Abstract
Purpose
To examine anatomical changes in idiopathic macular holes during surgery using handheld spectral-domain optical coherence tomography (SD-OCT).
Methods
Five eyes of 5 patients who underwent surgery for the repair of idiopathic macular holes were examined. The surgery included standard 25-gauge, 3-port pars plana vitrectomy, removal of the internal limiting membrane (ILM), fluid-air exchange, and 20% sulfur hexafluoride tamponade. Intraoperative SD-OCT images of the macular holes were obtained after ILM removal and under fluid-air exchange using a handheld SD-OCT. From SD-OCT images, the macular hole base diameter (MHBD) was measured and compared.
Results
All macular holes were successfully closed after the primary surgery. The mean MHBD under fluid-air exchange was significantly smaller than the mean MHBD after ILM removal and the preoperative mean MHBD. In 1 eye with a stage 3 macular hole, SD-OCT images revealed that the inner edges of the macular hole touched each other under fluid-air exchange.
Conclusion
Fluid-air exchange significantly reduced MHBD during surgery to repair macular holes. Fluid-air exchange may be an important step for macular hole closure as it reduces the base diameter of the macular hole.
Key Words: Spectral-domain OCT, Macular hole, Base diameter, Intraoperative OCT
Introduction
Spectral-domain optical coherence tomography (SD-OCT) enables physicians to noninvasively obtain detailed retinal images of various retinal diseases in a clinical environment. Intraoperative use of SD-OCT using an OCT-mounted surgical microscope or a handheld SD-OCT has been attractive because OCT images can be obtained immediately during vitreoretinal surgeries and used to detect retinal changes resulting from the surgical procedures, as well as to decide on the next procedures to be taken during surgery [1, 2, 3, 4].
Macular hole surgery has been successfully developed to obtain more than 90% closure rates after the primary surgery. Recently, several studies reported that macular holes could be closed with shorter periods of prone positioning or no prone positioning [5, 6, 7, 8]. Wykoff et al. [3] reported that, in their patient, the traumatic macular hole appeared to be closed in the OCT images after removal of the internal limiting membrane (ILM) during the surgery. Because the anatomical changes in macular holes during macular hole surgery are not clearly understood, intraoperative anatomical changes in macular holes using a handheld SD-OCT were examined in this study.
Patients and Methods
Patients
This interventional case study included 5 eyes of 5 consecutive patients with idiopathic full-thickness macular holes who visited Toyama University Hospital between November 2010 and February 2011. All patients were naïve to treatment for idiopathic macular holes and underwent the primary surgery at Toyama University Hospital. The patients were followed up for more than 1 month after the surgery with eye examinations of visual acuity and using SD-OCT.
Surgical Procedures
All patients underwent the standard surgery for idiopathic macular holes by a single surgeon (A.H.). Briefly, after local anesthesia with subtenon injection of 2% lidocaine, cataract surgery was performed before vitrectomy. Then, a standard 3-port pars plana vitrectomy was performed using a 25-gauge system. After the core vitrectomy, triamcinolone particles were used to visualize the vitreous and posterior vitreous detachment created in all eyes. Vitrectomy was performed using contact lenses, after which 0.05% indocyanine green solution was injected onto the posterior retinal surface and washed out immediately. Under a flat concave contact lens, the ILM was removed in the circular region around the macula up to about 2.5- to 3-disc diameters. The vitreous cavity was washed out again and the fluid was exchanged with air. Then, the air in the vitreous cavity was replaced with 20% sulfur hexafluoride (SF6) and the 3 ports were sutured with 10-0 vicryl. Finally, about 0.4 ml dexamethasone was injected into the subconjunctival space and antibiotic ointment was applied to the cul-de-sac. All patients were asked to lie in a prone position for several hours immediately after the surgery, after which they were allowed to take any position other than the supine position for 1 week.
OCT Examinations
A handheld SD-OCT (iVue-100®; Optovue Inc., Fremont, Calif., USA) was attached to a specially made OCT holder and used intraoperatively. The OCT images of all eyes were obtained with the retinal map program or the cross section program on 2 occasions during the macular hole surgery, with both these procedures taking place after ILM removal and under fluid-air exchange. The OCT images were taken several times in order to examine changes in the macular hole at every occasion. All patients underwent OCT examinations with an SD-OCT (RTVue-100®; Optovue Inc.) in the clinics before the macular hole surgery. From the OCT images, the macular hole base diameter (MHBD) was measured as the widest point of the inner/outer segment junction defect in each macular hole using SD-OCT caliper software [9]. The postoperative shapes of the macular holes were classified as bridge formation or simple closure, according to previous studies [5, 10]. SD-OCT examinations were performed at 7 to 10 days and at 1 month after surgery using an RTVue-100 SD-OCT at the clinics after more than half of the intraocular gas was absorbed and the fovea was observed through the intraocular fluid.
Statistical analyses were performed with Wilcoxon signed-rank test and a p value <0.05 was recognized as statistically significant.
Results
Baseline Results
All patients were female and their mean age was 62.2 ± 9.9 years (mean ± SD). All patients underwent simultaneous cataract surgery with the macular hole surgery, and all showed successful closure of macular holes at 7 to 10 days after the surgery. The mean visual acuity improved from 0.70 ± 0.30 to 0.58 ± 0.31 logMAR units at 7 to 10 days and to 0.32 ± 0.16 logMAR units at 1 month after the surgery (p = 0.07), as shown in table 1.
Table 1.
Visual acuity and macular hole parameters
| Case | PreO VA logMAR | MH stage | PreO MHBD μm | MHBD after ILM removal μm | MHBD under fluid-air exchange, μm | 7 to 10 days POVA logMAR | 1 month POVA logMAR | 1 month PO type of MH closure |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.00 | 3 | 815 | 801 | 574 | 0.70 | 0.30 | simple closure |
| 2 | 0.70 | 3 | 549 | 586 | 378 | 0.52 | 0.52 | simple closure |
| 3 | 0.40 | 3 | 722 | 716 | 528 | 0.52 | 0.40 | simple closure |
| 4 | 0.40 | 2 | 452 | 479 | 333 | 0.15 | 0.10 | simple closure |
| 5 | 1.00 | 2 | 853 | 855 | 608 | 1.00 | 0.30 | bridge formation |
ILM = Internal limiting membrane; MH = macular hole; MHBD = macular hole base diameter; PO = postoperative; PreO = preoperative; VA = visual acuity.
OCT Findings
The 5 treated eyes of the 5 patients consisted of 2 eyes with a stage 2 macular hole and 3 eyes with a stage 3 macular hole by Gass classification with preoperative SD-OCT. The size of the macular hole was expressed as MHBD [9]. The preoperative and intraoperative MHBD of each eye are shown in table 1. The preoperative MHBD was nearly the same as the intraoperative MHBD after ILM peeling in each eye, but the intraoperative MHBD under fluid-air exchange was smaller than that after ILM peeling in all eyes. The mean preoperative MHBD and intraoperative MHBD after ILM peeling were 678 ± 173 µm (range, 452–815 µm) and 690 ± 155 µm (range, 479–801 µm), respectively. However, the mean MHBD under fluid-air exchange was 484 ± 122 µm (range, 333–608 µm) and significantly smaller than that after ILM peeling (p < 0.05) or before the surgery (p < 0.05). One of the 3 eyes with a stage 3 macular hole (case 1) showed contact of the inner edges of the macular hole under the fluid-air exchange with SD-OCT (fig. 1). The remaining 2 eyes (cases 2 and 3) showed open macular holes under the fluid-air exchange (fig. 2).
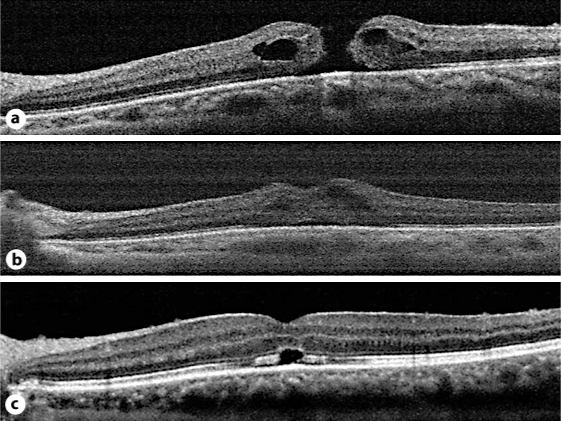
Fig. 1.
SD-OCT images of a macular hole from case 1. a Macular hole after ILM removal. b Macular hole under fluid-air exchange. The inner edges of the macular hole touched each other. c Macular hole at 7 postoperative days, showing a bridge formation.
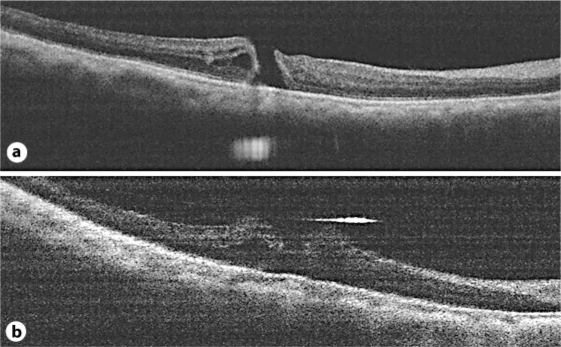
Fig. 2.
SD-OCT images of macular holes from cases 2 and 3. a Macular hole from case 2 under fluid-air exchange. b Macular hole from case 3 under fluid-air exchange. The macular holes were open under fluid-air exchange.
At 7 to 10 postoperative days in case 1, SD-OCT images showed that both edges of the macular hole were bridged and the retinal layers of the macular hole seemed continuous (fig. 1). According to previous reports on macular hole closure types [5, 10], 3 of the 5 eyes showed the bridge formation and 2 eyes showed the simple closure at 7 to 10 days postoperatively. At 1 month after the surgery, 4 of the 5 eyes showed the simple closure configuration. The remaining 1 eye still showed the bridge formation at 1 month postoperatively.
Discussion
In previous studies, the early changes in macular holes after vitrectomy surgery were characterized as follows: at 1 day after surgery, 77–93% of the eyes showed closure of the macular hole in subjects lying in a prone position based on OCT imaging [5, 8] and even as early as 3 hours after surgery, 3 of 5 eyes with macular holes showed hole closure based on OCT imaging [5].
In the present study, we have shown that the mean MHBD under fluid-air exchange became significantly smaller than that after ILM peeling or before the surgery, and that when using a handheld SD-OCT, the inner edges of the macular hole were observed to come into contact with each other under the fluid-air exchange in 1 of the 3 eyes with a stage 3 macular hole. In a case involving a 14-year-old boy with a traumatic full-thickness macular hole, there was nearly a complete closure of the macular hole immediately after vitrectomy and ILM peeling [3], whereas idiopathic macular holes in this study did not close before fluid-air exchange. We speculate that both anterior-posterior traction and tangential traction might be more involved in traumatic macular holes than in idiopathic macular holes.
Masuyama et al. [5] reported that the OCT patterns of macular hole closure showed the bridge formation in 9 of 12 eyes (75%) and the simple closure in 3 of 12 eyes (25%) within 7 days after macular hole surgery, whereas 12 of 16 eyes (75%) showed the simple closure and 4 of 16 eyes (25%) still showed the bridge formation at 1 month after the surgery. Takahashi and Kishi [10] showed that the bridge tissue needed an average of 2 months to attach to the retinal pigment epithelium after macular hole surgery. These results suggest that the reduction of MHBD caused by fluid-air exchange may be an important step in macular hole closure, and that the contact of both the inner edges of the macular hole after fluid-air exchange may result in the bridge formation of macular holes.
Intraoperative use of SD-OCT has been attractive because the OCT images provide valuable information which can assist in the choice of procedures during vitreoretinal surgery [1, 2]. In this study, OCT images were obtained both under fluid and under air during the macular hole surgery. With the air of the vitreous cavity, refractive errors were changed, but measurements of retinal thickness with OCT images were not affected until −10 diopters, except for inferior segments [11]. As only MHBD was measured and the SD-OCT software automatically focused the retina in each eye at each measurement, the effects of refractive errors on the measurements of MHBD seemed negligible.
Early detection of macular hole closure with SD-OCT after the surgery contributed to a reduction in the duration of prone positioning [5, 8]. Previous studies have reported closure rates of 88.6–90.5% following macular hole surgery without prone positioning [6, 7]. Further studies using intraoperative SD-OCT will be needed to clarify the detailed changes in macular holes in order to achieve higher closure rates of macular hole surgery without prone positioning.
Disclosure Statement
The authors have no financial or conflicting interests to disclose.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Binder S, Falkner-Radler CI, Hauger C, Matz H, Glittenberg C. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011 doi: 10.1097/IAE.0b013e3182019c18. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Dayani PN, Maldonado R, Farsiu S, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009;29:1457–1468. doi: 10.1097/IAE.0b013e3181b266bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wykoff CC, Berrocal AM, Schefler AC, Uhlhorn SR, Ruggeri M, Hess D. Intraoperative OCT of a full-thickness macular hole before and after internal limiting membrane peeling. Ophthalmic Surg Lasers Imaging. 2010;41:7–11. doi: 10.3928/15428877-20091230-01. [DOI] [PubMed] [Google Scholar]
- 4.Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116:2448–2456. doi: 10.1016/j.ophtha.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masuyama K, Yamakiri K, Arimura N, Sonoda Y, Doi N, Sakamoto T. Posturing time after macular hole surgery modified by optical coherence tomography images: a pilot study. Am J Ophthalmol. 2009;147:481–488. doi: 10.1016/j.ajo.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Carvounis PE, Kopel AC, Kuhl DP, Heffez J, Pepple K, Holz ER. Twenty-five-gauge vitrectomy using sulfur hexafluoride and no prone positioning for repair of macular holes. Retina. 2008;28:1188–1192. doi: 10.1097/IAE.0b013e318177f9a8. [DOI] [PubMed] [Google Scholar]
- 7.Yagi F, Sato Y, Takagi S, Tomita G. Idiopathic macular hole vitrectomy without postoperative face-down positioning. Jpn J Ophthalmol. 2009;53:215–218. doi: 10.1007/s10384-008-0642-7. [DOI] [PubMed] [Google Scholar]
- 8.Mittra RA, Kim JE, Han DP, Pollack JS. Sustained postoperative face-down positioning is unnecessary for successful macular hole surgery. Br J Ophthalmol. 2009;93:664–666. doi: 10.1136/bjo.2008.148544. [DOI] [PubMed] [Google Scholar]
- 9.Kusuhara S, Teraoka MF, Fujii S, Nakanishi Y, Tamura Y, Nagai A, Yamamoto H, Tsukahara Y, Negi A. Prediction of postoperative visual outcome based on hole configuration by optical coherence tomography in eyes with idiopathic macular holes. Am J Ophthalmol. 2004;138:709–716. doi: 10.1016/j.ajo.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi H, Kishi S. Tomographic features of early macular hole closure after vitreous surgery. Am J Ophthalmol. 2000;130:192–196. doi: 10.1016/s0002-9394(00)00456-6. [DOI] [PubMed] [Google Scholar]
- 11.Salchow DJ, Hwang AM, Li FY, Dziura J. Effect of contact lens power on optical coherence tomography of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2011;52:1650–1654. doi: 10.1167/iovs.10-6118. [DOI] [PubMed] [Google Scholar]