Abstract
X-linked adrenoleukodystrophy is a severe and progressive neurodegenerative disease caused by the peroxisomal transporter ATP-binding cassette, subfamily D, member 1 gene mutations. The defect of this gene product results in accumulation of very-long-chain fatty acids in organs and serum, central demyelination, and peripheral axonopathy. Although there are different magnetic resonance (MR) findings which reflect various phenotypes in adrenoleukodystrophy, some cases present with specific symmetrical occipital white-matter lesions. We describe a patient with adult-onset X-linked adrenoleukodystrophy with topographic disorientation, whose brain MR images revealed T2-signal hyperintensity along the occipito-pontine tract and lateral lemnisci, but not in the cortico-spinal tract in the brainstem. The occipito-pontine tract and lateral lemnisci were clearly detected using diffusion-tensor fiber tracking, suggesting that the topographic disorientation of this patient might be related to the occipito-pontine tract. MR tractography can effectively identify the occipito-pontine tract and may help to localize the fibers associated with clinical symptoms.
Key Words: Adrenoleukodystrophy, Occipito-pontine tract, Topographic disorientation, Tractography
Introduction
X-linked adrenoleukodystrophy (X-ALD) is a peroxisomal disorder caused by overstorage of very-long-chain fatty acids (VLCFAs) in the nervous system, adrenal cortex, and testis [1] resulting from genetic defects in the peroxisomal transporter ATP-binding cassette, subfamily D, member 1 (ABCD1) gene, an ABC transporter that encodes a peroxisomal membrane protein [2]. Magnetic resonance (MR) images of X-ALD patients often indicate abnormal findings in the periventricular, parietal, and occipital white matter; callosal splenium, and cortico-spinal tract [3, 4].
The occipito-pontine tract belongs to the occipito-parieto-temporo-pontine (OPTP) tracts, whose fibers connect the pons with the occipital, parietal, and temporal cortices, respectively, and run through the lateral third of the cerebral peduncle in the midbrain [5]. Although functions of the OPTP tracts are poorly understood, neuropathological analyses show that they are affected in X-ALD patients [6, 7]. However, most MR reports showed no [3, 4, 8] or only small [9] abnormalities in the OPTP tract. We clearly identified abnormal signals in the occipito-pontine tract using 3-tesla diffusion-tensor (DT) fiber tracking in an adult-onset X-ALD patient with topographic disorientation.
Methods and Case Report
Methods
MR images were collected with a 3-tesla MR imaging system (Magnetom Trio; Siemens, Erlangen, Germany) and a 12-channel head coil. Images were acquired with axial spin-echo T1-weighted [repetition time (TR) 600 ms, echo time (TE) 8.5 ms, excitations 1], turbo spin-echo T2-weighted (TR 3,600 ms, TE 96 ms, excitations 2, turbo factor 7), FLAIR (TR 9,000 ms, TE 81 ms, excitations 1, turbo factor 15, inversion time 2,500 ms), and DT imaging (DTI) sequences. T1-, T2-weighted, and FLAIR images were acquired with a slice thickness of 5 mm. DT images were obtained using a Stejskal-Tanner sequence with single-shot, spin-echo type echo-planar imaging (TR 8,500 ms, TE 88 ms, flip angle 90°, motion-probing gradient in 12 orientations, b value 1,000 smm−2, matrix 128 × 128 mm, and field of view 265 × 265 mm) in 64 consecutive, 2.1-mm-thick sections. DTI data were processed and analyzed offline. DT tractography was performed with the freely available software dTV (dTV v2.0, SR; volume-1 v1.72) for MR-DTI analysis (University of Tokyo Hospital, Tokyo, Japan) by using the fiber assignment by continuous tracking (FACT) method [10]. Propagation in each fiber tract was terminated if a voxel with an FA value <0.2 was reached or if the turning angles of 2 consecutive vectors were >70° during tracking.
To identify the fiber tract running through the hyperintense lesion in the lateral portion of the cerebral peduncle, tractography was generated with 1 region of interest (ROI) placed over the hyperintense lesion. The corticospinal tract was visualized with 2 ROIs (placed over the precentral gyrus and the middle portion of the cerebral peduncle).
Case Report
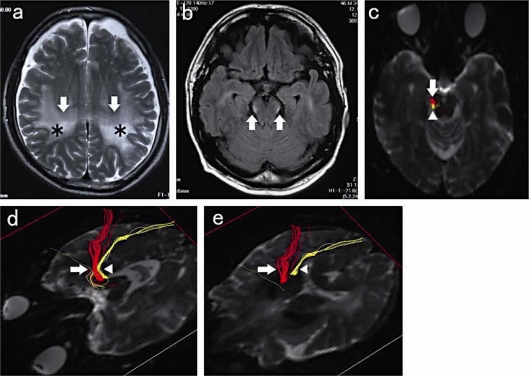
We report the case of a 46-year-old Japanese man whose wife first realized that he occasionally took the wrong way, got lost near his house, and made mistakes in reading and writing about 18 months prior to our seeing him. Thereafter, he often lost his way home and to the office and, about a year later, got lost within his office building and also made mistakes in reading, writing, and arithmetic. About 6 months later, he visited our outpatient clinic and was admitted to our hospital for investigation. Physical examination revealed no abnormal signs; eye fields and eye pursuit movements were normal in all directions, and a Mini-Mental State Examination score of 24 indicated that his intellectual function was within normal limits. However, he could not draw the room arrangement of his house or the way from home to the office. Laboratory tests were normal, except for elevated serum VLCFA ratios (C26/C22 = 1.318, C25/C22 = 0.034, and C24/C22 = 0.017), biochemically confirming a diagnosis of ALD. Visual evoked potentials were normal. T2-weighted and FLAIR brain MR images showed diffuse hyperintense lesions of the white matter, predominantly in the posterior regions of the parietal and occipital lobes (fig. 1a), and hyperintensities in the bilateral occipito-pontine tracts and lateral lemnisci (fig. 1b), which were seen more clearly using DT fiber tracking (fig. 1c–e).
Fig. 1.
a T2-weighted image showing large, hyperintense lesions in the bilateral cerebral white matter (arrow), predominantly in the parietal lobe (asterisk). b FLAIR image showing bilateral, small-spot, hyperintense lesions in the lateral portion of the cerebral peduncle (arrows). c–e DTI images. The hyperintense lesion in b corresponds with the yellow spot (arrowhead), not the red one (arrow), in c. DT fiber-tracking images using the right cerebral peduncle as the starting point showing the occipito-pontine tract and lateral lemnisci as yellow and the cortico-spinal tract as red in the midbrain (d) and internal-capsule (e) levels.
Discussion
Brain MR images of a number of adult-onset X-ALD patients exhibited tract involvement in the cortico-spinal, spino-thalamic, visual, and auditory pathways [8]. The brainstem auditory pathway is frequently involved in cerebral X-ALD [11]. Not only the occipito-pontine tract, but also the lateral lemnisci are involved in the hyperintense lesions (arrow) shown in figure 1b and the dorsal spot (arrowhead) in figure 1c [12, 13]. Although MR findings of ALD patients vary widely, the cortico-spinal tract is very frequently involved [3, 4, 8], while the OPTP tracts are involved much less frequently [9].
In contrast, a pathological investigation of an X-ALD autopsy case demonstrated that the OPTP tracts were among the most severely affected fibers, while the cortico-spinal tract was relatively well preserved in the midbrain [7]. As these two tracts are close to one another in the brainstem, the OPTP tracts may have been confused with the cortico-spinal tract as the source of the abnormal signal intensities. Tractography, however, is very effective for exactly identifying, clearly drawing, and following long fibers with abnormal intensities.
The occipito-pontine projection originates in cortical areas that respond to visual stimulation [14]. Though detailed functions of the occipito-pontine tract are not well understood, it was reported to be associated with eye pursuit movements [15] and may also be associated with visual perception. Although our patient's visual evoked potential examinations and eye pursuit movements were normal, abnormal intensities were clearly detected on images of the occipito-pontine tract. This might explain the patient's visual cognitive impairments (getting lost in familiar surroundings), although we should consider the caveat that symptoms, MR images, and pathological findings do not always correspond. In the late stage, the patient demonstrated extensive cerebral white-matter involvement; thus, his symptoms may also be related to injury from other parieto-occipital association fibers.
Topographic disorientation is one of the early signs of Alzheimer's disease, together with amnestic symptoms. A Mini-Mental State Examination score of 24 indicated that our patient's intellectual function was within normal limits. However, he could not draw the room arrangement of his house or the way from home to the office. To our knowledge, topographic disorientation at an early stage of the disease, as it was seen in this patient, has not been reported in X-ALD patients. The fact that intellectual function was relatively preserved and amnestic symptoms missing, while topographic disorientation was remarkable in this patient, might be characteristic of the disease.
Our results suggest that tractography is effective for observing the fibers involved in X-ALD and may be particularly useful for exact identification of the occipito-pontine tract in X-ALD with topographic disorientation. There are continuous advances in neuroimaging technology which make it more and more indispensable for clinical application. The role of neuroimaging progresses will become more important for the identification of fibers correlated with particular clinical symptoms.
Disclosure Statement
No support was given from any source relating to this research.
Acknowledgement
We thank Jun Tateishi (Kyushu University, Japan, welfare-based nursing homes for the elderly, Harukaze) for histopathological advice.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Moser HW, Smith KD, Watkins PA, Powers J, Moser AB. X-linked adrenoleukodystrophy. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. ed 8. New York, NY: McGraw Hill; 2001. pp. 3257–3301. [Google Scholar]
- 2.Mosser J, Douar AM, Sarde CO, Kioschis P, Feil R, Moser H, Poustka AM, Mandel JL, Aubourg P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361:726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- 3.Loes DJ, Fatemi A, Melhem ER, Gupte N, Bezman L, Moser HW, Raymond GV. Analysis of MRI patterns aids prediction of progression in X-linked adrenoleukodystrophy. Neurology. 2003;61:369–374. doi: 10.1212/01.wnl.0000079050.91337.83. [DOI] [PubMed] [Google Scholar]
- 4.Eichler F, Mahmood A, Loes D, Bezman L, Lin D, Moser HW, Raymond GV. Magnetic resonance imaging detection of lesion progression in adult patients with X-linked adrenoleukodystrophy. Arch Neurol. 2007;64:659–664. doi: 10.1001/archneur.64.5.659. [DOI] [PubMed] [Google Scholar]
- 5.Pizzini F, Beltramello A, Piovan E, Alessandrini F. Diffusion-weighted and diffusion tensor magnetic resonance brain imaging: principles and applications. Rivista di Neuroradiologia. 2003;16:207–220. [Google Scholar]
- 6.Schaumburg HH, Powers JM, Raine CS, Suzuki K, Richardson EP., Jr Adrenoleukodystrophy. A clinical and pathological study of 17 cases. Arch Neurol. 1975;32:577–591. doi: 10.1001/archneur.1975.00490510033001. [DOI] [PubMed] [Google Scholar]
- 7.Tateishi J, Sato Y, Suetsugu M, Takashiba T. Adrenoleukodystrophy with olivopontocerebellar atrophy-like lesions. Clin Neuropathol. 1986;5:34–39. [PubMed] [Google Scholar]
- 8.Kumar AJ, Köhler W, Kruse B, Naidu S, Bergin A, Edwin D, Moser HW. MR findings in adult-onset adrenoleukodystrophy. AJNR Am J Neuroradiol. 1995;16:1227–1237. [PMC free article] [PubMed] [Google Scholar]
- 9.Barkovich AJ, Ferriero DM, Bass N, Boyer R. Involvement of the pontomedullary corticospinal tracts: a useful finding in the diagnosis of X-linked adrenoleukodystrophy. AJNR Am J Neuroradiol. 1997;18:95–100. [PMC free article] [PubMed] [Google Scholar]
- 10.Mori S, van Zijl PC. Fiber tracking: principles and strategies – a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Kim HJ. Childhood X-linked Adrenoleukodystrophy: clinical-pathologic overview and MR imaging manifestations at initial evaluation and follow-up. Radiographics. 2005;25:619–631. doi: 10.1148/rg.253045118. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter MB. The Mesencephalon: Human Neuroanatomy. Baltimore: Williams and Wilkins; 1976. pp. 367–382. [Google Scholar]
- 13.Stieltjes B, Kaufmann WE, van Zijl PC, Fredericksen K, Pearlson GD, Solaiyappan M, Mori S. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage. 2001;14:723–735. doi: 10.1006/nimg.2001.0861. [DOI] [PubMed] [Google Scholar]
- 14.Albus K, Donate-Oliver F. Cells of origin of the occipito-pontine projection in the cat: functional properties and intracortical location. Exp Brain Res. 1977;28:167–174. doi: 10.1007/BF00237094. [DOI] [PubMed] [Google Scholar]
- 15.Wall M. Brainstem syndromes. In: Bradley WG, Daroff RB, Fenichel GM, Jankovic J, editors. Neurology in Clinical Practice, vol 1. ed 4. Boston: Butterworth-Heinemann; 1996. pp. 273–285. [Google Scholar]