Abstract
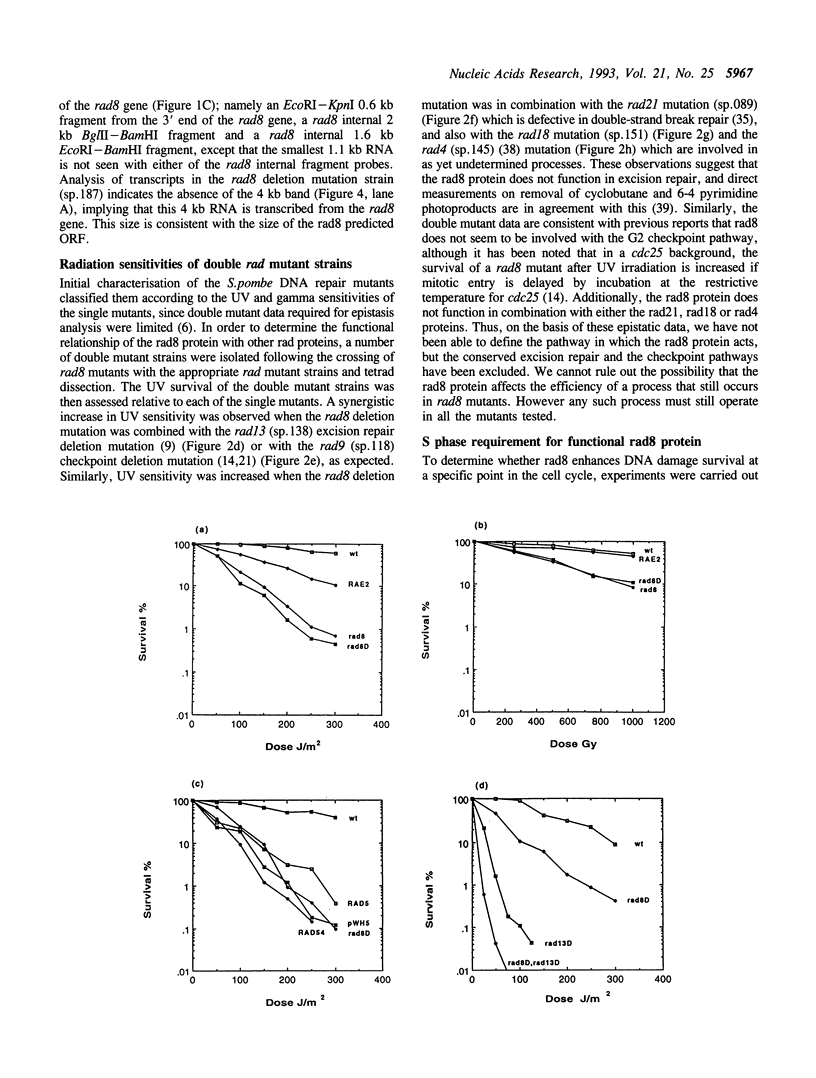
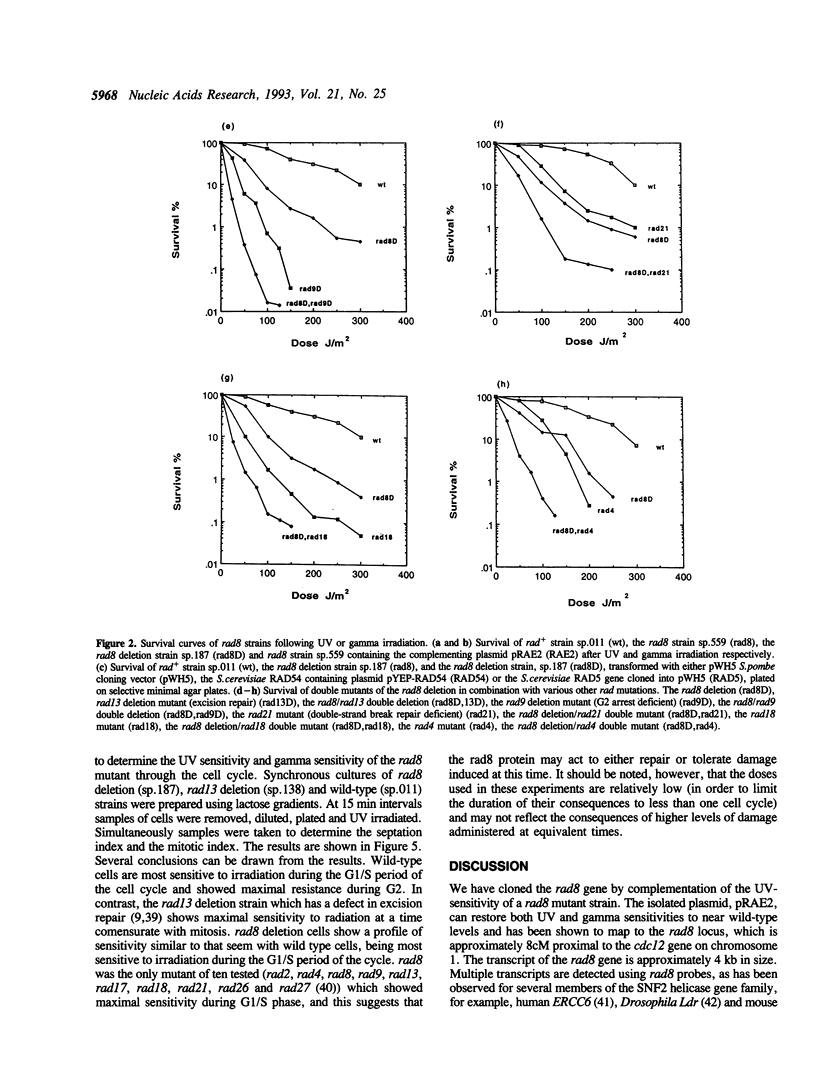
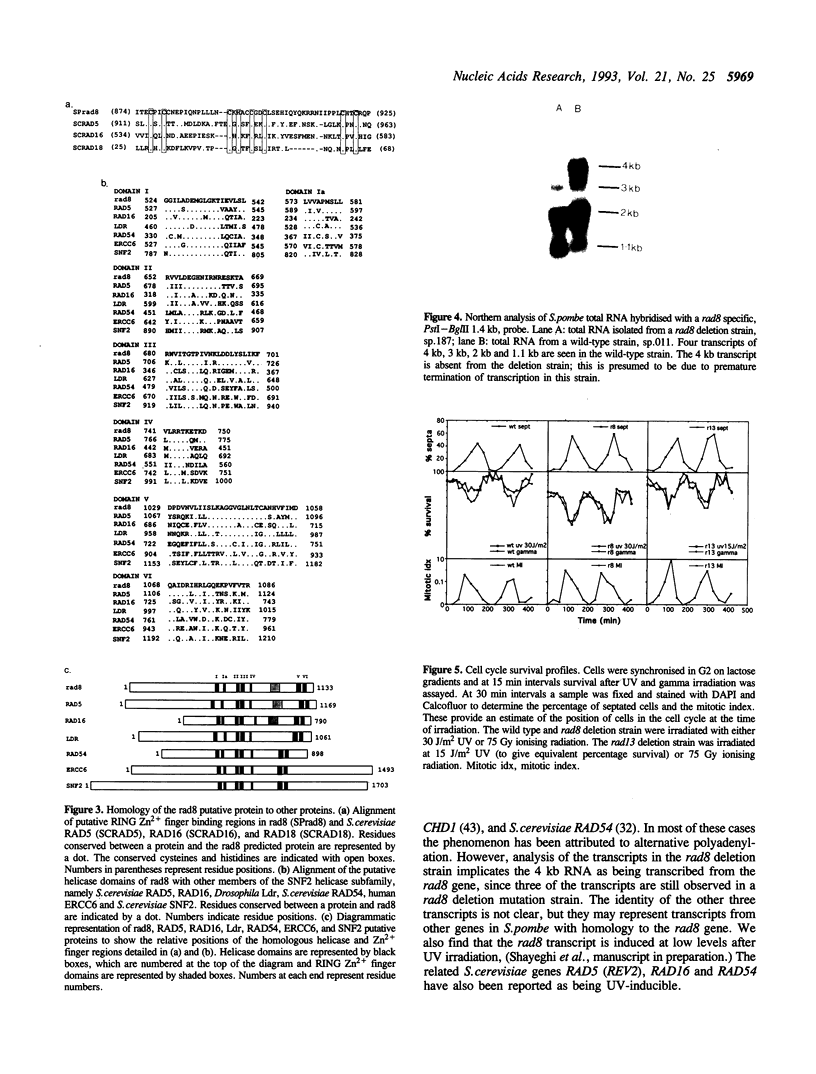
The Schizosaccharomyces pombe rad8 mutant is sensitive to both UV and gamma irradiation. We have cloned the rad8 gene by complementation of the UV sensitivity of a rad8.190 mutant strain. The gene comprises an open reading frame of 3.4 kb which does not contain any introns and is capable of encoding a 1133 amino acid protein of 129 kDa. Deletion of the gene indicates that it is not essential for cell viability. Recognisable motifs are present for a nuclear localisation signal, a RING finger and helicase domains. The predicted protein is a member of the SNF2 subfamily of proteins and shows particular homology to the Saccharomyces cerevisiae RAD5 protein. Double mutant analysis demonstrated that the rad8 mutant is not epistatic to mutants in the excision repair pathway (rad13) or checkpoint pathway (rad9). Analysis of radiation sensitivity though the cell cycle indicates that, unlike most other rad mutants, rad8 is most sensitive to irradiation during the G1/S period.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahne F., Baur M., Eckardt-Schupp F. The REV2 gene of Saccharomyces cerevisiae: cloning and DNA sequence. Curr Genet. 1992 Oct;22(4):277–282. doi: 10.1007/BF00317921. [DOI] [PubMed] [Google Scholar]
- Bang D. D., Verhage R., Goosen N., Brouwer J., van de Putte P. Molecular cloning of RAD16, a gene involved in differential repair in Saccharomyces cerevisiae. Nucleic Acids Res. 1992 Aug 11;20(15):3925–3931. doi: 10.1093/nar/20.15.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet N. C., Carr A. M. Fission yeast wee1 protein kinase is not required for DNA damage-dependent mitotic arrest. Nature. 1993 Aug 26;364(6440):824–827. doi: 10.1038/364824a0. [DOI] [PubMed] [Google Scholar]
- Barbet N., Muriel W. J., Carr A. M. Versatile shuttle vectors and genomic libraries for use with Schizosaccharomyces pombe. Gene. 1992 May 1;114(1):59–66. doi: 10.1016/0378-1119(92)90707-v. [DOI] [PubMed] [Google Scholar]
- Birkenbihl R. P., Subramani S. Cloning and characterization of rad21 an essential gene of Schizosaccharomyces pombe involved in DNA double-strand-break repair. Nucleic Acids Res. 1992 Dec 25;20(24):6605–6611. doi: 10.1093/nar/20.24.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P., Koonin E. V. An expanding family of helicases within the 'DEAD/H' superfamily. Nucleic Acids Res. 1993 Feb 11;21(3):751–752. doi: 10.1093/nar/21.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton B. C., Barbet N., Murray J., Watts F. Z., Koken M. H., Lehmann A. R., Carr A. M. Assignment of ten DNA repair genes from Schizosaccharomyces pombe to chromosomal NotI restriction fragments. Mol Gen Genet. 1991 Sep;228(3):470–472. doi: 10.1007/BF00260641. [DOI] [PubMed] [Google Scholar]
- Carr A. M., Sheldrick K. S., Murray J. M., al-Harithy R., Watts F. Z., Lehmann A. R. Evolutionary conservation of excision repair in Schizosaccharomyces pombe: evidence for a family of sequences related to the Saccharomyces cerevisiae RAD2 gene. Nucleic Acids Res. 1993 Mar 25;21(6):1345–1349. doi: 10.1093/nar/21.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. L., Kunisawa R., Thorner J. A presumptive helicase (MOT1 gene product) affects gene expression and is required for viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1992 Apr;12(4):1879–1892. doi: 10.1128/mcb.12.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas V., Stokes D. G., Perry R. P. A mammalian DNA-binding protein that contains a chromodomain and an SNF2/SWI2-like helicase domain. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2414–2418. doi: 10.1073/pnas.90.6.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery H. S., Schild D., Kellogg D. E., Mortimer R. K. Sequence of RAD54, a Saccharomyces cerevisiae gene involved in recombination and repair. Gene. 1991 Jul 31;104(1):103–106. doi: 10.1016/0378-1119(91)90473-o. [DOI] [PubMed] [Google Scholar]
- Fenech M., Carr A. M., Murray J., Watts F. Z., Lehmann A. R. Cloning and characterization of the rad4 gene of Schizosaccharomyces pombe; a gene showing short regions of sequence similarity to the human XRCC1 gene. Nucleic Acids Res. 1991 Dec 25;19(24):6737–6741. doi: 10.1093/nar/19.24.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont P. S., Hanson I. M., Trowsdale J. A novel cysteine-rich sequence motif. Cell. 1991 Feb 8;64(3):483–484. doi: 10.1016/0092-8674(91)90229-r. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C. Deoxyribonucleic acid repair in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Mar;52(1):70–102. doi: 10.1128/mr.52.1.70-102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C. Yeast genes involved in DNA-repair processes: new looks on old faces. Mol Microbiol. 1991 Oct;5(10):2303–2310. doi: 10.1111/j.1365-2958.1991.tb02074.x. [DOI] [PubMed] [Google Scholar]
- Girdham C. H., Glover D. M. Chromosome tangling and breakage at anaphase result from mutations in lodestar, a Drosophila gene encoding a putative nucleoside triphosphate-binding protein. Genes Dev. 1991 Oct;5(10):1786–1799. doi: 10.1101/gad.5.10.1786. [DOI] [PubMed] [Google Scholar]
- Grimm C., Kohli J., Murray J., Maundrell K. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol Gen Genet. 1988 Dec;215(1):81–86. doi: 10.1007/BF00331307. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H. Nucleotide excision repair I: from E. coli to yeast. Trends Genet. 1993 May;9(5):173–177. doi: 10.1016/0168-9525(93)90164-d. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H. Nucleotide excision repair. II: From yeast to mammals. Trends Genet. 1993 Jun;9(6):211–217. doi: 10.1016/0168-9525(93)90121-w. [DOI] [PubMed] [Google Scholar]
- Hoheisel J. D., Maier E., Mott R., McCarthy L., Grigoriev A. V., Schalkwyk L. C., Nizetic D., Francis F., Lehrach H. High resolution cosmid and P1 maps spanning the 14 Mb genome of the fission yeast S. pombe. Cell. 1993 Apr 9;73(1):109–120. doi: 10.1016/0092-8674(93)90164-l. [DOI] [PubMed] [Google Scholar]
- Johnson R. E., Henderson S. T., Petes T. D., Prakash S., Bankmann M., Prakash L. Saccharomyces cerevisiae RAD5-encoded DNA repair protein contains DNA helicase and zinc-binding sequence motifs and affects the stability of simple repetitive sequences in the genome. Mol Cell Biol. 1992 Sep;12(9):3807–3818. doi: 10.1128/mcb.12.9.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolstø A. B., Bork P., Kvaløy K., Lindback T., Grønstadt A., Kristensen T., Sander C. Prokaryotic members of a new family of putative helicases with similarity to transcription activator SNF2. J Mol Biol. 1993 Mar 20;230(2):684–688. doi: 10.1006/jmbi.1993.1185. [DOI] [PubMed] [Google Scholar]
- Laurent B. C., Treich I., Carlson M. The yeast SNF2/SWI2 protein has DNA-stimulated ATPase activity required for transcriptional activation. Genes Dev. 1993 Apr;7(4):583–591. doi: 10.1101/gad.7.4.583. [DOI] [PubMed] [Google Scholar]
- Laurent B. C., Treitel M. A., Carlson M. Functional interdependence of the yeast SNF2, SNF5, and SNF6 proteins in transcriptional activation. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2687–2691. doi: 10.1073/pnas.88.7.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman H. B., Riley R., Martel M. Isolation and initial characterization of a Schizosaccharomyces pombe mutant exhibiting temperature-dependent radiation sensitivity due to a mutation in a previously unidentified rad locus. Mol Gen Genet. 1989 Sep;218(3):554–558. doi: 10.1007/BF00332423. [DOI] [PubMed] [Google Scholar]
- Mitchison J. M., Carter B. L. Cell cycle analysis. Methods Cell Biol. 1975;11:201–219. [PubMed] [Google Scholar]
- Muris D. F., Vreeken K., Carr A. M., Broughton B. C., Lehmann A. R., Lohman P. H., Pastink A. Cloning the RAD51 homologue of Schizosaccharomyces pombe. Nucleic Acids Res. 1993 Sep 25;21(19):4586–4591. doi: 10.1093/nar/21.19.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. M., Carr A. M., Lehmann A. R., Watts F. Z. Cloning and characterisation of the rad9 DNA repair gene from Schizosaccharomyces pombe. Nucleic Acids Res. 1991 Jul 11;19(13):3525–3531. doi: 10.1093/nar/19.13.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. M., Doe C. L., Schenk P., Carr A. M., Lehmann A. R., Watts F. Z. Cloning and characterisation of the S. pombe rad15 gene, a homologue to the S. cerevisiae RAD3 and human ERCC2 genes. Nucleic Acids Res. 1992 Jun 11;20(11):2673–2678. doi: 10.1093/nar/20.11.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Peterson C. L., Herskowitz I. Characterization of the yeast SWI1, SWI2, and SWI3 genes, which encode a global activator of transcription. Cell. 1992 Feb 7;68(3):573–583. doi: 10.1016/0092-8674(92)90192-f. [DOI] [PubMed] [Google Scholar]
- Phipps J., Nasim A., Miller D. R. Recovery, repair, and mutagenesis in Schizosaccharomyces pombe. Adv Genet. 1985;23:1–72. doi: 10.1016/s0065-2660(08)60511-8. [DOI] [PubMed] [Google Scholar]
- Reynolds P. R., Biggar S., Prakash L., Prakash S. The Schizosaccharomyces pombe rhp3+ gene required for DNA repair and cell viability is functionally interchangeable with the RAD3 gene of Saccharomyces cerevisiae. Nucleic Acids Res. 1992 May 11;20(9):2327–2334. doi: 10.1093/nar/20.9.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P., Koken M. H., Hoeijmakers J. H., Prakash S., Prakash L. The rhp6+ gene of Schizosaccharomyces pombe: a structural and functional homolog of the RAD6 gene from the distantly related yeast Saccharomyces cerevisiae. EMBO J. 1990 May;9(5):1423–1430. doi: 10.1002/j.1460-2075.1990.tb08258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihs H. P., Jans D. A., Fan H., Peters R. The rate of nuclear cytoplasmic protein transport is determined by the casein kinase II site flanking the nuclear localization sequence of the SV40 T-antigen. EMBO J. 1991 Mar;10(3):633–639. doi: 10.1002/j.1460-2075.1991.tb07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley R., Subramani S., Young P. G. Checkpoint controls in Schizosaccharomyces pombe: rad1. EMBO J. 1992 Apr;11(4):1335–1342. doi: 10.1002/j.1460-2075.1992.tb05178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild D., Glassner B. J., Mortimer R. K., Carlson M., Laurent B. C. Identification of RAD16, a yeast excision repair gene homologous to the recombinational repair gene RAD54 and to the SNF2 gene involved in transcriptional activation. Yeast. 1992 May;8(5):385–395. doi: 10.1002/yea.320080506. [DOI] [PubMed] [Google Scholar]
- Tamkun J. W., Deuring R., Scott M. P., Kissinger M., Pattatucci A. M., Kaufman T. C., Kennison J. A. brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell. 1992 Feb 7;68(3):561–572. doi: 10.1016/0092-8674(92)90191-e. [DOI] [PubMed] [Google Scholar]
- Troelstra C., Hesen W., Bootsma D., Hoeijmakers J. H. Structure and expression of the excision repair gene ERCC6, involved in the human disorder Cockayne's syndrome group B. Nucleic Acids Res. 1993 Feb 11;21(3):419–426. doi: 10.1093/nar/21.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troelstra C., van Gool A., de Wit J., Vermeulen W., Bootsma D., Hoeijmakers J. H. ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne's syndrome and preferential repair of active genes. Cell. 1992 Dec 11;71(6):939–953. doi: 10.1016/0092-8674(92)90390-x. [DOI] [PubMed] [Google Scholar]
- Wright A., Maundrell K., Heyer W. D., Beach D., Nurse P. Vectors for the construction of gene banks and the integration of cloned genes in Schizosaccharomyces pombe and Saccharomyces cerevisiae. Plasmid. 1986 Mar;15(2):156–158. doi: 10.1016/0147-619x(86)90051-x. [DOI] [PubMed] [Google Scholar]
- al-Khodairy F., Carr A. M. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992 Apr;11(4):1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




