Abstract
Background:
Intravitreal injection of human mesenchymal stem cells has been shown to be effective in slowing the progression of diabetic retinopathy in an animal model of chemically induced diabetes mellitus. We studied changes in growth factor levels released from human mesenchymal stem cells in the vitreous cavity as well as changes in growth factor levels in host retinal neurons following intravitreal injection.
Methods:
Twenty-two Lewis rats were treated with an intravitreal human mesenchymal stem cell microinjection. Determination of neurotrophic factors released by human mesenchymal stem cells in the vitreous was carried out using real-time polymerase chain reaction.
Results:
Detectable levels of neurotrophic factors were identified postoperatively in the vitreous of all rats.
Conclusion:
Increased intravitreal and retinal concentrations of neuroprotective growth factors in rats confirm the neuroprotective activity of human mesenchymal stem cells in diabetic retinopathy.
Keywords: diabetic retinopathy, neuroprotection, apoptosis mechanism, stem cells, chemically induced diabetes mellitus
Introduction
Stem cells are nonspecialized cells capable of dividing and simultaneously giving rise to both new stem cells and precursors of a cell progeny which then develop into mature cells. Stem cells are classified into four groups, ie, embryonic, germinal embryonic, placental (umbilical cord), and adult cells. Human stem cells represent a large reservoir of anti-apoptotic and antineoangiogenic growth factors, and there is growing interest in the ophthalmologic field regarding the use of human stem cells in the management of a group of retinal diseases1,2 induced and supported by apoptotic and neoangiogenic mechanisms and mediated by proinflammatory growth factors, such as cytokines, chemokines, and vascular endothelial growth factor. In this study, we used placental human stem cells given that the placenta,3 specifically the amniotic membrane,4 represents an important source of human stem cells because of the abundance of cells which can be recovered and also the absence of ethical issues, considering that the placenta is a waste product.5 Histological study of placental tissue has shown that the amniotic membrane is made up of a layer of amniotic epithelial cells resting on a basal membrane and, more externally, on a stroma of connective tissue or amniotic mesoderm in which there are amnion mesenchymal stromal stem cells. The cells extracted from the placenta6 have an important immunomodulatory function. This property is due to the lack of the major histocompatibility complex II and to the expression of both costimulatory molecules in the membrane and of human leukocyte antigen G.7 Molecular analysis of factors expressed by amnion epithelial cells demonstrate that these cells express factors in common with specific membrane markers for human embryonic stem cells, such as octamer-binding transcription factor 4, a transcription factor essential for maintaining self-renewal of undifferentiated embryonic stem cells, and also produce fibroblast growth factor 4, which is encoded by the fibroblast growth factor gene and possesses broad mitogen and cell survival features. Furthermore, these human embryonic stem cells demonstrate positivity for stage-specific embryonic antigen 3 and 4, tumor rejection antigen 1–60 and 1–81, human proto-oncogene c-kit (a cell surface antigen), and nestin (a transcription factor critically involved in self-renewal of undifferentiated embryonic stem cells and their multipotency). Human embryonic stem cells do not express the stage-specific embryonic antigen 1 or the hematopoietic stem cell marker, CD34. Their proliferative capacity can be maintained in culture in Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum and epidermal growth factor. The aim of our research was to assess the tolerability and effectiveness of transplanted human placental stem cells in a rat model of chemically induced type 2 diabetes mellitus.8 The functional capacity of the transplant in preventing and/or slowing the progression of diabetic retinopathy was evaluated by measuring the neuroprotective, anti-apoptotic, and anti-angiogenic effects of growth factors liberated from human stem cells, and their possible role in slowing or blocking the retinal neoangiogenic process. Evolution of type 2 diabetes mellitus in a rat model was assessed daily using the glycemia index, water consumption, and variations in body weight, in order to identify the benefits of human placental stem cell therapy on the progression of diabetes.9 Diabetic retinopathy, in particular proliferative diabetic retinopathy, is characterized by preretinal neovascularization and fibrosis, vitreous hemorrhage, and tractional retinal detachment. Many authors believe that a great variety of proapoptotic growth factors, eg, vascular endothelial growth factor, are involved in activating and perpetuating the neovascular process. Many studies have demonstrated that the levels of many angiogenic factors are higher in the vitreous of patients affected by proliferative diabetic retinopathy than those in individuals without vasoproliferative retinopathies.
Methods
This preliminary interdisciplinary multicenter study was supported by the University of Bologna and carried out by the Department of General Surgery and Organ Transplants at the University of Bologna in collaboration with the Department of Ophthalmology at the University of Naples, and with the support of the Department of Ophthalmology at the US Army Health Clinic Camp Darby of Livorno. This study was approved by the ethical committee at the Sant’Orsola-Malpighi Hospital, University of Bologna, and all procedures were carried out under an institutionally approved protocol in accordance with the ethical principles and standards of the Federation of European Animal Science Association, following the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the current legislation on preclinical research in Italy. All the regulations of the Centralized Veterinary Service of Bologna were respected. Fifteen young adult male syngenetic Lewis rats, weighing 180–200 g (mean 188.56 ± standard deviation [SD] 7.14 g) were included in this randomized preclinical study and were rendered diabetic using streptozotocin. Glycemia10 was set at 90–120 (mean 98.3 ± 3.4) mg/dLat baseline (time 0, beginning of the study). Animals which did not meet these requirements were excluded. All rats selected for study were further subdivided into two groups, ie, Group A and Group B. Group A (n = 12) underwent an intravitreal human placental stem cell implant, and Group B (n = 3) served as the control. The rats in both groups were rendered diabetic by a single intraperitoneal injection of streptozotocin11 65 mg/kg. Starting from the day after streptozotocin injection, evening glycemia was evaluated daily and rats with a glycemia index ≥250 mg/dL for 3 consecutive days were considered to have developed type 2 diabetes mellitus. Chemically-induced diabetes in this rat model was monitored by a glycemic check in both groups, taking daily blood samples from the caudal vein. Consumption of water and variation in bodyweight were also recorded. Rats treated with streptozotocin demonstrated a marked increase in daily water consumption up to 150–200 (mean 163.99 ± 1.9) mL/day 21 days after injection, while control values were 20–23 mL/day. Streptozotocin-treated diabetic rats reached glycemiclevels of 250–350 (mean 301.87 ± 4.1) mg/dL 21 days after injection whereas the nondiabetic rats had glycemic levels of 110–130 mg/dL. Treatment with streptozotocin also interrupted growth (see Figure 1). At 21 days after treatment with streptozotocin, all rats in Group A received an intravitreal transplant of human placental stem cells. Rats in Group B (sham-operated) were not treated, and were just monitored for water consumption, glycemia index, and bodyweight changes, in order to control the evolution of type 2 diabetes. The cell populations we transplanted were representative of human placental stem cells, ie, having elevated staminality, and were isolated from healthy placental tissue at term. We observed and studied the in vitro potential of these cell populations to differentiate into retinal phenotypes, with the aim of evaluating the differentiation potential of these cells in a diabetic animal model. In our study, we injected adult mesenchymal stem cells into the vitreous of the diabetic rats. Adult mesenchymal stem cells have a vast differentiation potential, with suppressive immunomodulatory activity via expression of immunomodulatory molecules, such as human leukocyte antigen G and interleukin-10. Moreover, adult mesenchymal stem cells were capable of inserting into rat tissue without inducing rejection phenomena. The cell populations were tagged with fluorescent markers in order to differentiate between them for in vivo transplantation. The markers we used were carbocyanine dyes with hydrophilic/hydrophobic characteristics. These dyes are usually used in living tissues and cells because they insert into the membrane and diffuse rapidly, staining the entire cell surface. We used 3,3′-dioctadecyloxacarbocyanine and 1-1′-dioctadecyl – 3,3,3′,3′ tetramethy 1 indodicarbocyanine. The advantages of these dyes are their manageability and thermal stability. Release of insulin in response to elevated glucose concentrations was tested in vitro using radioimmunoassay. One week before treating the animals, in vitro expansion of the human placental stem cells was carried out in culture. This procedure allowed us to maintain a stable phenotype with a high proliferative capacity. Characterization of the human placental stem cells and their differentiating potential was then performed by cytofluorimetric analysis. The profile of genetic expression was also studied in relation to nerve growth factor, brain-derived neurotrophic factor, ciliary-derived neurotrophic factor, glial-derived neurotrophic factor, and basic fibroblast growth factor.12,13 The day before intravitreal transplant, the human placental stem cells were trypsinized with 1 mL of trypsin, after aspiration of the culture medium and having washed the cells with about 3 mL of phosphate-buffered saline. After approximately 1 minute, the cells began to detach and were then stained with 3,3′-dioctadecyloxacarbocyanine fluorescent dye and resuspended in serum-free medium supplemented with epidermal growth factor and basic fibroblast growth factor at a concentration of 50,000 cells/μL. The cells were kept on ice for 4 hours before transplanting. Before receiving the transplant, Equithesin® was administered to all diabetic rats in Group A for general anesthesia.14 Equithesin was prepared according to the doses reported in Table 1, adhering to the order of components as listed. Before adding each successive component, a check was performed to ensure that each preceding component was well dissolved. The dose administered was 0.4 mL/100 g, adjusted for the weight of each individual rat. Before the surgical procedure, each rat was settled on a stereotactic table, and a single drop of iodopovidone 10% was instilled into the conjunctival sac to disinfect the periorbital and periocular skin, thereby preventing onset of endophthalmitis. After 5 minutes, another drop of iodopovidone 5% was instilled into the eye. When performing the surgical procedure, a microinjector pump connected to a flow rate system was used to control the flux of human placental stem cells, so that a maximum concentration of stem cells could be reached in the vitreous. The flow rate was maintained at 50,000 cells/μL. Intravitreal injection of the resuspended human placental stem cells was carried out via the pars planaat the level of the inferotemporal quadrant, 1 mm from the limbus. A drop of iodopovidone 5% was instilled in the operated eye after the intravitreal stem cell implant. The animals were monitored during postoperative reawakening and daily in the 7-day postsurgical period, to be able to identify any signs of suffering as early as possible. Postoperative discomfort was minimal. Evaluation of the diabetic rats in group B (sham operated) was also performed; the same surgical procedure was carried out in these rats using 0.2 μL physiological solution only. Immediately after the intravitreal transplant, all the rats were started ontopical antibiotic prophylaxis using tobramycin eye drops, instilling one drop in each operated eye three times daily for 10 days postoperatively. On postoperative day 21, all the diabetic rats in Group A underwent fluorangiography examination for clinical evaluation of diabetic retinopathy. Diabetic rats in Group B underwent the same examination. Fluorescein was injected at the caudal level. We evaluated the diabetic retinopathy from a clinical point of view in order to assess the eventual stabilization/regression of diabetic retinopathy, paying particular attention to retinal neovascularization and other signs of disease. This evaluation demonstrated the presence of neovascularization, ischemic areas, and microaneurysms present in the retina of the Group B diabetic rats. Moreover, vitreous levels of anti-apoptotic growth factors were quantified by real-time polymerase chain reaction using an enzyme-linked immunosorbent assay kit for vitreous growth factors. Multiplex bead assays were used for measurement of different growth factors and cytokines, in particular levels of basic fibroblastic growth factor, nerve growth factor, brain-derived neurotrophic factor, ciliary-derived neurotrophic factor,15 and glial-derived neurotrophic factor in the vitreal chamber. The increase in the levels of these factors in rats with transplanted human stem cells underscore the potential neuroprotective effects against oxidative damage of high growth factor levels released from stem cells.16–18 Vitreous samples (0.2 μL each sample) were used undiluted and immediately, in order to avoid vitreous condensation and solidification. Real-time polymerase chain reaction was performed according to the manufacturer’s instructions. Because of the limited volume of some of the samples, a minimum of duplicate tests were undertaken for each assay. Saline was substituted for the vitreous implant in the negative controls.
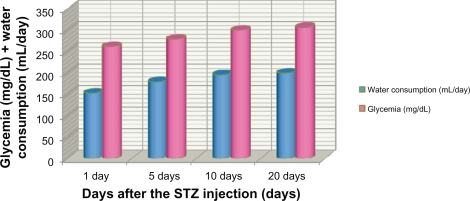
Figure 1.
Variation in water consumption and glycemia after treatment with streptozotocin. Rats treated with streptozotocin demonstrated a notable increase in daily water consumption, up to values of 150–200 mL/day at 21 days after treatment (mean 163.99 ± 1.9 mL/day), while reference values were 20–23 mL/day. Rats treated with streptozotocin reached mean glycemic levels of 250–350 mg/dL at 21 days after treatment (301.87 ± 4.1 mg/dL) unlike the nondiabetic rats (standard values 110–130 mg/dL).
Table 1.
Equithesin® anesthesia. Equithesin was prepared according to the doses reported, respecting the order of the components. Before adding each successive component, it was necessary to check that each preceding component was well dissolved. The dose administered was 0.4 mL/100 g, adjusted in relationship to the weight of each individual rat
| Component | Dose |
|---|---|
| H2O distilled | 20 mL |
| Propylene glycol | 19.8 mL |
| Ethanol | 5 mL |
| Sodium pentobarbital | 0.4860 g |
| Magnesium sulfate | 1.0625 g |
| Chloral hydrate | 2.1245 g |
Statistical analysis
Statistical analysis was performed on all data using analysis of variance on XLSTAT2010 software (Addinsoft USA, New York). The level of statistical significance was set at P < 0.05.
Results
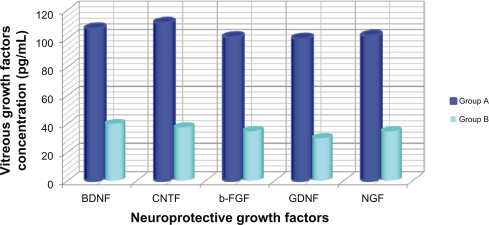
Fluorangiography examination showed that rats receiving the intravitreal human placental stem cell transplant had a marked improvement in the evolution of diabetic retinopathy. Group A showed markedly decreased areas of hypofluorescence and hypoperfusion, due to the ischemia leading to evolution of diabetic retinopathy and absence of leakage phenomena, which indicates slowing and/or stabilization of diabetic retinopathy. On fluorangiographic examination, Group B rats demonstrated retinal hyperfluorescence (leakage) associated with significant areas of hypofluorescence, and microaneurysms, neovascularization, and ischemic areas, both preoperatively and postoperatively. The fluorangiographic examination was strictly qualitative, so was performed by the same operator to reduce the chances of observational errors. Moreover, determination of neurotrophic and growth factors having an anti-apoptotic, antineoangiogenic, and neuroprotective role and released by the transplanted human placental stem cells in Group A were evaluated using vitreous real-time polymerase chain reaction. The growth factors released were ligands of two large families of receptors, ie, the family of transmembrane receptors with tyrosine kinase activity and the family of neurotrophic receptors. The growth factors we evaluated were basic fibroblastic growth factor, nerve growth factor, brain-derived neurotrophic factor, ciliary-derived neurotrophic factor, and glial-derived neurotrophic factor. All these growth factors were detected in all vitreous samples tested. Real-time polymerase chain reaction showed notable levels of vitreous expression of neurotrophic factors. Qualitative and quantitative analysis of these factors showed that the greatest peaks of concentration at the endovitreal level were reached by brain-derived neurotrophic factor and ciliary-derived neurotrophic factor in rats with transplanted human stem cells. Significantly higher levels of basic fibroblast growth factor, nerve growth factor, brain-derived neurotrophic factor, ciliary-derived neurotrophic factor, and glial-derived neurotrophic factor were observed in the vitreous samples of rats in group A compared with Group B controls. All neuroprotective growth factor levels were significantly higher (P < 0.05) in rats affected by diabetic retinopathy and with transplanted human stem cells than in Group B controls. Both brain-derived neurotrophic factor and ciliary-derived neurotrophic factor reached levels of 107.99 pg/mL ± 0.002 (P < 0.05) in Group A rats, while the median level of brain-derived neurotrophic factor and ciliary-derived neurotrophic factor in Group B controls was 38.7 pg/mL ± 0.023. This difference was statistically significant (P < 0.05). Furthermore, a significant negative association was observed between vascular endothelial growth factor and proinflammatory cytokine levels and the progression of the diabetic retinopathy after transplantation in Group A rats (Figure 2).
Figure 2.
Vitreous concentration of neuroprotective growth factor in Group A (treated) and in Group B (controls). Brain-derived neurotrophic factor, ciliary-derived neurotrophic factor, basic fibroblast growth factor, glial-derived neurotrophic factor, and nerve growth factor are significant neuroprotective growth factors tested in the vitreous in both Group A and Group B. They were significantly higher (P < 0.05) in rats affected by diabetic retinopathy treated by human stem cell transplant compared with Group B controls.
Discussion
We had not been optimistic that a single stem cell injection could provide enough antioxidant activity to have detectable effects on the development of diabetic retinopathy in an animal model. Therefore, we were surprised to find that a single intravitreal injection of human placental stem cells resulted in a significant decrease of retinal apoptosis in rats rendered diabetic by means of a single injection of streptozotocin compared with the controls. This preliminary study provides additional support for the hypothesis that oxidative damage is an important contributor to retinal damage in diabetic retinopathy.19 Critical analysis of the results shows that human placental stem cells currently represent the safest and most controlled biological pathway for the release of chemical mediators, such as growth factors with specific anti-apoptotic and antineoangiogenic roles, in an animal model of diabetes mellitus. The procedure we used allowed control of progression of chemically induced diabetic retinopathy. Furthermore, no deaths, rejection, intolerance phenomena, or postoperative complications were encountered, nor were any adverse reactions encountered on fluorangiographic examination. This study describes a simple methodology with possible clinical implications for humans in the future. Our finding that a decrease in retinal apoptotic phenomena and oxidative damage contributes to preventing development of diabetic retinopathy in rats has important clinical implications. Moreover, this challenging approach to the treatment of diabetic retinopathy using an animal model has several advantages, in that it is simple to perform, is not surgeon-dependent, is portable and relatively inexpensive, is not as invasive as other surgical techniques, and gives a rapid and very good response. We did not identify any disadvantages, and no side effects or rejection phenomena were noticed during the study. It provides a therapeutic target that may apply in the future to patients affected by this disease. The clinical and genetic heterogeneity that constitutes diabetic retinopathy is a problem when attempting to develop treatments targeting the primary defect involved in this disorder. The documented slowing down of diabetic retinopathy in this animal model, as well as the antineoangiogenic effects, indicates an effective neuroprotective and anti-inflammatory role at the retinal level. The increased concentrations of neuroprotective growth factors seen in the vitreous camera following intravitreal injection of stem cells, allow us to hypothesize a possible neuroretinal protective role for these factors. Additional studies are necessary in order to expand upon these results so as to make this therapeutic approach relevant to humans. Human placental stem cells taken from a healthy placenta at term and implanted in the vitreous chamber by simple intravitreal injection could be an attractive and innovative therapeutic option in the management of diabetic retinopathy. Furthermore, in this way, trauma at the ocular level could be avoided. The interest shown by ophthalmologists in this retinal pathology has grown exponentially, given the epidemiological importance of diabetic retinopathy, which is the retinal pathology associated with the highest rate of blindness in Italy and in the rest of the world, and given the absence of long-term definitive therapeutic approaches. It will be necessary to increase the number of researchers involved in our study group to reach the clinical phase of experimentation for this pathology in the future.20,21
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Joe AW, Gregory-Evans K. Mesenchymal stem cells and potential applications in treating ocular disease. Curr Eye Res. 2010;35(11):941–952. doi: 10.3109/02713683.2010.516466. [DOI] [PubMed] [Google Scholar]
- 2.Jeganathan VS, Palanisamy M. Treatment viability of stem cells in ophthalmology. Curr Opin Ophthalmol. 2010;21(3):213–217. doi: 10.1097/ICU.0b013e32833867ad. [DOI] [PubMed] [Google Scholar]
- 3.Tsagias N, Koliakos I, Lappa M, et al. Placenta perfusion has hematopoietic and mesenchymal progenitor stem cell potential. Transfusion. 2011 Mar 7; doi: 10.1111/j.1537-2995.2011.03077.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Parolini O, Caruso M. Preclinical studies on placenta-derived cells and amniotic membrane: An update. Placenta. 2011;32(Suppl 2):S186–S195. doi: 10.1016/j.placenta.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Abdulrazzak H, Moschidou D, Jones G, et al. Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. J R Soc Interface. 2010;7(Suppl 6):S689–S706. doi: 10.1098/rsif.2010.0347.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marongiu F, Gramignoli R, Sun Q, et al. Isolation of amniotic mesenchymal stem cells. Curr Protoc Stem Cell Biol. 2010. Chapter 1: Unit 1E.5. [DOI] [PubMed]
- 7.Menier C, Rouas-Freiss N, Favier B, et al. Recent advances on the non-classical major histocompatibility complex class I HLA-G molecule. Tissue Antigens. 2010;75(3):201–206. doi: 10.1111/j.1399-0039.2009.01438.x. [DOI] [PubMed] [Google Scholar]
- 8.Volarevic V, Arsenijevic N, Lukic ML, et al. Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29(1):5–10. doi: 10.1002/stem.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remuzzi A, Cornolti R, Bianchi R, et al. Regression of diabetic complications by islet transplantation in the rat. Diabetologia. 2009;52(12):2653–2661. doi: 10.1007/s00125-009-1537-y. [DOI] [PubMed] [Google Scholar]
- 10.Daly M. Sugars, insulin sensitivity, and the postprandial state. Am J Clin Nutr. 2003;78(4):865S–872S. doi: 10.1093/ajcn/78.4.865S. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Yang SH, Oh JM, et al. Pharmacokinetics of drugs in rats with diabetes mellitus induced by alloxan or streptozocin: Comparison with those in patients with type I diabetes mellitus. J Pharm Pharmacol. 2010;62(1):1–23. doi: 10.1211/jpp.62.01.0001. [DOI] [PubMed] [Google Scholar]
- 12.Liu WJ, Jin HY, Lee KA, et al. Neuroprotective effect of the glucagon-like peptide-1 receptor agonist, synthetic exendin-4, in streptozotocin-induced diabetic rats. Br J Pharmacol. 2011 Feb 16; doi: 10.1111/j.1476-5381.2011.01272.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inberg A, Linial M. Protection of pancreatic beta-cells from various stress conditions is mediated by DJ-1. J Biol Chem. 2010;285(33):25686–25698. doi: 10.1074/jbc.M110.109751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunnett SB, Torres EM, Richards H, et al. Effects of surgical anaesthesia on the viability of nigral grafts in the rat striatum. Cell Transplant. 1998;7(6):567–572. doi: 10.1177/096368979800700607. [DOI] [PubMed] [Google Scholar]
- 15.Aloe L, Rossi S, Manni L. Altered expression of nerve growth factor and its receptors in the kidneys of diabetic rats. J Nephrol. 2011 Mar 4; doi: 10.5301/JN.2011.6418. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Mahdy RA, Nada WM, Hadhoud KM, et al. The role of vascular endothelial growth factor in the progression of diabetic vascular complications. Eye (Lond) 2010;24(10):1576–1584. doi: 10.1038/eye.2010.86. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Li Y, Liu Y, et al. Endothelial progenitor cells (EPCs) mobilized and activated by neurotrophic factors may contribute to pathologic neovascularization in diabetic retinopathy. Am J Pathol. 2010;176(1):504–515. doi: 10.2353/ajpath.2010.081152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talahalli A, Zarini S, Sheibani N, et al. Increased synthesis of leukotrienes in the mouse model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51(3):1699–1708. doi: 10.1167/iovs.09-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couri CE, Voltarelli JC. Stem cell therapy for type 1 diabetes mellitus: A review of recent clinical trials. Diabetol Metab Syndr. 2009;1(1):19. doi: 10.1186/1758-5996-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haller JA, Bandello F, Belfort R, Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117(6):1134–1146. doi: 10.1016/j.ophtha.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Bandello F, Blumenkranz MS, Jiao J, et al. 12-Month evaluation of dexamethasone intravitreal implant in patients with macular edema due to central retinal vein occlusion. Abstr 6397. Association for Research in Vision and Ophthalmology annual meeting; May 2–6, 2010; Fort Lauderdale, FL. [Google Scholar]