INTRODUCTION
Viral respiratory tract infections are a major cause of asthma exacerbations in adults and children.1,2 Rhinoviruses (HRV) are frequently identified, especially among severe exacerbations requiring hospitalization.3,4 The precise mechanism that HRV elicits asthma exacerbations has not been fully explained, but many theories exist.5 The role of leukotrienes in the process has been postulated as elevations have been noted during viral infections, including those caused by HRVs.6 Furthermore, leukotriene receptor antagonists have been shown in a few studies to reduce asthma symptoms or exacerbations with colds in children.7-9
These studies suggest the possibility that montelukast, a leukotriene receptor antagonist, could reduce the severity of HRV illnesses in asthma subjects and if so, the mechanism by which this occurs. To test this hypothesis, we conducted a pilot study in which a group of subjects with allergic asthma were randomized to receive either montelukast or placebo, and then experimentally inoculated with HRV-16. The primary outcome was the infection-related change in asthma control diary scores. Secondary outcomes included cold symptoms, and analysis of secretions for viral shedding, leukotrienes and cellular inflammation. Experimental infection with HRV-16 was used to minimize confounding from response variation to the many strains of HRV as well as other viruses found in the community.
METHODS
Subjects
Between November 2006 and December 2008, 171 subjects were recruited by advertisement and tested for HRV-16 serology; 63 had no detectable antibody to HRV-16, and 42 seronegative consented to the study procedures. At visit 2, 19 subjects were removed from the study for the following reasons: 13 had no evidence of methacholine reactivity (PC20>8) or reversible airway obstruction, 3 had FEV1 below the study entry criteria, 2 had clinical symptoms of a cold that day and 1 had been started on omaluzimab since the first visit. At visit 3, three additional subjects dropped out; 2 could not make the visits, and 1 had a low FEV1. Twenty subjects (8 who received montelukast treatment and 12 who received placebo treatment) completed the study. One patient was not included in the statistical analysis because of a positive test for HRV-16 antibody at the randomization visit. Nineteen subjects were included in the statistical analysis.
The study included healthy adults between 18 and 65 years who had a diagnosis of mild persistent asthma for greater than six months, were allergic based on at least one positive skin prick to a standard panel of common allergens (wheal size > 3 mm greater than negative control), were able to produce induced sputum, and had pre-bronchodilator FEV1 ≥80% predicted at baseline. Subjects used only inhaled short-acting beta-agonist inhalers as needed. Subjects qualified based on reversibility post-bronchodilator ≥ 12% or bronchial hyper responsiveness to methacholine PC20 ≤8 mg/ml.10 Subjects were excluded if they had any of the following: history of severe asthma exacerbations with respiratory infections, serum HRV-16 antibody titer ≥ 1 at screening, use of asthma controller medication such as montelukast or inhaled corticosteroid, current smoker or more than 5 pack-year history, currently receiving immunotherapy, pregnant, breastfeeding, or currently participating in another clinical trial. All subjects provided written informed consent, and the study was approved by University of Wisconsin Health Sciences Institutional Review Board. The trial was registered on clinicaltrials.gov.
Study design
The study was a randomized double-blind placebo-controlled trial designed to test whether montelukast would lessen asthma symptoms associated with experimental inoculation of seronegative volunteers with mild allergic asthma with HRV-16. At screening, subjects had a physical examination, allergy skin prick testing, blood draw for HRV-16-neutralizing antibody, urine pregnancy test, pulmonary function tests before and after inhalation of albuterol, and diary cards were distributed. Specimens were collected at baseline, during the acute cold and upon recovery (Table I). Peak flow meters were issued at the beginning of the study. Subjects filled out peak flow data, asthma and cold symptom diaries daily for 7 days prior to randomization, 7 days prior to inoculation and then 14 days after inoculation. Nasal lavage, sputum and blood were collected at each visit after screening. Cell counts with differential were done on nasal lavage, sputum and blood samples. Viral titers and RNA were quantitated in samples of nasal lavage samples. An attempt was made to measure cysteinyl leukotriene in the sputum but based on quality control measures; leukotriene values were not stable after freezing.
Table I.
Study Design
| Screen | Randomize | Acute Cold | Convalescence | ||||
|---|---|---|---|---|---|---|---|
| Visit | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Day | −14 | −7 | 0 | 3 | 8 | 14 | 21 |
| Randomize | X | ||||||
| RV16 Inoculation |
X | ||||||
| Nasal Lavage | X | X | X | X | X | X | |
| Sputum Induction | X | X | X | X | X | X | |
| Blood Draw | X | X | X | X | X | X | |
| Spirometry | X | X | X | X | X | X | X |
HRV 16 Inoculation
None of the subjects had neutralizing antibody to HRV16 at the time of screening, although 1 subject had a low titer of HRV16-neutralizing antibody detected in the serum obtained on the day of inoculation (1.4). This patient was excluded from our analysis. Samples on the day of inoculation were tested for other respiratory viruses by multiplex PCR (Respiratory MultiCode Assay, EraGen BioSciences, Madison, WI).11 If a HRV was detected, partial sequencing was performed to determine strain.12 HRV16 inoculation was performed with 1,000 TCID50 units administered by spraying 0.25 mL by atomizer into each nostril as previously described.13
Cold and asthma symptom scores
The Jackson scale was utilized to assess the 13 cold symptoms (cough, nasal discharge, sneezing, stuffy nose, sore throat, headache, malaise, chilliness, shaking chills, fever, laryngitis, aching joints or muscles, and watery or burning eyes) on a four-point scale (0=not present, 1=mild, 2=moderate, 3=severe).14 The symptoms were scored twice daily, and the highest value for each symptom was used to calculate the total daily highest symptom score (TDHSS, maximum possible daily score=39). Cold symptoms were also recorded on the validated Wisconsin Upper Respiratory Symptom Survey-21 (www.fammed.wisc.edu/wurss).15
Asthma symptoms were assessed twice a day with patients completing a validated daytime diary card prior to bed and a nocturnal diary card upon awakening.16 For the daytime diary, subjects answered four questions: how often they experienced asthma symptoms (0=none of the time, 6=all of the time), how much their asthma symptoms bothered them (0=not at all, 6=severely bothered), how much activity they could do (0=more than usual, 6=less than usual) and how often their asthma affected their activities (0=none of the time, 6=all of the time). Daily scores were calculated as the average of the four questions and an overall score for the week was assessed as the average of the daily scores. For the nocturnal diary, subjects recorded how often they woke up with asthma symptoms (0=none, 1=once, 2=more than once, 3=awake all night). Weekly average scores were computed from the nocturnal diary. Additionally, subjects measured peak flows and recorded albuterol use upon awakening and just prior to bed.
Nasal lavage, sputum induction and virology
Nasal lavage13, sputum induction14 and processing of samples14 were performed as reported previously. Viral titers from nasal lavage were calculated after four tissue culture tubes containing WI38 cells (human lung diploid cells, ViroMed, Minnetonka, MN) were inoculated for each serial 10-fold dilution of sample (100-10−7) and incubated while rolling at 33°C for 10 days. Tubes were read at 24 hours and then every other day up to day 10. TCID50 was calculated as the concentration that was capable of infecting 50% of the tubes. Viral titers were expressed as TCID50/mL. Cell counts and differentials were made from nasal lavage and sputum samples after treatment with 0.1% dithiothreitol.
Total RNA was extracted from nasal lavage samples and reverse transcribed, and the quantity of HRV RNA was measured as reported previously.17 The standard curve was developed by extracting HRV16 dilutions of known concentration ranging from 1 PFU to 1 × 106 PFU; 1 PCR unit on the standard curve was defined as 1 PFU (~130 copies of RNA).
Statistical Analysis
Group characteristics and outcomes including height, weight, age, peak flow, sputum and nasal cell counts and percentages, cold and asthma symptom scores, viral shedding and RNA, and albuterol puffs, were compared using the Wilcoxon rank-sum test. Categorical outcomes including race, gender, and detection of nasal eosinophils were compared using Fisher’s exact test. For daily-recorded data (symptom scores, peak flow, albuterol use) both the mean and maximum were calculated for each of four study weeks (baseline, randomization, acute, convalescent) and compared between groups. Continuous outcomes are summarized as median [1st quartile, 3rd quartile]. A two-sided p-value less than 0.05 was regarded as statistically significant.
RESULTS
Subject characteristics
Twenty subjects completed the study; 9 received montelukast and 11 received placebo. (Table II). One subject from the montelukast group had no detectable virus in nasal secretions, but that subject had a virus detected in nasal lavage by RT-PCR at inoculation and was therefore excluded from the analysis.
Table II.
Demographics
| Placebo N=11 |
Montelukast N=8 |
P-value | |
|---|---|---|---|
| Height (cm) | 173 [169, 177] | 176 [174, 183] | 0.20 |
| Weight (kg) | 70 [62, 90] | 73 [65, 80] | 0.84 |
| Age (yrs) | 21 [20, 23] | 20 [19, 21] | 0.50 |
| Gender | 4F, 7M | 3F, 5M | 1.00 |
| Race | 10C, 1AA | 8C | 1.00 |
| PEF (ml/min) | 445 [402, 566] | 469 [439, 519] | 0.49 |
When comparing the placebo and montelukast groups, patients were predominately in their twenties (median [1st quartile, 3rd quartile] age in years 21 [20, 23], 20 [19, 21], p=0.50) and were mainly Caucasian (10 Caucasian, one African American vs. 8 Caucasian, p=1.00). There was a similar male to female ratio with 4 females and 7 males in the placebo group and 3 females and 5 males in the montelukast group (p=1.00). Height (173 [169, 177] vs. 176 [174, 183], p=0.20) and weight (70 [62, 90] vs. 73 [65, 80], p=0.84) were not significantly different.
Cold symptoms
Subjects in both groups had similar peak cold symptom scores during HRV infection (TDHSS: placebo 5.9 [4.2, 10.1] vs. montelukast 7.3 [5.6, 7.9]; p=0.74). When comparing the placebo group to the montelukast group, both had an increase in cold symptom scoring from randomization to acute illness and there was no significance in the change observed between these two groups (change from randomization to acute phase in placebo 4.9 [4.0, 8.2] vs. montelukast 5.3 [4.3, 6.2]; p=0.70). Similar results were seen when assessing the WURSS-21 diary completed by both groups. Mean symptom scores during acute illness in placebo group 15.8 [9.0, 32.1] were not significantly different from the montelukast group (12.9 [11.0, 24.5]; p=1.00) Again, there was an increase in symptoms scores from randomization to acute illness and no significant difference between the two groups (change from randomization to acute phase in placebo 11.5 [7.3, 31.4] vs. montelukast 12.7 [9.2, 23.7], p=0.90).
Viral shedding
Subjects in both groups had similar peak viral shedding during the infection (placebo log10 3.5 [2.5, 4.5] TCID50/mL vs. montelukast log10 2.5 [2.3, 4.5], p=0.50). Both groups had similar levels of nasal RV-16 RNA at the peak of the acute cold on day 3 (placebo vs. montelukast: log10 7.9 [7.2, 8.6] vs. 8.2 [6.8, 8.8] PCR units; p=0.84) and upon recovery on day 10 (placebo vs. montelukast: log10 6.1 [5.2, 6.8] vs. 5.9 [5.7, 6.7] PCR units; p=0.97). There was a significant decrease in nasal HRV RNA between the acute cold and recovery in both groups (placebo p=0.002; montelukast p=0.04).
Asthma symptoms and peak flows
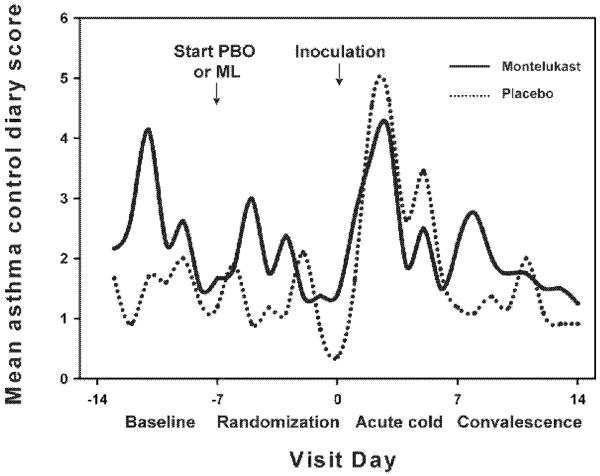
No subject developed a severe asthma exacerbation during this study that required oral corticosteroids, urgent or emergency room care, or hospitalization. The placebo and montelukast groups had no significant differences in mean daily asthma symptoms at baseline (placebo vs. montelukast: 1.0 [0.2, 1.4] vs. 0.3 [0.0, 3.8], p=0.83), randomization (0.3 [0.0, 1.6] vs. 0.5 [0.0, 2.0], p=0.90), during the acute illness phase (2.3 [0.0, 4.9]; 2.1 [0.1, 4.1], p=0.83) or during convalescence (0.1 [0.0, 1.3] vs. 0.7 [0.0, 2.6], p=0.77). Albuterol use was similarly low between the groups at baseline (0.0 [0.0, 0.1] puffs for placebo and 0.1 [0.0, 0.2] puffs for montelukast, p=0.42) and during the acute cold (0.0 [0.0, 0.5] puffs for placebo and 0.3 [0.1, 0.6] puffs for montelukast, p=0.32).
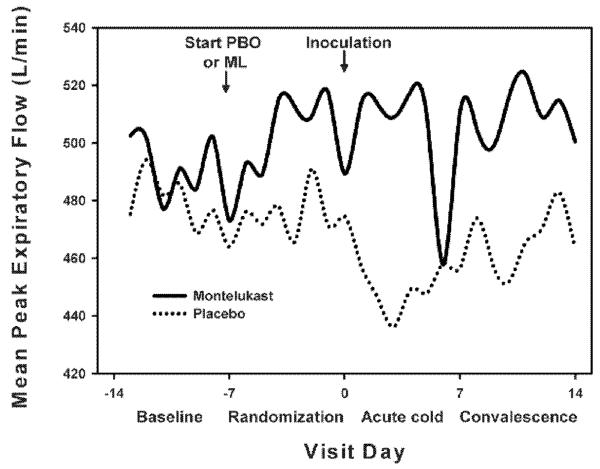
Peak expiratory flows (PEF) obtained did not differ between the two groups at any one point in time. However, when comparing change in mean PEF from randomization to acute illness, the infection-induced drop in mean PEF was greater in the placebo group (placebo −31 [−37, −6] vs. montelukast: 5 [−13, 19] l/min, p=0.05). The infection-induced drop in maximum PEF was similarly affected (placebo −16 [−27, −15] vs. montelukast −3 [−7, 1] l/min, p=0.01).
Cellular inflammation
Montelukast treatment had no effects on eosinophil counts during the acute cold (Table III). During the recovery phase (14 days after inoculation) eosinophils were increased in the placebo group, but not in the treatment group (placebo 2.0% [0.3, 2.7], montelukast 0.3% [0.1, 0.3], p=0.05, Table III.) Eosinophils were also detectable in nasal lavage in some subjects but no significant difference was observed between the two groups during any point in the study.
Table III.
Effect of Montelukast on Virus-Induced Changes in Sputum Eosinophils (% of total cell count)
| Visit | Placebo N=11 |
Montelukast N=8 |
P-value |
|---|---|---|---|
| 2 (day −7) | 0.3 [0.0, 1.2] | 0.7 [0.4, 6.0] | 0.30 |
| 3 (day 0) | 0.3 [0.3, 1.7] | 0.7 [0.7, 1.3] | 0.68 |
| 4 (day 3) | 0.5 [0.2, 3.4] | 0.3 [0.0, 1.0] | 0.62 |
| 5 (day 8) | 0.4 [0.3, 0.7] | 0.3 [0.0, 0.6] | 0.31 |
| 6 (day 14) | 2.0 [0.3, 2.7] | 0.3 [0.1, 0.3] | 0.05 |
| 7 (day 21) | 0.9 [0.3, 2.4] | 0.0 [0.0, 1.5] | 0.25 |
In sputum, neutrophils were detectable and no significance difference was observed between the two groups at baseline, during the acute infection or during convalescence. In nasal lavage samples, a significant difference was observed in neutrophils both 3 days (% neutrophils in placebo vs. montelukast: 80 [72, 88] vs. 91 [87, 93], p=0.05) and 8 days (% neutrophils in placebo vs. montelukast: 91 [84, 91] vs. 92 [91, 94], p=0.05) after inoculation with HRV16.
DISCUSSION
Previous studies have shown that starting treatment with montelukast prior to a known viral illness season can lessen asthma symptoms.8 The primary goal of this study was to use a model of experimental infection to determine whether montelukast would reduce virus-induced asthma and cold symptoms in subjects with mild allergic asthma, and to explore possible mechanisms for beneficial effects. In fact, montelukast had no significant effects on virus-induced asthma or cold symptoms; however, there were some changes in preselected secondary outcomes. When patients were started on montelukast prior to inoculation with HRV16, a decrease in peak expiratory flow was observed in the placebo group between randomization and the acute cold period. The group of patients on montelukast did not have a drop in their PEF which suggests montelukast had a protective effect on lung function. There was no appreciable effect on viral shedding. These findings suggest that leukotrienes may be involved in virus-induced reductions in lung function, and this effect is not mediated by a reduction in viral replication.
Montelukast also affected the course of eosinophilic inflammation. Eosinophils tend to increase during the convalescence phase following an acute URI in patients with allergic airway disease,18 and in the montelukast group, there was a decrease in the number of sputum eosinophils during the convalescence phase. This finding is in agreement with other studies showing that montelukast can affect eosinophilic inflammation in the airways. For example, a recent study conducted in Korea showed a significant decrease in eosinophil derived neurotoxin (EDN) levels in patients started on montelukast after an RSV infection. Furthermore, montelukast-induced reductions in EDN levels were associated with a significant decrease in wheezing episodes in follow-up.19 Furthermore, the presence of eosinophils in the airways has been identified as a risk factor for more severe colds in previous studies.10,20 These findings suggest that montelukast effects to reduce airway eosinophils could contribute to clinical benefits in the context of viral infection.
Previous studies of montelukast effects during acute colds have produced conflicting results. In patients with acute RSV bronchiolitis, montelukast had no effects on respiratory symptoms, including wheezing and cough.21,22 In contrast, school-age children with mild allergic asthma were treated with either montelukast or placebo prior to starting school in the fall.23 This was done in anticipation of exposure to HRV upon returning to school. Children were monitored for symptoms after a natural community-acquired cold, and montelukast therapy was associated with a significant decrease in days with worsening asthma symptoms and unscheduled physician visits. Furthermore, in preschool children with recurrent wheeze, starting montelukast at the first sign of upper respiratory illness produced a modest reduction in wheezing, trouble breathing, and activity limitation.24 These findings suggest that cysteinyl leukotrienes contribute to cold-induced asthma exacerbations. In the present study, we serially monitored cold symptom scores and viral shedding after inoculation and found no evidence that montelukast affected the course of upper respiratory illness. This leads us to believe montelukast does not potentiate antiviral pathways that are important for HRV infections. In our study, there were no differences in lower airway symptoms either, although lower airway symptoms are generally mild after experimental inoculation of volunteers with mild asthma, and this low signal may have impaired our ability to detect montelukast effects on virus-induced chest symptoms.
Advantages of this study design are that standardized inoculation procedures limit the variability related to infection dose, strain, and timing. Inoculating patients with HRV allows for precise monitoring of viral shedding, inflammatory changes and symptoms at specified intervals relative to the time of inoculation. We also excluded patients taking other controller medications that could mask effects of virus on the upper or lower airways. One limitation to this study is that the study subjects had mild asthma and were relatively stable at the time of inoculation. Responses to infection would be more pronounced in patients with more severe or poorly controlled asthma. Patients with moderate to severe asthma may be most likely to benefit from prevention of asthma exacerbation from viral illnesses, and this limits our ability to generalize our findings to all patients with asthma. In addition, this was a pilot study, and the small sample size limits the statistical power of the study and does not allow for subgroup analysis.
Overall, we found that montelukast did not improve asthma control or cold symptom scores caused by experimental rhinovirus infection. Secondary findings provide further evidence that montelukast can attenuate eosinophilic inflammation in the airway, and also indicate that leukotriene inhibitors may protect lung function during viral respiratory infections. Based on these findings and other studies linking blood and sputum eosinophils to more severe viral illnesses,20 additional studies may be warranted to determine whether montelukast might be useful in preventing asthma exacerbations with viral illness in patients who have eosinophilic airway inflammation.
Figure 1.
Mean asthma symptom score at baseline, after randomization, during the acute cold, and during recovery in subjects on montelukast (solid line) and those on placebo (dotted line).
Figure 2.
Median morning peak flows at baseline, after randomization, during the acute cold, and during recovery in subjects on montelukast (solid line) and those on placebo (dotted line).
Acknowledgments
This study was supported by an investigator initiated research grant from Merck Inc Clinical Trials Registry www.clinicaltrials.gov NCT00359073 and in part by a grant from the NIH, award number: T32 AI007635.
Glossary of abbreviations and acronyms
- HRV
Human Rhinovirus
- FEV1
Forced expiratory volume in one second
- PC20
Provocative concentration of methacholine producing a 20% decline in FEV1
- PEF
Peak expiratory flow
- PCR
Polymerase chain reaction
- TCID50
Tissue Culture Infectious Dose that produces pathological change in 50% of cell cultures
- TDHSS
Total daily highest symptom score
- RNA
Ribonucleic acid
- PFU
Plaque-forming unit
- WURSS-21
Wisconsin Upper Respiratory Symptom Survey
- URI
Upper respiratory infection
- EDN
Eosinophil derived neurotoxin
Footnotes
The authors listed above have no conflicts of interest to claim outside of the funding from Merck to conduct the study.
References
- 1.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedlander SL, Busse WW. The role of rhinovirus in asthma exacerbations. J Allergy Clin Immunol. 2005;116:267–273. doi: 10.1016/j.jaci.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Miller EK, Edwards KM, Weinberg GA, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123:98–104. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly JT, Busse WW. Host immune responses to rhinovirus: mechanisms in asthma. J Allergy Clin Immunol. 2008;122:671–682. doi: 10.1016/j.jaci.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gentile DA, Fireman P, Skoner DP. Elevations of local leukotriene C4 levels during viral upper respiratory tract infections. Ann Allergy Asthma Immunol. 2003;91:270–274. doi: 10.1016/S1081-1206(10)63529-6. [DOI] [PubMed] [Google Scholar]
- 7.Bisgaard H, Zielen S, Garcia-Garcia ML, et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med. 2005;171:315–322. doi: 10.1164/rccm.200407-894OC. [DOI] [PubMed] [Google Scholar]
- 8.Johnston NW, Mandhane PJ, Dai J, et al. Attenuation of the September epidemic of asthma exacerbations in children: a randomized, controlled trial of montelukast added to usual therapy. Pediatrics. 2007;120:e702–e712. doi: 10.1542/peds.2006-3317. [DOI] [PubMed] [Google Scholar]
- 9.Robertson CF, Price D, Henry R, et al. Short-course montelukast for intermittent asthma in children: a randomized controlled trial. Am J Respir Crit Care Med. 2007;175:323–329. doi: 10.1164/rccm.200510-1546OC. [DOI] [PubMed] [Google Scholar]
- 10.DeMore JP, Weisshaar EH, Vrtis RF, et al. Similar colds in subjects with allergic asthma and nonatopic subjects after inoculation with rhinovirus-16. J Allergy Clin Immunol. 2009;124:245–52. 252. doi: 10.1016/j.jaci.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WM, Grindle K, Pappas T, et al. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol. 2007;45:2626–2634. doi: 10.1128/JCM.02501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee WM, Kiesner C, Pappas T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarjour NN, Gern JE, Kelly EA, et al. The effect of an experimental rhinovirus 16 infection on bronchial lavage neutrophils. J Allergy Clin Immunol. 2000;105:1169–1177. doi: 10.1067/mai.2000.106376. [DOI] [PubMed] [Google Scholar]
- 14.Gern JE, Vrtis R, Grindle KA, et al. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 15.Barrett B, Brown R, Mundt M, et al. The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid. J Clin Epidemiol. 2005;58:609–617. doi: 10.1016/j.jclinepi.2004.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santanello NC, Barber BL, Reiss TF, et al. Measurement characteristics of two asthma symptom diary scales for use in clinical trials. Eur Respir J. 1997;10:646–651. [PubMed] [Google Scholar]
- 17.Mosser AG, Vrtis R, Burchell L, et al. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med. 2005;171:645–651. doi: 10.1164/rccm.200407-970OC. [DOI] [PubMed] [Google Scholar]
- 18.Calhoun WJ, Dick EC, Schwartz LB, et al. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94:2200–2208. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CK, Choi J, Kim HB, et al. A randomized intervention of montelukast for post-bronchiolitis: effect on eosinophil degranulation. J Pediatr. 2010;156:749–754. doi: 10.1016/j.jpeds.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Rakes GP, Arruda E, Ingram JM, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 21.Bisgaard H, Flores-Nunez A, Goh A, et al. Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children. Am J Respir Crit Care Med. 2008;178:854–860. doi: 10.1164/rccm.200706-910OC. [DOI] [PubMed] [Google Scholar]
- 22.Proesmans M, Sauer K, Govaere E, et al. Montelukast does not prevent reactive airway disease in young children hospitalized for RSV bronchiolitis. Acta Paediatr. 2009;98:1830–1834. doi: 10.1111/j.1651-2227.2009.01463.x. [DOI] [PubMed] [Google Scholar]
- 23.Sears MR, Johnston NW. Understanding the September asthma epidemic. J Allergy Clin Immunol. 2007;120:526–529. doi: 10.1016/j.jaci.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bacharier LB, Phillips BR, Zeiger RS, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol. 2008;122:1127–1135. doi: 10.1016/j.jaci.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]