Abstract
Purpose:
To describe the pharmacokinetics of intravitreal bevacizumab (Avastin®) in rabbits.
Methods:
The right eye of 20 rabbits was injected intravitreally with 1.25 mg/0.05 mL bevacizumab. Both eyes of four rabbits each time were enucleated at days 1, 3, 8, 15, and 29. Bevacizumab concentrations were measured in serum, aqueous humor, and vitreous.
Results:
Maximum vitreous (406.25 μg/mL) and aqueous humor (5.83 μg/mL) concentrations of bevacizumab in the right eye were measured at day 1. Serum bevacizumab concentration peaked at day 8 (0.413 μg/mL) and declined to 0.032 μg/mL at 4 weeks. Half-life values in right vitreous, right aqueous humor, and serum were 6.61, 6.51, and 5.87 days, respectively. Concentration of bevacizumab in the vitreous of the noninjected eye peaked at day 8 (0.335 ng/mL) and declined to 0.218 ng/mL at 4 weeks. In the aqueous humor of the noninjected eye, maximum concentration of bevacizumab was achieved at day 8 (1.6125 ng/mL) and declined (to 0.11 ng/mL) at 4 weeks.
Conclusion:
The vitreous half-life of 1.25 mg/0.05 mL intravitreal bevacizumab was 6.61 days in this rabbit model. Maximum concentrations of bevacizumab were reached at day 1 in both vitreous and aqueous humor of the right eye and at day 8 in the serum. Very low concentrations of bevacizumab were measured in the fellow noninjected eye.
Keywords: bevacizumab, pharmacokinetics, rabbits, intravitreal
Introduction
Bevacizumab (Avastin®, Genentech, South San Francisco, CA) is a humanized monoclonal antibody that inhibits human vascular endothelial growth factor (VEGF). VEGF is an endothelial cell-specific mitogen required for pathological angiogenesis observed in tumor growth and metastatic spread. Therefore, bevacizumab has been approved by the US Food and Drug Administration as an adjunct treatment for metastatic colorectal cancer.1 Bevacizumab is composed of 214 amino acids with a molecular mass of 149 kDa. Although some studies2 suggest that molecules exceeding 100 kDa cannot cross the retinal layers into the subretinal space, a number of other studies3,4 have demonstrated that bevacizumab can efficiently diffuse through the retinal layers into the choroidal space where it can inhibit neovascularization. In this regard, intravitreal injections of bevacizumab for off-label use have been shown to be beneficial in eyes with macular edema secondary to neovascular age-related macular degeneration (AMD),5,6 diabetic retinopathy,7–9 and central or branch retinal vein occlusion.10–12
The pharmacokinetics and distribution of bevacizumab after intravitreal injection of 1.25 mg/0.05 mL in rabbits have been studied.13,14 Interestingly, in these studies, very low concentrations of bevacizumab were detected in the fellow noninjected eye, approaching the detection limit of the methods used. In our study, we aimed to measure the concentrations of bevacizumab in both the injected and noninjected eye with more sensitive methods. It is important to know the exact pharmacokinetic profile of bevacizumab to optimize doses, assess its safety profile, and achieve the best therapeutic results not only in the injected but also in the noninjected eye.
Methods
Approval was obtained from the Ethics Committee of the National and Kapodistrian University of Athens and the Veterinary Address of Prefecture of Athens. The procedures adhered to the guidelines of the Association for Research in Vision and Ophthalmology for animal use in ophthalmic and vision research and were in accordance with the standards set down in the Guide for the Care and Use of Laboratory Animals. Twenty-four male New Zealand rabbits with a mean weight of 2.8975 kg (±0.2028 kg) were anesthetized with 20 mg/kg of intramuscular ketamine hydrochloride and 3 mg/kg of intramuscular xylazine hydrochloride. Ophthalmic drops of 1% alcaine hydrochloride were placed on the conjunctiva of 20 right eyes and then povidone iodine 5% was instilled in these eyes. These right eyes were injected intravitreally 2 mm behind the surgical limbus in the superotemporal quadrant with bevacizumab 1.25 mg/0.05 mL using a 30-gauge needle. The 20 left eyes received no intravitreal injections and acted as controls. Eyes were monitored closely daily for any signs of adverse effects. Four rabbits received no intravitreal injections in the right eye and acted as controls to determine the background levels in the detection of bevacizumab.
Four of the rabbits that received intravitreal injections of bevacizumab were sacrificed at each of the following days: 1, 3, 8, 15, and 29. Rabbits were first anesthetized with 20 mg/kg of intramuscular ketamine hydrochloride and 3 mg/kg of intramuscular xylazine hydrochloride and then a sample of arterial blood was drawn from the central artery of their ears just before the euthanasia. Rabbits were then sacrificed with pentobarbital overdose (1.2 mL/kg). Serum was obtained by allowing the blood sample to clot at room temperature for 1 hour followed by centrifugation. Serum was then frozen at −80°C until tested. The vitreous was taken with a 2.5 mL syringe while aqueous humor was withdrawn into an insulin syringe. All samples were immediately frozen at −80°C until tested. The four rabbits that acted as control animals were sacrificed and samples were obtained as mentioned above at day 0.
Bevacizumab assay
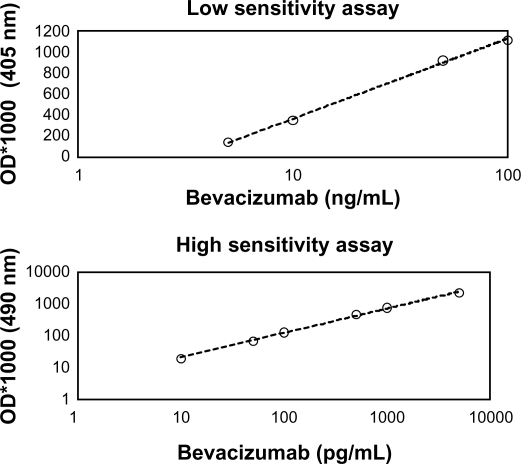
Bevacizumab concentrations were measured using enzyme-linked immunosorbent assay (ELISA). Two ELISA methods were used, one of low sensitivity (LS ELISA, linear range: 5 ng/mL to 0.1 μg/mL, Figure 1A) and one of high sensitivity (HS ELISA, linear range: 10pg/mL to 5 ng/mL, Figure 1B). ELISA plates (Costar high binding) were coated with 100 μL/well of rec-hVEGF (R&D Systems, Minneapolis, MN) at a concentration of 0.2 μg/mL in carbonate-bicarbonate buffer (pH = 9.6). After washing with PBS (200 μL/well), the plates were blocked with 200 μL/well of bovine albumin 2% in PBS (BB: blocking buffer). Afterwards, the plates were washed and the samples were added (100 μL/well) in various dilutions ranging from 1:10,000 (vitreous/right eye day1) to 1:1 (vitreous/left eye day 29) and incubated for 2 hours at room temperature (RT). The plates were then washed again and for i) LS ELISA: 100 μL/well of anti-human Fab specific antibody conjugated to horse radish peroxidase (HRP) (Sigma-Aldrich, St Louis, MO) (1:1200 in BB) was added to the wells. After washing, 100 μL of ABTS [2, 2′-azino-bis (3-ethylbenzthiazoline-6-sulfonicacid)] substrate was added to the wells and the color development was measured at 405 nm, ii) HS ELISA: 100 μL/well of anti-human Fab specific antibody conjugated to alkaline phosphatase (AP) (Jackson Immuno research, West Grove, PA) (1:1200 in BB) was added to the wells. Subsequently, the plates were washed four times with PBS and the Invitrogen’s ELISA amplification system was used to enhance the detection of bevacizumab. Briefly, the plates were incubated with 50 μL/well of substrate (reduced NADPH) for 20 minutes followed by the addition of 50 μL/well of amplifier (alcohol dehydrogenase and diaphorase). The color was quantified at 490 nm.
Figure 1.
Development of specific assays for the quantification of bevacizumab: A) low sensitivity ELISA with linear range 5 to 100 ng/mL; B) high sensitivity ELISA with linear range 10 to 5000 pg/mL.
Abbreviation: OD, optical density.
The detection limit of the designed assay was evaluated using decreasing concentrations of bevacizumab. The detection limit was found to be 0.01 ng/mL (with the high-sensitivity assay). Intra- and inter-assay variations were determined using bevacizumab at concentrations 10, 50, and 100 ng/mL for the low-sensitivity assay and 0.1, 0.5, and 1.0 ng/mL for the high-sensitivity assay. Then, intra- and inter-assay variations were calculated using the formula:
where CV = coefficient of variation, SD = standard deviation and X = mean.
Each dilution of bevacizumab was run eight times to calculate the coefficient of variation. Mean intra-assay and inter-assay CV were found to be 3.96% and 6.75% for the low-sensitivity assay and 4.4% and 8.19% for the high-sensitivity assay, respectively.
ELISA assay for detection of anti-bevacizumab antibodies (rabbit anti-Idiotypic antibodies)
We additionally performed an ELISA assay for detection of anti-bevacizumab antibodies in order to quantify their effect on bevacizumab concentration. According to this method, ELISA plates (Costar high binding) were coated with 100 μL/well of bevacizumab at a concentration of 0.4 μg/mL in carbonate-bicarbonate buffer (pH = 9.6). After washing with PBS (200 μL/well), the plates were blocked with 200 μL/well of bovine albumin 2% in PBS (BB:blocking buffer).
Subsequently, the serum samples were added (100 μL/well) in 1:5 dilution and incubated for 2 hours at RT. The plates were then washed again and 100 μL/well of anti-rabbit Fc specific antibody conjugated to AP (1:1200 in BB) was added to the wells. Afterwards, the plates were washed with PBS (200 μL/well) and the Invitrogen ELISA amplification system was used according to the procedure described above. Finally, the color was quantified at 490 nm.
Pharmacokinetic methods
Bevacizumab vitreous humor, aqueous humor, and serum concentration–time data were each fit by standard noncompartmental analysis to determine half-life (t1/2), area under the curve (AUC0→∞) and bevacizumab vitreous clearance using WinNonlin Pro (v2.1; Pharsight, Mountain View, CA). The terminal elimination rate constant was determined by least-squares regression of the ln (serum concentration)–time data for the last four time points.
Results
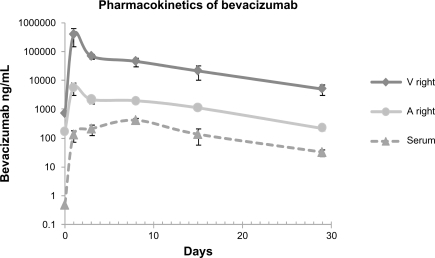
Data were obtained from the 48 eyes of 24 rabbits. There were no signs of ocular inflammation or other adverse events. The change in concentration over time for bevacizumab in the vitreous and aqueous humor of injected eye and in serum after intravitreal injection is illustrated in Figure 2. A peak concentration of 406.25 μg/mL was achieved in the vitreous 1 day after intravitreal injection of 1.25 mg/0.05 mL bevacizumab. Half-life of bevacizumab in the right vitreous was 6.61 days (Table 1). A concentration of 5.17 μg/mL was maintained in the vitreous 29 days after injection. Bevacizumab concentrations in the aqueous humor of the injected eye reached a peak concentration of 5.835 μg/mL 1 day after drug administration as well. A concentration of 0.225 μg/mL was maintained in aqueous humor 29 days after injection. In serum, a maximum concentration of 0.413 μg/mL was achieved 8 days after drug injection and the concentration fell to 0.032 μg/mL 29 days after drug administration. Half-life of bevacizumab in the right aqueous humor and serum were 6.51 and 5.87 days, respectively (Table 1).
Figure 2.
Bevacizumab concentration in the vitreous, aqueous humor, and serum after intravitreal injection of 1.25 mg/0.05 mL of bevacizumab in rabbits. Samples were taken from the aqueous humor and vitreous of the injected right eye. Values at day 0 indicate background levels of bevacizumab detection in control animals.
Table 1.
Analysis of distribution of bevacizumab in the aqueous, vitreous, and serum after intravitreal injection of 1.25 mg/0.05 mL bevacizumab in the right eye
| Compartment | t1/2 (days) | Tmax (days) | Cmax (ng/mL) | % of vitreous Cmax | AUC0→∞ (μg/mL * day) | Exposure to bevacizumab as a % of vitreous exposure |
|---|---|---|---|---|---|---|
| Vitreous (R) | 6.61 | 1 | 406250 | – | 1455.1 | – |
| Aqueous (R) | 6.51 | 1 | 5835 | 1.44 | 44.8 | 3.08 |
| Serum | 5.87 | 8 | 413 | 0.10 | 5.3 | 0.36 |
| Aqueous (L) | 5.56 | 8 | 1.61 | 0.0004 | 0.018 | 0.001 |
| Vitreous (L) | – | 8 | 0.34 | 0.00008 | 0.017 | 0.001 |
Abbreviations: R, right eye; L, left eye; Tmax, day when concentration of bevacizumab peaked; Cmax, maximum concentration; AUC, area under the curve.
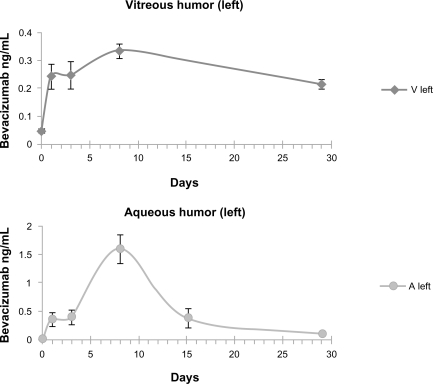
Very low concentrations of the drug were detected in the fellow noninjected eye. The levels of bevacizumab in the aqueous humor of the noninjected eye peaked at 8 days after intravitreal injection with a concentration of 1.61 ng/mL and declined to 0.11 ng/mL at 29 days. For the vitreous of the noninjected eye, the levels of bevacizumab ranged from 0.245 ng/mL at 1 day after injection to a maximum of 0.335 ng/mL at 8 days and then declined to 0.218 ng/mL at 29 days. Figure 3 shows the change in concentration of bevacizumab in the aqueous and vitreous humor of the fellow noninjected eye.
Figure 3.
Bevacizumab concentration in the vitreous and the aqueous humor of the noninjected left eye after intravitreal injection of 1.25 mg/0.05 mL bevacizumab into the fellow eye. Values at day 0 indicate background levels of bevacizumab detection in control animals.
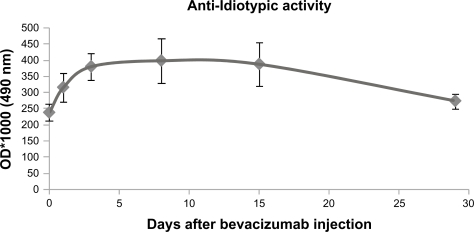
The results of high sensitivity ELISA assay for the detection of anti-bevacizumab antibodies are illustrated in Figure 4. There was a 30% increase of anti-bevacizumab antibodies at day 1, 60% at day 3, and 70% at day 8. Subsequently, the anti-bevacizumab activity of antibodies gradually decreased (to 120% of the initial levels at day 29), following the elimination of bevacizumab from blood circulation. Taking into account the absolute optical density values of the measurements of the high sensitivity ELISA, compared with the high sensitivity assay for bevacizumab, we can conclude that the concentration of anti-bevacizumab antibodies in serum is at least 200-fold lower than the bevacizumab concentration. Therefore, anti-bevacizumab antibodies cannot have an important effect on bevacizumab concentration because of their low concentration.
Figure 4.
Quantification of anti-bevacizumab antibodies in serum of rabbits at day 0 (before intravitreal injection of bevacizumab) and at days 1, 3, 8, 15, and 29 after injection.
Abbreviation: OD, optical density.
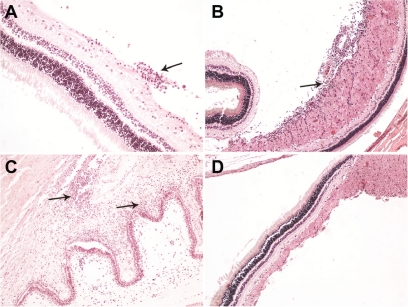
The cytotoxicity of the drug is currently under investigation in our laboratory. Our preliminary results show no important histological changes in all the anatomical parts that were examined by light microscopy (Figure 5). In both injected and noninjected eyes, no signs of necrosis or degeneration of the retina were noticed and the retinal thickness was unchanged. Only some specimens of the right injected eyes revealed chronic inflammatory infiltrations (indicated by arrows in Figure 5) consisting of lymphocytes, plasma cells, and rarely eosinophils. The left noninjected eyes had no histological changes as seen by light microscopy.
Figure 5.
Light micrographs of 4μm thick sections stained with hematoxylin-eosin revealed chronic inflammatory infiltrations (A) especially in the nerve fiber layer of the retina around the optic disc (B) and in iris (C) in a few specimens. No other histological differences were evident between injected and noninjected eyes in the rest of the specimens (D).
Discussion
In our study, we aimed to describe the pharmacokinetics of 1.25 mg/0.05 mL intravitreal bevacizumab in rabbits. This is the dose that is currently being used in human eyes and is widely described in the literature.4,7–9,11,13–16 The assay we used measures free bevacizumab and does not detect complexes of bevacizumab with bound VEGF. Assays which detect only the free drug are used widely in pharmacologic research and results are usually considered good representations of total drug concentration. However, an underestimation of the total bevacizumab concentration by our results cannot be excluded because of partial denaturation or proteolytic degradation of bevacizumab which may take place in samples.
Studies of pharmacokinetics of intravitreal bevacizumab in rabbits have already been published.13,14 Nevertheless, it is very important to make measurements as accurate as possible in order to know the exact pharmacokinetics of bevacizumab and optimize drug dosing and therapeutic results in both injected and noninjected eyes.
Our results show that the maximum concentration of bevacizumab in the right vitreous is measured at day 1 after injection (406 μg/mL) and then declines, but it remains at high levels 29 days after injection (5 μg/mL). Maximum concentration of bevacizumab in right aqueous was measured at day 1 as well and not at day 3 as in the Bakri et al study, in which it was much lower (5.8 μg/mL instead of 37.7 μg/mL).13 Maximum concentration of bevacizumab in serum is measured 8 days after intravitreal injection due to its gradual distribution from the right eye to serum (vitreousR→aqueousR→serum).13 On the same day (day 8), we measured the maximum concentration of bevacizumab in the left aqueous because bevacizumab is very easily and quickly distributed from serum to left aqueous (serum → aqueousL).13 The concentration of bevacizumab in the left vitreous reached a peak concentration at day 8 and then gradually declined, not constantly raised as in the Bakri et al study.13 The reduction of bevacizumab concentration in the left vitreous after day 8 is caused by the reduction of drug concentration in serum and left aqueous.
In our rabbit model we used high sensitivity ELISA (linear range 10 pg/mL to 5 ng/mL) in order to measure drug concentrations in the left eye and low sensitivity ELISA (with linear range between 5–100 ng/mL) to measure drug concentrations in the right eye. In this regard, we avoided dilutions in the order of 1 million-fold, required for the measurement of bevacizumab with the HS assay in the right eye, which would inevitably be accompanied by a 1 million-fold increase of the measurement errors. On the other hand, the HS assay (with detection limit at 5 pg/mL) was necessary to assure the accurate detection of the very low concentrations of bevacizumab in the noninjected eye. This is the reason we measured considerably lower concentrations in the fellow eye compared with those of the Bakri et al study.13
We also measured considerably lower concentrations in the aqueous and vitreous of the fellow noninjected eye and higher concentrations in the aqueous and vitreous of the injected eye compared with the values in the Nomoto et al study.14 In addition, we measured lower concentrations in serum and we found that the maximum concentration of bevacizumab in serum was achieved at day 8 and not at day 14, as in the Nomoto et al study.14 In our assay, hVEGF was used in order to capture and quantify ‘active’ bevacizumab molecules with a detection limit of 0.01 ng/mL. Nomoto et al used anti-rabbit IgG (Heavy + Light chains) to capture antibodies with a detection limit of 0.1 ng/mL. In that case, both native and denatured bevacizumab molecules as well as their fragments were quantified with questionable ability to recognize hVEGF. Moreover, we obtained samples more frequently during the first month (days 1, 3, 8, 15, and 29) and thus we demonstrated a more detailed pharmacokinetic profile of bevacizumab during the first month compared with the Nomoto et al study. Finally, our results depict the variations between animals and the development of anti-bevacizumab antibodies.
The variability of results between animals was very low for the right eye but considerably higher for the left eye (see error bars in Figures 2 and 3). The maximum variability was observed at day 15 for one of the rabbits (rabbit with identification number R13), which had considerably higher concentration of bevacizumab in the left eye than the other three rabbits on the same day (0.63 ng/mL in the left vitreous and 0.8 ng/mL in the left aqueous, while the other three rabbits had an average of 0.19 ng/mL and 0.25 ng/mL in the left vitreous and left aqueous on the same day, respectively). These very high concentrations of bevacizumab in rabbit R13 may have been caused by a higher distribution rate of drug from the right vitreous to serum and then to the left aqueous and vitreous, most probably due to inflammation, infection, or traumatic damage of vessels in the injection area.
As we have already mentioned, very low concentrations of bevacizumab were detected in the fellow noninjected eye. It is very important to know if these concentrations are able to achieve therapeutic results in this eye. Wang et al17 found that bevacizumab displayed a dose-dependent inhibition in an in vitro endothelial cell proliferation assay, with an estimated median inhibition concentration of 22 ng/mL. In our study, the maximum concentration of bevacizumab in the vitreous of the noninjected eye was much lower (0.335 ng/mL). Our findings suggest that intravitreal injection of 1.25 mg/0.05 mL bevacizumab in one eye cannot achieve therapeutic concentrations in the fellow noninjected eye, even though Avery et al9 reported that some patients with bilateral proliferative diabetic retinopathy had regression of neovascularization in both eyes when injected with intravitreal bevacizumab in only one eye.
Rabbits have been widely used in order to study intravitreal pharmacokinetics of drugs.18–20 There are some differences between humans and rabbits which may affect the study of the drug’s pharmacokinetics. The most important are that humans have a larger vitreous cavity (4.5 mL instead of 1.5 mL in rabbits), a larger serum compartment, and a more vascular retina. Krohne et al,21 in a study of pharmacokinetics of intravitreal bevacizumab in humans, found that after a single intravitreal injection of 1.5 mg bevacizumab, the concentration of the drug in the aqueous humor peaked on the first day after injection (33.3 μg/mL) and dropped to less than 5 μg/mL at day 28. These concentrations are much higher than those in our study (5.8 and 0.2 μg/mL, respectively). Zhu et al15 measured vitreous levels of bevacizumab after intravitreal injection of 1.25 mg of bevacizumab and found that maximum concentration was achieved at day 2 after injection instead of day 1 in our study. Furthermore, the maximum concentration of bevacizumab was 165 μg/mL instead of 406 μg/mL in our study.
In our study, we used nonvitrectomized eyes. In vitrectomized eyes, clearance of the drug may be markedly increased. After intravitreal injection of triamcinolone acetonide in rabbits, the half-life in the aqueous humor was reported to be 2.89 days in nonvitrectomized eyes and 1.57 days in vitrectomized eyes.22 In humans, Beer et al23 reported that the half-life of the same drug in the aqueous humor after intravitreal injection of triamcinolone acetonide was 18.6 days in nonvitrectomized eyes compared with 3.2 days in vitrectomized eyes. Similar effects should be expected for bevacizumab in vitrectomized eyes and may result in a lack of clinical effect of bevacizumab, as reported by Yanyali et al16 in vitrectomized human eyes with diabetic macular edema.
Besides bevacizumab, its derivative drug ranibizumab (Lucentis®; Genentech; South San Francisco, CA) is now widely used as intravitreal anti-VEGF therapy for neovascular AMD. Bevacizumab has a larger molecular weight (149 kDa)24 than ranibizumab (48 kDa)25 and its penetration into the retina may be slower than ranibizumab’s. Its clearance from the vitreous may be slower as well, resulting in a longer half-life in the human eye, thus requiring less frequent application. In rabbits, Bakri et al reported a vitreous half-life of 2.88 days after intravitreal injection of 0.5 mg ranibizumab,26 which indeed is shorter than the half-life reported for bevacizumab (4.32 days) in the same animal model.13 In monkeys, the vitreous elimination half-life of ranibizumab was determined by Gaudreault et al to be 2.6 days after bilateral intravitreal injection of 0.5 mg of the drug.27 With the increasing use of intravitreal injections of anti-VEGF substances for various ocular diseases, accurate information on their pharmacokinetics is very important and will help to optimize reinjection intervals and achieve the maximum therapeutic results.
Footnotes
Disclosure
No author has a proprietary interest in any of the products mentioned in the article.
References
- 1.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Mordenti J, Cuthbertson RA, Ferrara N, et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125l-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999;27:536–544. doi: 10.1177/019262339902700507. [DOI] [PubMed] [Google Scholar]
- 3.Shahar J, Avery RL, Heilweil G, et al. Electrophysiologic and retinal penetration studies following intravitreal administration of bevacizumab (Avastin) Retina. 2006;26:262–269. doi: 10.1097/00006982-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Dib Eduardo, Maia Mauricio, Longo-Maugeri Ieda Maria, et al. Subretinal Bevacizumab Detection after Intravitreous Injection in Rabbits. Invest Ophthalmol Vis Sci. 2008;49:1097–1100. doi: 10.1167/iovs.07-1225. [DOI] [PubMed] [Google Scholar]
- 5.Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112:1035–1047. doi: 10.1016/j.ophtha.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Ladewig MS, Ziemssen F, Jaissle G, et al. Intravitreal bevacizumab for neovascular age-related macular degeneration. Ophthalmologe. 2006;103:463–470. doi: 10.1007/s00347-006-1352-5. [DOI] [PubMed] [Google Scholar]
- 7.Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006;26:999–1005. doi: 10.1097/01.iae.0000247165.38655.bf. [DOI] [PubMed] [Google Scholar]
- 8.Diabetic Retinopathy Clinical Research Network. Scott IU, Edwards AR, Beck RW, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114:1860–1867. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695–1705. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 10.Jaissle GB, Ziemssen F, Petermeier K, et al. Bevacizumab for treatment of macular edema secondary to retinal vein occlusion. Ophthalmologe. 2006;103:471–475. doi: 10.1007/s00347-006-1355-2. [DOI] [PubMed] [Google Scholar]
- 11.Priglinger SG, Wolf AH, Kreutzer TC, et al. Intravitreal bevacizumab injections for treatment of central retinal vein occlusion: six-month results of a prospective trial. Retina. 2007;27:1004–1012. doi: 10.1097/IAE.0b013e3180ed458d. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36:331–335. [PubMed] [Google Scholar]
- 13.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin) Ophthalmology. 2007;114:855–859. doi: 10.1016/j.ophtha.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Nomoto H, Shiraga F, Kuno N, et al. Pharmacokinetics of bevacizumab after topical, sub conjunctival, and intravitreal administration in rabbits. Invest Ophthalmol Vis Sci. 2009;50:4807–4813. doi: 10.1167/iovs.08-3148. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Qi, Ziemssen Focke, Henke-Fahle Sigrid, et al. Vitreous levels of bevacizumab and vascular endothelial growth factor-A in patients with choroidal neovascularization. Ophthalmology. 2008;115:1750–1755. doi: 10.1016/j.ophtha.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Yanyali A, Aytug B, Horozoglu F, Nohutcu AF. Bevacizumab (Avastin) for diabetic macular edema in previously vitrectomized eyes. Am J Ophthalmol. 2007;144:124–126. doi: 10.1016/j.ajo.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7:335–345. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- 18.Iyer MN, He F, Wensel TG, et al. Clearance of intravitreal moxifloxacin. Invest Ophthalmol Vis Sci. 2006;47:317–319. doi: 10.1167/iovs.05-1124. [DOI] [PubMed] [Google Scholar]
- 19.Fauser S, Kalbacher H, Alteheld N, et al. Pharmacokinetics and safety of intravitreally delivered etanercept. Graefes Arch Clin Exp Ophthalmol. 2004;242:582–586. doi: 10.1007/s00417-004-0895-x. [DOI] [PubMed] [Google Scholar]
- 20.Scholes GN, O’ Brien WJ, Abrams GW, Kubicek MF. Clearance of triamcinolone from vitreous. Arch Ophthalmol. 1985;103:1567–1569. doi: 10.1001/archopht.1985.01050100143037. [DOI] [PubMed] [Google Scholar]
- 21.Krohne T, Eter N, Holz F, Meyer C. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol. 2008;146:508–512. doi: 10.1016/j.ajo.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Chin HS, Park TS, Moon YS, Oh JH. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina. 2005;25:556–560. doi: 10.1097/00006982-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Beer PM, Bakri SJ, Singh RJ, et al. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110:681–686. doi: 10.1016/S0161-6420(02)01969-3. [DOI] [PubMed] [Google Scholar]
- 24.European Medicines Agency (EMEA) Avastin (bevacizumab): scientific discussion. Available at: http://www.emea.europa.eu/humandocs/PDFs/EPAR/avastin/17199204en6.pdf. Accessed September 28, 2007.
- 25.European Medicines Agency (EMEA) Lucentis (ranibizumab): scientific discussion. Available at: http://www.emea.europa.eu/humandocs/PDFs/EPAR/lucentis/H-715-EN6.pdf. Accessed September 28, 2007.
- 26.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis) Ophthalmology. 2007;114:2179–2182. doi: 10.1016/j.ophtha.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–733. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]