Abstract
Background
Light-to-moderate alcohol consumption is associated with a reduced risk of coronary heart disease (CHD). This protective effect of alcohol, however, may be confined to middle-aged or older individuals. CHD Incidence is low in men younger than 40 and in women younger than 50 years and for this reason, study cohorts rarely have the power to investigate effects of alcohol on CHD risk in younger adults. This study examined whether the beneficial effect of alcohol on CHD depends on age.
Methods and results
A pooled analysis of eight prospective studies from North America and Europe including 192,067 women and 74,919 men free of cardiovascular diseases, diabetes, and cancers at baseline. Average daily alcohol intake was assessed at baseline using a food frequency or diet history questionnaire. An inverse association between alcohol and risk of coronary heart disease was observed in all age groups: hazard ratios among moderately drinking men (5.0–29.9 g/day) aged 39–50, 50–59, and 60+ years were 0.58 (95% C.I. 0.36 to 0.93), 0.72 (95% C.I. 0.60–0.86), and 0.85 (95% C.I. 0.75 to 0.97) compared with abstainers. However, the analyses indicated a smaller incidence rate difference (IRD) between abstainers and moderate consumers in younger adults (IRD=45 per 100,000; 90% C.I. 8 to 84), than in middle-aged (IRD=64 per 100,000; 90% C.I. 24 to 102) and older adults (IRD=89 per 100,000; 90% C.I. 44 to 140). Similar results were observed in women.
Conclusions
Alcohol is also associated with a decreased risk of CHD in younger adults; however, the absolute risk was small compared with middle-aged and older adults.
Keywords: Alcohol, Coronary disease, Age groups, Epidemiology
The association between alcohol intake and coronary heart disease (CHD) has been thoroughly investigated over the past decades, both with regard to the amount and type consumed.1–4 In recent years, the importance of drinking pattern has been considered as well.5–7 In general, alcohol intake is consistently linked with a lower risk of CHD. Age-specific incidence rates of CHD vary considerably, being very low in men younger than 40 and in women younger than 50 years.8 For this reason, the statistical power to investigate effects of alcohol on CHD in younger adults is limited. Most results are obtained from cohorts consisting of middle-aged and older adults and only few studies have addressed the effects of alcohol on CHD in younger adults.9;10 In principle, the aetiology of CHD among younger adults may differ from the aetiology among older individuals; for instance, relatively more cases of CHD among younger adults may be attributable to genetic causes.11;12 Hence, alcohol may not necessarily protect against CHD in this age group. We pooled the data from eight studies to increase sample size, and enable the investigation of associations between alcohol intake and CHD in subsets of populations defined by age group.
METHODS
Study population
The analyses were based on data from the Pooling Project on Diet and Coronary Disease. The inclusion criteria were: A prospective study with at least 150 incident coronary cases, assessment of usual dietary intake, and a validation study of the diet assessment method. The following 12 studies met these criteria and agreed to share data: Adventists health study (AHS),13 Atherosclerosis risk in communities study (ARIC),14 Alpha-tocopherol, beta-carotene cancer prevention study (ATBC),15 Finnish mobile clinic health examination (FMC),16 Glostrup population study (GPS),17 Health professionals follow-up study (HPFS),18 Israeli ischemic heart disease study (IIHD),19 Iowa women’s health study (IWHS),20 Nurses’ health study (NHS),21 Västerbotten intervention program (VIP),22 and Women’s health study (WHS).23 The AHS only included non-drinkers, and the FMC and IIHD studies were excluded from the present analysis due to missing information on alcohol intake. In addition, IWHS was excluded from main analyses due to self-reported information on CHD. The eight remaining studies are presented in Table 1. The NHS was divided in two segments thereby taking advantage of repeated assessments on dietary intake and the long follow-up period. The two segments are referred to as NHSa (1980–86) and NHSb (1986–96), respectively. The second segment only comprises women who remained free of CHD after the first follow-up period, and cases are included in whichever segment they occurred.
Table 1.
Baseline Characteristics of Included Studies.
| No. of CHD cases
|
Alcohol intake
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Study | Baseline cohort* | Year of questionnaire | Mean age, years (limits) | Person-years of follow-up | CHD deaths | Total CHD events | Median†, g/day (5th-95th percentile) | Abstainers, % |
| ARIC
| ||||||||
| Men | 5217 | 1987–89 | 54.6 (45–64) | 45,652 | 51 | 267 | 12.1 (1.9–56.6) | 45.8 |
| Women | 6462 | 1987–89 | 53.9 (45–64) | 58,019 | 18 | 122 | 6.8 (1.5–30.2) | 69.8 |
|
| ||||||||
| ATBC
| ||||||||
| Men | 21,141 | 1984–88 | 57.3 (50–69) | 121,813 | 534 | 1339 | 13.7 (0.8–62.3) | 10.2 |
|
| ||||||||
| GPS
| ||||||||
| Men | 1658 | 1974–95 | 51.9 (35–80) | 14,365 | 79 | 102 | 20.8 (2.7–72.6) | 6.2 |
| Women | 1666 | 1974–95 | 51.5 (35–80) | 14,605 | 34 | 34 | 8.9 (1.3–35.1) | 18.0 |
|
| ||||||||
| HPFS
| ||||||||
| Men | 41,754 | 1986–88 | 53.4 (39–77) | 383,206 | 421 | 1273 | 9.7 (1.0–46.1) | 23.0 |
|
| ||||||||
| NHSa
| ||||||||
| Women | 81,415 | 1980–82 | 47.1 (35–66) | 513,915 | 97 | 397 | 5.6 (0.8–35.0) | 31.4 |
|
| ||||||||
| NHSb
| ||||||||
| Women | 61,706 | 1986–88 | 52.6 (39–66) | 607,049 | 208 | 696 | 4.9 (0.9–35.9) | 34.2 |
|
| ||||||||
| VIP
| ||||||||
| Men | 9521 | 1992–96 | 49.1 (39–70) | 39,230 | 38 | 134 | 4.4 (0.2–15.8) | 4.7 |
| Women | 10,555 | 1992–96 | 49.3 (39–70) | 43,872 | 4 | 23 | 1.7 (0.1–7.3) | 11.4 |
|
| ||||||||
| WHS
| ||||||||
| Women | 37,272 | 1992–95 | 53.9 (38–89) | 190,755 | 10 | 152 | 3.7 (0.9–28.4) | 40.0 |
|
| ||||||||
|
Total
| ||||||||
| Men | 79,291 | 1974–96 | 54.0 (35–80) | 604,266 | 1123 | 3115 | 9.6 (0.9–50.4) | 18.5 |
| Women | 199,076 | 1974–96 | 50.4 (35–89) | 1,428,216 | 371 | 1424 | 4.7 (0.8–35.0) | 33.9 |
ARIC, Atherosclerosis Risk in Communities Study; ATBC, Alpha-Tocopherol Beta-Carotone Cancer Prevention Study; GPS, Glostrup Population Study; HPFS, Health Professionals Follow-up Study; NHSa, Nurses’ Health Study 1980–86; NHSb, Nurses’ Health Study 1986–96; VIP, Västerbotten Intervention Program; WHS, Women’s Health Study
Sample size after exclusion of participants with baseline cardiovascular diseases, cancers, diabetes, and missing information on alcohol intake
Median values were calculated for drinkers only
Measurements
Average daily alcohol intake was assessed at baseline using a food frequency or diet history questionnaire inquiring about typical intake of alcoholic beverages. For each beverage, grams of daily alcohol intake were calculated based on information on amount andfrequency and the alcohol content of the beverage. Study-specific conversion factors for the alcohol content were used. A standard drink contains approximately 10–15 grams of pure alcohol. The total alcohol intake was given by the sum of the beverage-specific intakes.
The outcome of interest was incident CHD events (both fatal and non-fatal). All eight of the included studies have used validated methods to define nonfatal and fatal CHD cases.24
Statistical methods
Participants were excluded if they reported energy intakes beyond 3 standard deviations from the study-specific, log-transformed mean energy intake of the baseline population (1% of the study population). Persons aged less than 35 years or with a history of cardiovascular disease, diabetes, or cancers (other than non-melanoma skin cancer) were also excluded. Participants were followed from baseline to date of CHD-event, date of death or end of follow-up whichever occurred first. Follow-up periods longer than 10 years (ARIC and GPS) were truncated to reduce heterogeneity. Individual studies were combined using an aggregated pooled analysis technique allowing for calculation of a single exposure-effect estimate while adjusting for study origin.25
The hazard ratios of CHD were ascertained by the Cox proportional hazards regression model with age as underlying time scale, allowing for delayed entry (left censoring).26 Absolute risks (incidence rates) describing the scale of CHD according to sex, age, and level of alcohol intake were estimated by means of Poisson regression.27 Absolute risk differences were calculated and 90% confidence limits were derived by bootstrap estimation (5,000 replications) with the 5th and 95th percentiles of the distribution as lower and upper limits.
We performed primary analyses considering the risk of CHD in categories of alcohol consumption (0, 0.1–4.9, 5.0–14.9, 15.0–29.9, 30.0–59.9, ≥60.0 g/day in women and 0, 0.1–4.9, 5.0–14.9, 15.0–29.9, 30.0–59.9, 60.0–89.9 ≥90.0 g/day in men) both for each individual study and for the pooled cohort. The study population was analysed separately in the following three age groups: 39–49.9 years, 50–59.9 years, and 60+ years. Age was updated during follow-up and participants were assigned to the appropriate age category; thus each person could contribute person-time at risk to more than one age category.
Additional analyses exploring the risk of CHD per alcohol increment (1 g/day) were performed. Alcohol was modelled continuously using second-degree fractional polynomials, thus allowing for a single turning point (the nadir) in the risk function. Following the results of Corrao and others,1 a model describing the dose-response relationship of alcohol on CHD including both a linear and root-squared term of alcohol was applied.
The P-value for the test for trend was obtained by assigning the median value within categories of alcohol intake, and using this variable as a continuous variable. SAS statistical package, version 9.1, was used for all analyses.28
We harmonised the variables of the different studies and the following set of potential confounders was identified based on the method of causal diagrams as suggested by Greenland and others29: Educational level (< high school, high school, > high school), smoking (never smokers, ex-smokers, and current smokers of 1–4, 5–14, 15–24, or ≥ 25 cigarettes per day), BMI (< 18.5, 18.5–24.9, 25.0–29.9, and ≥ 30 kg/m2), total energy intake (kcal/day), and energy-adjusted quintiles of cholesterol, dietary fibre, saturated fat, monounsaturated fat, polyunsaturated fat intake. Physical activity measures varied across the cohorts, either measured according to an energy expenditure score of weekly time spent on various activities during the past year (ARIC, HPFS, NHS, VIP, and WHS) or according to the intensity of the average weekly physical activity during the past 12 months (ATBC, GPS). These measures were harmonized to a 5-level variable from 1 (least active) to 5 (most active).30–32 In addition, models were stratified by study origin and baseline year to account for differences in follow-up procedures or questionnaire design as well as period effects. Information on postmenopausal hormone therapy use was unavailable in VIP; therefore the main analyses for women did not include adjustment for this factor. Sensitivity analyses included measures of self-reported history of physician diagnosed elevated cholesterol (dyslipidemia) and hypertension (yes/no).
Results
Baseline characteristics
Baseline characteristics of participants from the eight included studies are shown in Table 1. In total, the pooled study population comprised 199,076 women and 79,291 men who experienced 1424 and 3115 coronary events during 1,428,216 and 604,266 person-years of follow-up, respectively. Baseline age of participants varied from 35 to 89 years in women and from 35 to 80 years in men with a mean age of 50.4 and 54.0, respectively. The proportion of non-drinkers varied substantially between studies from 11.4 % to 53.0 % in women and from 4.7 % to 45.8 % in men. Median alcohol intakes varied from 4.4to 20.8g/day in men and from 1.7to 8.9g/day in women.
Table 2 shows characteristics of participants included in the pooled cohort according to alcohol consumption. Heavier alcohol intake was associated with higher proportions of smokers, physical inactivity, hypertension, and lower median intakes of fat and fibre, while a moderate alcohol intake was associated with the highest median cholesterol intake compared to abstainers and heavy drinkers. In men, a higher frequency of those with low educational level was observed among participants in the highest alcohol group. Similar distributions of covariates according to alcohol intake were observed across cohorts.
Table 2.
Baseline Characteristics of Pooled Cohort According to Daily Alcohol Intake.
| Alcohol intake, g/day
|
|||||||
|---|---|---|---|---|---|---|---|
| Total | Non-drinkers | 0.1–4.9 | 5.0–14.9 | 15.0–29.9 | 30.0–59.9 | ≥ 60.0 | |
| Women | |||||||
| Number* | 192,067 | 65,121 | 67,187 | 39,177 | 12,258 | 7418 | 906 |
| CHD events | 1365 | 596 | 390 | 241 | 65 | 58 | 15 |
| Age, mean (SD) years | 50.4 (7.6) | 51.0 (7.7) | 49.8 (7.7) | 50.1 (7.5) | 50.5 (7.4) | 51.1 (7.3) | 51.3 (7.2) |
| Education, low (%)† | 8276 (4) | 2186 (3) | 4719 (7) | 936 (2) | 322 (3) | 96 (1) | 17 (2) |
| Smokers (%) | 44,513 (23) | 11,971 (18) | 14,547 (22) | 10,454 (27) | 3628 (30) | 3426 (46) | 487 (54) |
| BMI, mean (SD) kg/m2 | 25.0 (10.1) | 26.0 (5.3) | 25.1 (4.5) | 24.0 (3.8) | 23.7 (3.6) | 23.9 (3.8) | 24.4 (4.2) |
| Physical inactivity (%) | 66,248 (25) | 24,382 (37) | 21,858 (33) | 12,760 (33) | 3859 (31) | 2971 (40) | 418 (46) |
| Diet, median (5th–95th pct): | |||||||
| Polyunsaturated fat‡ g/day | 5.4 (3.3–8.4) | 5.5 (3.4–8.6) | 5.4 (3.4–8.4) | 5.4 (3.4–8.4) | 5.3 (3.2–8.3) | 4.8 (2.8–7.9) | 4.0 (2.2–6.9) |
| Monounsat. fat‡ g/day | 13.1 (8.1–20.5) | 13.1 (7.9–20.7) | 13.0 (8.3–20.5) | 13.2 (8.4–20.5) | 12.9 (8.2–19.7) | 12.3 (7.7–19.0) | 10.6 (6.4–16.8) |
| Saturated fat ‡ g/day | 12.8 (7.7–20.0) | 12.7 (7.5–20.1) | 13.0 (8.0–20.2) | 12.9 (7.9–20.1) | 12.6 (7.6–19.7) | 11.9 (7.2–18.6) | 10.3 (5.7–16.8) |
| Fibre‡ g/day | 15.4 (8.4–25.7) | 15.7 (8.5–26.7) | 15.9 (8.9–26.1) | 15.0 (8.3–24.4) | 14.2 (7.8–23.1) | 12.2 (6.7–20.4) | 10.3 (5.0–18.9) |
| Cholesterol‡ mg/day | 254 (139–458) | 252 (134–464) | 249 (137–451) | 264 (148–460) | 263 (151–464) | 252 (143–443) | 222 (121–394) |
| Total energy kcal/day | 1603 (900–2627) | 1579 (871–2637) | 1581 (893–2589) | 1614 (915–2608) | 1678 (977–2689) | 1758 (1070–2749) | 2008 (1283–3050) |
| Hypertension (%) | 35,637 (19) | 13,566 (21) | 11,695 (17) | 6271 (16) | 2137 (17) | 1695 (23) | 273 (30) |
| Dyslipidemia | 20,850 (11) | 8389 (13) | 6599 (10) | 3787 (10) | 1217 (10) | 731 (10) | 127 (14) |
| Non-drinkers | 0.1–4.9 | 5.0–14.9 | 15.0–29.9 | 30.0–59.9 | 60.0–89.9 | ≥ 90.0 | ||
|---|---|---|---|---|---|---|---|---|
| Men | ||||||||
| Number* | 74,720 | 13,904 | 19,030 | 20,556 | 11,375 | 7789 | 1546 | 520 (1) |
| CHD events | 2961 | 623 | 737 | 751 | 449 | 311 | 59 | 31 |
| Age, mean (SD) years | 54.0 (8.4) | 54.4 (8.6) | 53.3 (8.7) | 53.6 (8.5) | 54.6 (7.6) | 55.2 (7.6) | 55.2 (7.6) | 55.8 (6.2) |
| Education, low (%)† | 20,773 (28) | 2513 (18) | 6125 (32) | 5580 (27) | 3590 (32) | 2283 (29) | 450 (29) | 232 (45) |
| Smokers (%) | 28,144 (38) | 3338 (24) | 6086 (32) | 7409 (36) | 5688 (50) | 4254 (55) | 952 (62) | 417 (80) |
| BMI, mean (SD) kg/m2 | 25.8 (8.2) | 26.0 (3.8) | 25.8 (3.4) | 25.7 (3.4) | 25.8 (3.4) | 25.9 (3.5) | 26.1 (3.9) | 26.5 (4.1) |
| Physical inactivity (%) | 18,850 (25) | 3740 (27) | 4411 (23) | 4489 (22) | 2956 (26) | 2421 (31) | 572 (37) | 261 (80) |
| Diet, median (5th–95th pct): | ||||||||
| Polyunsaturated fat‡ g/day | 5.4 (3.3–8.9) | 5.5 (3.3–9.0) | 5.3 (3.4–8.8) | 5.4 (3.4–8.9) | 5.4 (3.2–9.0) | 5.2 (3.0–8.8) | 4.6 (2.6–8.2) | 4.1 (2.2–7.8) |
| Monounsat. fat‡ g/day | 12.8 (8.5–16.8) | 12.9 (8.0–17.3) | 12.8 (8.6–16.8) | 12.9 (8.8–16.8) | 12.9 (9.0–16.6) | 12.4 (8.3–16.3) | 11.3 (7.4–15.4) | 10.5 (6.8–15.5) |
| Saturated fat‡ g/day | 13.2 (7.5–23.8) | 12.3 (6.9–22.8) | 13.6 (7.7–24.0) | 13.3 (7.7–24.0) | 13.9 (7.8–24.5) | 13.2 (7.4–23.5) | 12.7 (6.5–21.6) | 13.3 (6.5–21.0) |
| Fiber‡ g/day | 19.8 (11.5–32.3) | 20.7 (11.6–35.7) | 21.0 (12.7–33.5) | 20.1 (12.3–31.8) | 19.1 (11.5–30.1) | 17.0 (10.1–27.4) | 14.6 (8.3–24.5) | 12.9 (6.6–21.2) |
| Cholesterol‡ mg/day | 322 (174–573) | 315 (161–568) | 303 (166–536) | 322 (177–566) | 349 (193–602) | 343 (190–610) | 323 (176–606) | 324 (167–544) |
| Total energy kcal/day | 2135(1149–3671) | 1929(1044–3438) | 2047(1120–3537) | 2111(1173–3598) | 2311(1274–3823) | 2387(1344–3911) | 2652(1518–4138) | 3054(1570–4662) |
| Hypertension | 14,388 (19) | 2759 (20) | 3292 (17) | 3634 (18) | 2246 (20) | 1902 (24) | 421 (27) | 134 (26) |
| Dyslipidemia§ | 5076 (9) | 1145 (10) | 1114 (8) | 1312 (9) | 720 (11) | 609 (13) | 147 (17) | 29 (16) |
After exclusion of participants with missing information on any of the relevant covariates
Defined as less than high school;
Energy-adjusted; n=total number of cases
This information was not available for ATBC
Differences in distribution of covariates across levels of alcohol consumption were tested by ANOVA (age, BMI), Kruskal-Wallis (dietary factors), and chi-square tests (education, smoking, physical activity, hypertension, and dyslipidemia). All tests showed statistically significant differences (P < 0.0001)
Alcohol consumption and risk of CHD
Table 3 shows the relative risks (hazard ratios) of CHD by categories of alcohol intake for each of the studies, and a pooled estimate for women and men, respectively. In women, an inverse relation between alcohol intake and CHD was found in each individual study except for Västerbotten Intervention Program. The confidence bounds around risk estimates for this particular study were very broad, as the reference category included two cases only. In men, an inverse relation was observed in all studies. In the pooled analysis, we observed a significantly lower risk of CHD among women with an alcohol intake of up to 60 g/day and among men with an alcohol intake of up to 90 g/day.
Table 3.
Study-Specific and Pooled Hazard Ratios of CHD for Categories of Daily Alcohol Intake.
| Hazard ratio (95% CI) by daily alcohol intake, g/day
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Women | Non- drinkers (CHD=606) | 0.1–4.9 (CHD=397) | 5.0–14.9 (CHD=242) | 15.0–29.9 (CHD=65) | 30.0–59.9 (CHD=60) | ≥ 60.0 (CHD=15) | P for trend | |
| Study-specific | N | |||||||
| ARIC | 6406 | 1.00 (reference) | 0.84 (0.44, 1.59) | 0.58 (0.29, 1.17) | 0.78 (0.30, 2.00) | NA§ | NA§ | 0.0738 |
| GPS | 1509 | 1.00 (reference) | 0.75 (0.27, 2.06) | 0.89 (0.31, 2.55) | 1.15 (0.31, 4.23) | 0.52 (0.05, 5.21) | NA§ | 0.8418 |
| NHSa | 79,479 | 1.00 (reference) | 0.73 (0.57, 0.94) | 0.72 (0.55, 0.95) | 0.59 (0.38, 0.94) | 0.49 (0.29, 0.82) | 1.64 (0.77, 3.49) | 0.1548 |
| NHSb | 60,083 | 1.00 (reference) | 0.78 (0.65, 0.94) | 0.67 (0.54, 0.84) | 0.47 (0.32, 0.69) | 0.57 (0.39, 0.82) | 0.60 (0.26, 1.38) | 0.0003 |
| VIP | 9758 | 1.00 (reference) | 2.26 (0.28,18.47) | 2.17 (0.12,38.67) | NA§ | NA§ | NA§ | 0.8654 |
| WHS | 34,832 | 1.00 (reference) | 1.02 (0.69, 1.50) | 0.81 (0.49, 1.35) | 0.37 (0.11, 1.19) | 0.58 (0.18, 1.90) | 1.32 (0.18, 9.95) | 0.1768 |
| Pooled | ||||||||
| Age-adj.* | 192,067 | 1.00 (reference) | 0.78 (0.69, 0.89) | 0.73 (0.62, 0.84) | 0.57 (0.44, 0.74) | 0.80 (0.61, 1.05) | 1.71 (1.02, 2.86) | 0.1112 |
| Smoking-adj.† | 192,067 | 1.00 (reference) | 0.75 (0.65, 0.85) | 0.62 (0.53, 0.72) | 0.47 (0.36, 0.61) | 0.52 (0.39, 0.68) | 1.02 (0.61, 1.70) | <0.0001 |
| Multivariable‡ | 192,067 | 1.00 (reference) | 0.78 (0.69, 0.90) | 0.68 (0.59, 0.80) | 0.52 (0.40, 0.67) | 0.53 (0.39, 0.70) | 0.93 (0.55, 1.58) | <0.0001 |
| Men | Non- drinkers (CHD=623) | 0.1–4.9 (CHD=737) | 5.0–14.9 (CHD=751) | 15.0–29.9 (CHD=449) | 30.0–59.9 (CHD=311) | 60.0–89.9 (CHD=59) | ≥ 90.0 (CHD=31) | P for trend | |
|---|---|---|---|---|---|---|---|---|---|
| Study-specific | |||||||||
| ARIC | 5166 | 1.00 (reference) | 1.16 (0.78, 1.71) | 1.29 (0.93, 1.79) | 0.87 (0.57, 1.32) | 0.73 (0.44, 1.22) | 0.74 (0.27, 2.04) | 1.40 (0.56, 3.54) | 0.4499 |
| ATBC | 21,119 | 1.00 (reference) | 0.86 (0.71, 1.03) | 0.86 (0.72, 1.03) | 0.70 (0.58, 0.85) | 0.61 (0.49, 0.77) | 0.48 (0.32, 0.74) | 0.80 (0.51, 1.27) | 0.0001 |
| GPS | 1294 | 1.00 (reference) | 0.84 (0.30, 2.32) | 0.54 (0.21, 1.38) | 0.60 (0.24, 1.50) | 0.44 (0.16, 1.18) | 0.37 (0.09, 1.52) | NA§ | 0.0304 |
| HPFS | 38,654 | 1.00 (reference) | 1.00 (0.85, 1.17) | 0.75 (0.63, 0.88) | 0.69 (0.56, 0.86) | 0.66 (0.52, 0.83) | 0.65 (0.41, 1.01) | 0.17 (0.02, 1.23) | <0.0001 |
| VIP | 8486 | 1.00 (reference) | 0.50 (0.28, 0.93) | 0.24 (0.11, 0.48) | 0.25 (0.07, 0.91) | 0.73 (0.09, 5.85) | NA§ | NA§ | 0.009 |
| Pooled | |||||||||
| Age-adj.* | 74,719 | 1.00 (reference) | 0.95 (0.85, 1.06) | 0.84 (0.75, 0.93) | 0.75 (0.66, 0.85) | 0.74 (0.64, 0.85) | 0.70 (0.53, 0.91) | 0.96 (0.67, 1.39) | <0.0001 |
| Smoking-adj.† | 74,719 | 1.00 (reference) | 0.94 (0.84, 1.05) | 0.81 (0.72, 0.90) | 0.71 (0.63, 0.81) | 0.66 (0.58, 0.76) | 0.60 (0.46, 0.79) | 0.81 (0.57, 1.18) | <0.0001 |
| Multivariable‡ | 74,719 | 1.00 (reference) | 0.96 (0.86, 1.08) | 0.83 (0.74, 0.92) | 0.72 (0.64, 0.82) | 0.66 (0.57, 0.76) | 0.58 (0.44, 0.77) | 0.77 (0.53, 1.13) | <0.0001 |
ARIC, Atherosclerosis risk in Communities; GPS, Glostrup population study; NHSa/NHSb, Nurses’ Health Study 1980–86/1986–96; VIP, Västerbotten Intervention Programme; WHS, Women’s Health Study
Also adjusted for year of baseline questionnaire
Also adjusted for age, year of baseline questionnaire
Multivariable hazard ratios were adjusted for age, year of baseline questionnaire, smoking, BMI, education, physical activity, energy intake, polyunsaturated fat, monounsaturated fat, saturated fat, fibre, cholesterol intake, and study origin
NA§ indicates ‘not applicable’ due to limited number of cases
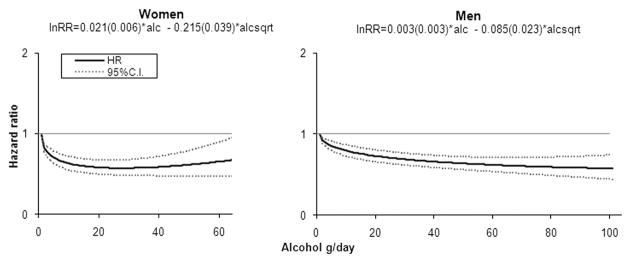
We also performed analyses describing the risk of CHD according to alcohol consumption modelled as a continuous variable(Figure 1). In both men and women, the reduction in CHD risk was observed at low to moderate levels of alcohol. The relative risk of CHD was 0.58 (95% CI: 0.49, 0.68) in women and 0.69 (95% CI: 0.62, 0.76) in men with a daily intake of 30 g/day per day, corresponding to around 2–3 drinks. Higher levels of alcohol consumption were not associated with any discernible additional protection in women, and with only modest protection in men.
Figure 1.
Relative Risk Functions (95% CI) Describing the Dose-response Relation Between Alcohol Intake and Risk of CHD.
(Analyses were adjusted for year of baseline questionnaire, education, smoking, BMI, physical activity, total energy intake, polyunsaturated fat, monounsaturated fat, saturated fat, fibre, and cholesterol intake. The fitted model (S.E.) is reported. The 99-percentile of cases was 64 g/day in women and 90 g/day in men. The highest alcohol intake with observed cases was 90 g/day in women and 215 g/day in men.)
Alcohol consumption and risk of CHD in age strata
We estimated the risks of CHD according to alcohol intake separately for three age groups (≤50, 50–59, and ≥60 years) (Figure 2). In all age groups, and for both men and women, a decreased risk of CHD according to alcohol intake was observed; however, with broader confidence bounds for the youngest age group. The test for interaction between alcohol and age was not statistically significant in either women (p=0.34) or men (p=0.25). We also modelled the risk continuously for the different age groups and observed similar shapesof the curves (data not shown).
Figure 2.
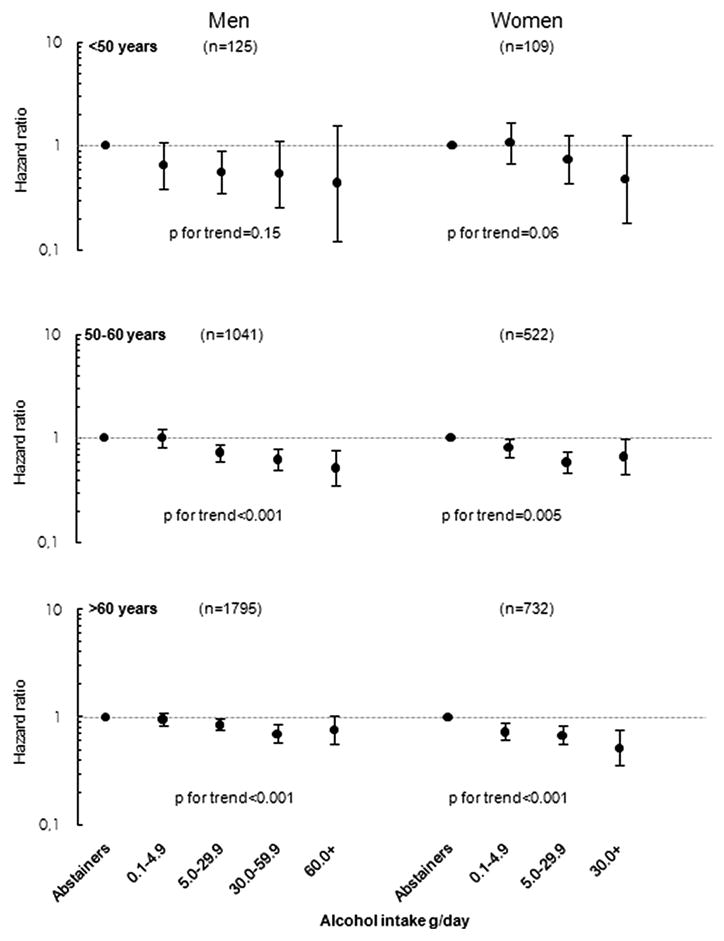
Sex-and Age-Specific Pooled Hazard Ratios of CHD for Categories of Daily Alcohol Intake.
(Multivariable hazard ratios were adjusted for year of baseline questionnaire, education, smoking, BMI, physical activity, total energy intake, polyunsaturated fat, monounsaturated fat, saturated fat, fibre, and cholesterol intake. N indicates number cases for each sex-and age group.)
Incidence rates of CHD in women and men according to alcohol intake and age are displayed in Figure 3. As expected, the incidence of CHD was much lower in the younger compared to older participants. The incidence rates of CHD among female abstainers in the three age groups were 11 (95% CI: 1, 109), 41 (95% CI: 1, 400), 103 (95% CI: 9, 1018) per 100,000, respectively. In male abstainers, the incidence rates were 114 (95% CI: 77, 171), 262 (95% CI: 201, 343), and 454 (95% CI: 354, 553) per 100,000 for the three age groups, respectively. In all age groups, and in both men and women, the incidence rate was lower among participants with a low to moderate alcohol intake compared with abstainers. In women, the incidence rate differences between drinking 0 g/day and 5.0–29.9 g/day were 3 (90% CI: −1, 25), 16 (90% CI: 0, 111), and 35 (90% CI: 0, 250). For men, corresponding incidence rate differences were 45 (90% CI: 8, 84), 64 (90% CI: 24, 102) and 89 (90% CI: 44, 140) per 100,000.
Figure 3.
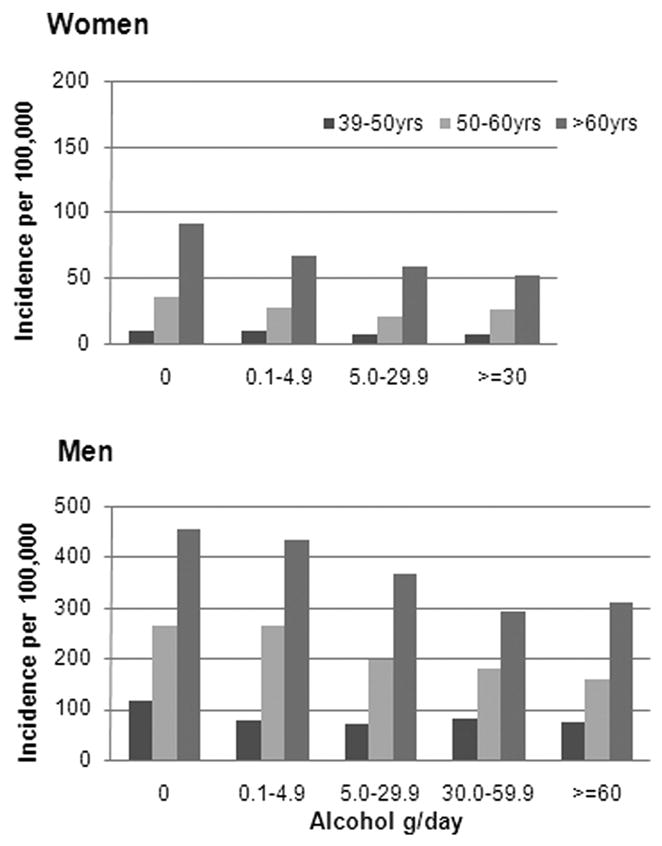
Incidence Rates of CHD According to Age and Alcohol Intake.
(Analyses were adjusted for year of baseline questionnaire, education, smoking, BMI, physical activity, total energy intake, polyunsaturated fat, monounsaturated fat, saturated fat, fibre, and cholesterol intake.)
Sensitivity analyses
Heterogeneity between study-specific effects was assessed by including an interaction term between alcohol and study origin under the null hypothesis of no between-study differences in the relative risk of CHD by alcohol intake,1;25 with no sign of heterogeneity detected (p=0.95 in women and p=0.12 in men). In addition, comparing pooled risk estimates after systematically excluding each study at a time, confirmed that no single study strongly influenced the pooled estimates.33 Hence, the pooled hazard ratios are considered appropriate summaries of the study-specific data. Performing a test for interaction between age (time-scale) and alcohol consumption did not yield violations of the proportional hazards assumption for either women (p=0.10) or men (p=0.22).
Separate analyses were performed for fatal and non-fatal events to examine whether the effect of alcohol on CHD differed according to the severity of the outcome. The results of this analysis did not reveal obvious differences between the two measures of outcome, although a tendency towards an elevated risk of fatal CHD was observed for the highest alcohol category in both women and men (data not shown).
To examine the possibility that latent baseline symptoms of CHD might reduce the alcohol intake, thereby biasing the results, we performed analyses where the first two or four years after baseline were excluded. This did not attenuate the estimates (data not shown).
Additional analyses for women were also performed to examine if adjustment for postmenopausal hormone replacement therapy had an impact on results. This involved excluding participants with unavailable information on this particular covariate (n=9799; 5% of the study population). In the remaining cohorts, postmenopausal hormone replacement therapy did not appreciably affect the association between alcohol and risk of CHD. Also, the inclusion of history of hypertension and dyslipidemia as covariates in the model did not affect the risk estimates of CHD according to alcohol consumption. Further, an analysis was performed including the Iowa Women’s Health Study (n=29,801). This inclusion also did not change the hazard ratios appreciably (data not shown).
A test for interaction between alcohol and smoking was performed to examine whether the estimates of alcohol’s effect on CHD risk differed according to smoking status. Smoking did not modify the association between alcohol and CHD in either men (P=0.79) or women (P=0.14). Additional analyses including non-smokers only were performed separately for women (n=100,144) and men (n=46,576) for alcohol levels of 0, 0.1–14.9, 15.0–29.9, and ≥30.0 g/day showing similar associations (data not shown).
Discussion
In this pooled cohort of eight prospective studies, we observed a lower risk of coronary heart disease among men and women with a light to moderate alcohol intake compared with non-drinkers, and this finding was consistent across different age groups without significant variations in dose-response.
The current knowledge on the effect of alcohol on CHD in younger adults is sparse. In a study based on the Honolulu Heart Program, the authors compared CHD risk according to conventional risk factors in middle-aged and older men (45–93 years). Compared with non-drinkers, they observed a lower risk of CHD among drinkers in middle-aged but not among older participants (75+ years), and concluded accordingly that the relation between alcohol and CHD weakened with age. The study, however, was limited by the simple categorisation of alcohol intake into drinkers versus non-drinkers and included men only.10
Several plausible explanations for the lowered risk of CHD among moderate drinkers exist. Among those explanations, the evidence is probably strongest for a mechanism involving alcohol increasing high-density lipoprotein (HDL) cholesterol and reducing plasma fibrinogen levels, thereby reducing platelet aggregability.7;34 The hypothesised J-shaped relation between alcohol intake and diabetes could also explain some of the benefit from alcohol intake.35;36 Also, alcohol has an effect on PAI-1 that would tend to reduce thrombosis.37
Previous studies have suggested that the causes of CHD in younger adults differ from the mechanisms involved with CHD in older persons. Results from the Honolulu Heart Program indicated that the effect of hypertension, body mass index (BMI), and cholesterol on CHD differed according to age. For instance, the relative risk of CHD in hypertensive men declined from 3.7 in those aged 45–54 years to 1.7 in those aged 75 years or more. Similarly, associations between BMI and total cholesterol weakened with advancing age.10 The Coronary Artery Risk in Young Adults (CARDIA) study of men and women aged 33–45 years found that alcohol intake was associated with expected dose-response between alcohol and HDL cholesterol levels and an inverse relation between alcohol and fibrinogen levels.9 They also observed an increased risk of coronary calcification with greater alcohol consumption. Since coronary calcification is a marker of atherosclerosis this result in young adults is not consistent with the results of the present study.9
Another aspect of alcohol consumption related to age is drinking patterns. Younger adults may tend to binge-drink more often than older persons, and this may increase their risk of CHD;5;7;9 however, findings from the CARDIA study mentioned above did not indicate a protective effect of alcohol intake on coronary calcification in younger adults even after excluding binge-drinkers.9
Our findings suggest a J-shaped curve in women; but in men, the risk did not increase significantly at high amounts of alchohol. Biomarkers that mediate the association between alcohol and decreased risk of CHD, such as high-density lipoprotein and fibrinogen, are found to explain a larger proportion of the association among men than among women which may indicate that alcohol has specific effects on such mediators according to sex.7 Other biological explanations for sex-specific associations include differences in alcohol pharmacokinetics (i.e. processing and elimination of alcohol in the body), which depend largely on body composition.38;39 However, as the risk curves of this study were modelled separately for the genders comparisons between the two are not straight forward.
The present study is one of few existing studies focusing on the effects of alcohol on CHD according to age. Our work was based on a large body of data with thorough measurements of alcohol intake and relevant covariates. A strength was the availability of diet data in the Pooling Project of Diet and Coronary Disease that enabled adjustment for potential dietary confounders. The size of the study population allowed us to perform subset analyses exploring the association between alcohol and CHD in strata of younger men and women – aspects even large individual cohorts do not have the power to address. The findings of the present study are strengthened by the prospective design, which provided information on the sequence of events allowing for conclusions on causality – assuming proper confounding control. Potential confounders of the association between alcohol and CHD were carefully selected on basis of Directed Acyclic Graphs, ensuring a minimally sufficient set of covariates. Further, the inclusion criteria of the Pooling Project of Diet and Coronary Disease enabled adjustment for relevant dietary factors, for which most previous studies did not control. The pooled analyses included both cohorts and intervention studies from North America and Europe, and similar effects were observed across the studies. Finally, an advantage of the Pooling Project is the inclusion of previously unpublished results thereby reducing the risk of publication bias.
However, several limitations of the study should also be considered. We focused on the importance of amount of alcohol consumed; however, other aspects of patterns of alcohol intake may be equally important and were not addressed. Our study only included information on current alcohol consumption and confounders at baseline. For this reason, the reference category of abstainers may contain former drinkers who quit because of existing illness, which could cause a moderate alcohol intake to appear more protective than it is. Although several studies including only lifelong or long-term abstainers in this category have confirmed a protective effect of alcohol even among healthy individuals,6;40 the “sick-quitter” hypothesis is relevant in the present context, as older age groups may include more abstainers who stopped drinking due to illness, e.g. hypertension.
As mentioned above, patterns of alcohol consumption (e.g. choice of alcohol type and frequency of consumption) may differ considerably with age which we did not account for. Our findings of protective effects of alcohol on CHD in all examined age-groups may indicate that neither type of alcohol or frequency of consumption modify the influence of alcohol on CHD considerably. However, future research based on observational studies should place emphasis on other measures of alcohol intake such as frequency of alcohol consumed. Further, cohort studies with information on lifetime alcohol intake or repeated measurements of alcohol intake and potential confounders could contribute with valuable insight, as changes in alcohol intake over a given period may be of great importance. Additional experimental studies are also needed in order to expand the knowledge on biological mechanisms.
Further, this study only focused on CHD events. Overall effects of alcohol on all-cause morbidity and mortality must be considered in order to be able to optimize alcohol guidelines for different age groups of the population. The lower risk of all-cause mortality is mainly expected to be caused by the effects of alcohol on CHD. In this study, a lower risk of CHD was observed in all examined age-groups in moderate alcohol consumers compared to abstainers; however, the absolute risk of CHD was rather low in the youngest age group. Thus, considering the increasing contribution of CHD to all-cause mortality with age, it is reasonable to assume that the protective effect of alcohol on all-cause mortality is mostly pronounced in older age-groups. This issue has been addressed in a few previuos studies indicating that the protective effect of alcohol consumption on mortality in general is confined to middle-aged and older individuals.4;41;42 Unfortunately, information on all-cause mortality was not collected in the database of the Pooling Project on Diet and CHD.
In summary, this study supports current knowledge that alcohol in moderate amounts protects against coronary heart disease in both men and women. Our findings further suggest that this effect is also present in younger age groups. However, younger adults are at low risk for CHD and the beneficial effects obtained by a moderate alcohol intake may be negligible compared to the increased risk of for instance traffic accidents and cancer. Recommendations on alcohol intake among younger adults should consider all-cause mortality and morbidity.
Acknowledgments
Funding: This research was supported by research grant NIH NHLBI R01 HL58904. The Unit for Dietary Studies was funded by the FREJA (Female Researchers in Joint Action) program of the Danish Medical Research Council.
Footnotes
DISCLOSURES: None.
References
- 1.Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and coronary heart disease: a meta-analysis. Addiction. 2000;95:1505–1523. doi: 10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- 2.Grønbæk M, Becker U, Johansen D, Gottschau A, Schnohr P, Hein HO, Jensen G, Sørensen TI. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann Intern Med. 2000;133:411–419. doi: 10.7326/0003-4819-133-6-200009190-00008. [DOI] [PubMed] [Google Scholar]
- 3.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs CS, Stampfer MJ, Colditz GA, Giovannucci EL, Manson JE, Kawachi I, Hunter DJ, Hankinson SE, Hennekens CH, Rosner Bl. Alcohol consumption and mortality among women. N Engl J Med. 1995;332:1245–1250. doi: 10.1056/NEJM199505113321901. [DOI] [PubMed] [Google Scholar]
- 5.Tolstrup J, Jensen MK, Tjønneland A, Overvad K, Mukamal KJ, Grønbæk M. Prospective study of alcohol drinking patterns and coronary heart disease in women and men. BMJ. 2006;332:1244–1248. doi: 10.1136/bmj.38831.503113.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukamal KJ, Conigrave KM, Mittleman MA, Camargo CA, Jr, Stampfer MJ, Willett WC, Rimm EB. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N Engl J Med. 2003;348:109–118. doi: 10.1056/NEJMoa022095. [DOI] [PubMed] [Google Scholar]
- 7.Mukamal KJ, Jensen MK, Grønbæk M, Stampfer MJ, Manson JE, Pischon T, Rimm EB. Drinking frequency, mediating biomarkers, and risk of myocardial infarction in women and men. Circulation. 2005;112:1406–1413. doi: 10.1161/CIRCULATIONAHA.105.537704. [DOI] [PubMed] [Google Scholar]
- 8.Stamler J. Established major risk factors: historical overview. In: Marmot M, Elliott P, editors. Coronary heart disease epidemiology -from aetiology to public health. New York: Oxford University Press; 2005. pp. 18–31. [Google Scholar]
- 9.Pletcher MJ, Varosy P, Kiefe CI, Lewis CE, Sidney S, Hulley SB. Alcohol consumption, binge drinking, and early coronary calcification: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 2005;161:423–433. doi: 10.1093/aje/kwi062. [DOI] [PubMed] [Google Scholar]
- 10.Abbott RD, Curb JD, Rodriguez BL, Masaki KH, Yano K, Schatz IJ, Ross GW, Petrovitch H. Age-related changes in risk factor effects on the incidence of coronary heart disease. Ann Epidemiol. 2002;12:173–181. doi: 10.1016/s1047-2797(01)00309-x. [DOI] [PubMed] [Google Scholar]
- 11.Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 12.Zdravkovic S, Wienke A, Pedersen NL, Marenberg ME, Yashin AI, de Faire U. Heritability of death from coronary heart disease: a 36-year follow-up of 20 966 Swedish twins. J Intern Med. 2002;252:247–254. doi: 10.1046/j.1365-2796.2002.01029.x. [DOI] [PubMed] [Google Scholar]
- 13.Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med. 1992;152:1416–1424. [PubMed] [Google Scholar]
- 14.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 16.Knekt P, Reunanen A, Jarvinen R, Seppanen R, Heliovaara M, Aromaa A. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol. 1994;139:1180–1189. doi: 10.1093/oxfordjournals.aje.a116964. [DOI] [PubMed] [Google Scholar]
- 17.Hoyer AP, Engholm G. Serum lipids and breast cancer risk: a cohort study of 5,207 Danish women. Cancer Causes Control. 1992;3:403–408. doi: 10.1007/BF00051352. [DOI] [PubMed] [Google Scholar]
- 18.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 19.Balogh M, Medalie JH, Smith H, Groen JJ. The development of a dietary questionnaire for an ischemic heart disease survey. Isr J Med Sci. 1968;4:195–203. [PubMed] [Google Scholar]
- 20.Munger RG, Folsom AR, Kushi LH, Kaye SA, Sellers TA. Dietary assessment of older Iowa women with a food frequency questionnaire: nutrient intake, reproducibility, and comparison with 24-hour dietary recall interviews. Am J Epidemiol. 1992;136:192–200. doi: 10.1093/oxfordjournals.aje.a116485. [DOI] [PubMed] [Google Scholar]
- 21.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 22.Hallmans G, Agren A, Johansson G, Johansson A, Stegmayr B, Jansson JH, Lindahl B, Rolandsson O, Söderberg S, Nilsson M, Johansson I, Weinehall L. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort -evaluation of risk factors and their interactions. Scand J Public Health Suppl. 2003;61:18–24. doi: 10.1080/14034950310001432. [DOI] [PubMed] [Google Scholar]
- 23.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women’s Health Study. J Womens Health Gend Based Med. 2000;9:19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 24.Pereira MA, O’Reilly E, Augustsson K, Fraser GE, Goldbourt U, Heitmann BL, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch Intern Med. 2004;164:370–376. doi: 10.1001/archinte.164.4.370. [DOI] [PubMed] [Google Scholar]
- 25.Smith-Warner SA, Spiegelman D, Ritz J, Albanes D, Beeson WL, Bernstein L, Berrino F, van den Brandt PA, Buring JE, Cho E, Colditz GA, Folsom AR, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Harnack L, Horn-Ross PL, Krogh V, Leitzmann MF, McCullough ML, Miller AB, Rodriguez C, Rohan TE, Schatzkin A, Shore R, Virtanen M, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Zhang SM, Hunter DJ. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163:1053–1064. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 26.Clayton D, Hills M. Cox’s method for follow-up studies. Statistical models in epidemiology. New York: Oxford; 1993. pp. 298–306. [Google Scholar]
- 27.Woodward M. Modelling follow-up data. In: Woodward M, editor. Epidemiology -study design and data analysis. Boca Raton: Chapman & Hall; 2005. pp. 601–671. [Google Scholar]
- 28.SAS Institute Inc. SAS/STAT Software, version 9.1 for Windows. Cary, NC: SAS Institute Inc; 2002. [Google Scholar]
- 29.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 30.Devore EE, Kang JH, Okereke O, Grodstein F. Physical Activity Levels and Cognition in Women with Type 2 Diabetes. Am J Epidemiol. 2009;170:1040–1047. doi: 10.1093/aje/kwp224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation. 2003;107:2435–9. doi: 10.1161/01.CIR.0000066906.11109.1F. [DOI] [PubMed] [Google Scholar]
- 32.Hemilä H, Kaprio J, Albanes D, Virtamo J. Physical activity and the risk of pneumonia in male smokers administered vitamin E and beta-carotene. Int J Sports Med. 2006;27:336–41. doi: 10.1055/s-2005-865670. [DOI] [PubMed] [Google Scholar]
- 33.Morton LM, Hartge P, Holford TR, Holly EA, Chiu BC, Vineis P, Stagnaro E, Willett EV, Franceschi S, La Vecchia C, Hughes AM, Cozen W, Davis S, Severson RK, Bernstein L, Mayne ST, Dee FR, Cerhan JR, Zheng T. Cigarette smoking and risk of non-Hodgkin lymphoma: a pooled analysis from the International Lymphoma Epidemiology Consortium (interlymph) Cancer Epidemiol Biomarkers Prev. 2005;14:925–933. doi: 10.1158/1055-9965.EPI-04-0693. [DOI] [PubMed] [Google Scholar]
- 34.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klatsky AL. Alcohol, cardiovascular diseases and diabetes mellitus. Pharmacol Res. 2007;55:237–247. doi: 10.1016/j.phrs.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care. 2005;28:719–725. doi: 10.2337/diacare.28.3.719. [DOI] [PubMed] [Google Scholar]
- 37.Lee KW, Lip GY. Effects of lifestyle on hemostasis, fibrinolysis, and platelet reactivity: a systematic review. Arch Intern Med. 2003;163:2368–2392. doi: 10.1001/archinte.163.19.2368. [DOI] [PubMed] [Google Scholar]
- 38.Makelä P, Paljärvi T, Poikolainen K. Heavy and nonheavy drinking occasions, all-cause and cardiovascular mortality and hospitalizations: a follow-up study in a population with a low consumption level. J Stud Alcohol. 2005;66:722–8. doi: 10.15288/jsa.2005.66.722. [DOI] [PubMed] [Google Scholar]
- 39.Mumenthaler MS, Taylor JL, O’Hara R, Yesavage JA. Gender differences in moderate drinking effects. Alcohol Res Health. 1999;23:55–64. [PMC free article] [PubMed] [Google Scholar]
- 40.Klatsky AL, Armstrong MA, Friedman GD. Risk of cardiovascular mortality in alcohol drinkers, ex-drinkers and nondrinkers. Am J Cardiol. 1990;66:1237–1242. doi: 10.1016/0002-9149(90)91107-h. [DOI] [PubMed] [Google Scholar]
- 41.White IR, Altmann DR, Nanchahal K. Alcohol consumption and mortality: modelling risks for men and women at different ages. BMJ. 2002;325:191. doi: 10.1136/bmj.325.7357.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gmel G, Gutjahr E, Rehm J. How stable is the risk curve between alcohol and all-cause mortality and what factors influence the shape? A precision-weighted hierarchical meta-analysis. Eur J Epidemiol. 2003;18:631–42. doi: 10.1023/a:1024805021504. [DOI] [PubMed] [Google Scholar]