effect of docetaxel with cisplatin or vinorelbine on lung cancer cell lines. Anticancer Res. 1999;19:291–299. [PubMed] [Google Scholar]
- 17.Kelloff GJ, Crowell JA, Hawk ET, Steele VE, Lubet RA, Boone CW, Covey JM, Doody LA, Omenn GS, Greenwald P, et al. Clinical development plan: tea extracts. Green tea polyphenols, epigallocatechin gallate. J Cell Biochem Suppl. 1996;26:236–257. [PubMed] [Google Scholar]
- 18.Bushman JL. Green tea and cancer in humans: a review of the literature. Nutr Cancer. 1998;31:151–159. doi: 10.1080/01635589809514697. [DOI] [PubMed] [Google Scholar]
- 19.Mukhtar T, Wang Z, Katiyer SK, Agarwal R. Tea components: antimutagenic and anticarcinogenic effects. Prev Med. 1992;21:351–360. doi: 10.1016/0091-7435(92)90042-g. [DOI] [PubMed] [Google Scholar]
- 20.Katiyer SK, Agarwal R, Mukhtar H. Inhibition of spontaneous and photo-enhanced lipid peroxidation in mouse epidermal microsomes by epicatechin derivatives from green tea. Cancer Lett. 1994;79:61–66. doi: 10.1016/0304-3835(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 21.Nishida H, Omori M, Fukutomi Y, Ninomiya M, Nishiwaki S, Suganuma M, Moriwaki H, Muto Y. Inhibitory effects of (-)-epigallocatechin gallate on spontaneous hepatoma in C3H/HeNCrj mice and human hepatoma-derived PLC/PRF/5 cells. Jpn J Cancer Res. 1994;85:221–225. doi: 10.1111/j.1349-7006.1994.tb02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagesaka Y, Sugiura T, Miwa Y, Yamaguchi K, Kyuki K. Effect of tea-leaf saponin on blood pressure of spontaneously hypertensive rats. Yakugaku Zasshi. 1996;116:388–395. doi: 10.1248/yakushi1947.116.5_388. [DOI] [PubMed] [Google Scholar]
- 23.Sano M, Takahashi Y, Yoshino K, Shimoi K, Nakamura Y, Tomita I, Oguni I, Konomoto H. Effect of tea (Camellia sinensis L. on lipid peroxidation in rat liver and kidney: a comparison of green and black tea feeding. Biol Pharm Bull. 1995;18:1006–1008. doi: 10.1248/bpb.18.1006. [DOI] [PubMed] [Google Scholar]
- 24.Nagata T. Studies on useful components of tea in leaves of the genus Camellia. Bull Natl Res Inst Tea. 1986;21:60–120. [Google Scholar]
- 25.Sadzuka Y, Sugiyama T, Sonobe T. Efficacies of tea components on Dox induced antitumor activity and reversal of multidrug resistance. Toxicol Lett. 2000;114:155–162. doi: 10.1016/s0378-4274(99)00290-8. [DOI] [PubMed] [Google Scholar]
- 26.Sadzuka Y, Sugiyama T, Hirota S. Modulation of cancer chemotherapy by green tea. Clin Cancer Res. 1998;4:153–156. [PubMed] [Google Scholar]
- 27.Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Comparative effects of EGCG, green tea and a nutrient mixture on the patterns of MMP-2 and MMP-9 expression in cancer cell lines. Oncol Rep. 2010;24:747–757. doi: 10.3892/or_00000917. [DOI] [PubMed] [Google Scholar]
- 28.Farabegoli F, Papi A, Orlandi M. (-)-Epigallocatechin-3-gallate down-regulates EGFR, MMP-2, MMP-9 and EMMPRIN and inhibits the invasion of MCF-7 tamoxifen-resistant cells. Biosci Rep. 2011;31:99–108. doi: 10.1042/BSR20090143. [DOI] [PubMed] [Google Scholar]
- 29.Suganuma M, Okabe S, Kai Y, Sueoka N, Sueoka E, Fujiki H. Synergistic effects of (-)-epigallocatechin gallate with (-)-epicatechin, sulindac, or tamoxifen on cancer-preventive activity in the human lung cancer cell line PC-9. Cancer Res. 1999;59:44–47. [PubMed] [Google Scholar]
- 30.Hollstein M, Rice K, Greenblatt MS, Soussi T, Fuchs R, Sorlie T, Hovig E, Smith-Sorensen B, Montesano R, Harris CC. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994;22:3551–3555. [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR. The p53 network. J Biol Chem. 1998;273:14–23. doi: 10.1074/jbc.273.1.1. [DOI] [PubMed] [Google Scholar]
- 32.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 33.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 34.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumors. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 35.Levrero M, De Laurenzi V, Costanzo A, Gong J, Wang JY, Melino G. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci. 2000;113:1661–1670. doi: 10.1242/jcs.113.10.1661. [DOI] [PubMed] [Google Scholar]
- 36.Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, Minty A, Chalon P, Lelias JM, Dumont X, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J, Jiang J, Zhou W, Chen X. The potential tumor suppressor p73 differentially regulates cellular p53 target genes. Cancer Res. 1998;58:5061–5065. [PubMed] [Google Scholar]