Abstract
OBJECTIVE: To analyze the costs of nitrofurantoin use compared to those of other antibiotics recommended for treatment of uncomplicated urinary tract infection (UTI).
PATIENTS AND METHODS: We used a decision analysis model to perform cost-minimization and sensitivity analyses to determine the level of trimethoprim-sulfamethoxazole (TMP-SMX) and fluoroquinolone resistance that would favor the use of nitrofurantoin as a first-line empirical treatment of uncomplicated UTIs. The model used a program perspective to evaluate costs.
RESULTS: Nitrofurantoin was cost-minimizing when the prevalence of fluoroquinolone resistance exceeded 12% among uropathogens or the prevalence of TMP-SMX resistance exceeded 17%. On 2-way sensitivity analysis, variables that had a significant impact on our cost-minimization threshold included cost of antibiotics and probability of clinical cure with antibiotics.
CONCLUSION: From a payer perspective, nitrofurantoin appears to be a reasonable alternative to TMP-SMX and fluoroquinolones for empirical treatment of uncomplicated UTIs, especially given the current prevalence of antibiotic resistance among community uropathogens. On the basis of efficacy, cost, and low impact on promoting antimicrobial resistance, clinicians should consider nitrofurantoin as a reasonable alternative to TMP-SMX and fluoroquinolones for first-line therapy for uncomplicated UTIs.
From a payer perspective, nitrofurantoin appears to be a reasonable alternative to trimethoprim-sulfamethoxazole and fluoroquinolones for empirical treatment of uncomplicated urinary tract infections, especially given the current prevalence of antibiotic resistance among community uropathogens.
AWP = average wholesale price; IDSA = Infectious Diseases Society of America; TMP-SMX = trimethoprim-sulfamethoxazole; UTI = urinary tract infection
Uncomplicated urinary tract infections (UTIs) in women are a common problem in primary care. In 1995, 10.8% of women in the United States aged 18 years or older had a UTI, resulting in 11.3 million prescriptions written for the treatment of UTI and an annual social cost of $1.6 billion.1
For many years, trimethoprim-sulfamethoxazole (TMP-SMX) was the preferred antibiotic for the treatment of UTI, given its efficacy and low cost.2 However, development of a high prevalence of TMP-SMX resistance among uropathogens3-5 has discouraged use of this drug in many communities. An effective alternative for many clinicians has been the fluoroquinolone class of antibiotics, which achieve high concentrations in the urine and have excellent activity against most uropathogens.2 Of note, the 2011 Infectious Diseases Society of America (IDSA) guidelines on the treatment of UTIs discourage use of fluoroquinolones for acute, uncomplicated UTI.6 Experts are concerned about overuse of fluoroquinolones leading to increased prevalence of fluoroquinolone-resistant pathogens that has major implications for the treatment of more serious infections, such as community-acquired pneumonia, health care–associated pneumonia, and complicated UTIs.
Nitrofurantoin has been used for decades as an alternative treatment of uncomplicated UTIs. Additionally, nitrofurantoin has retained a high prevalence of sensitivity to most uropathogens and has a favorable side-effect profile.7 A recently published randomized trial demonstrated that nitrofurantoin for 5 days is as effective as TMP-SMX for 3 days in treating acute UTI in women.8 Moreover, nitrofurantoin, like TMP-SMX, is less expensive than fluoroquinolones in many communities.9
For editorial comment, see page 477
Previous analyses have attempted to determine optimal therapy for uncomplicated UTIs on the basis of cost-minimization models and the prevalence of antimicrobial resistance among uropathogens. The first such investigation compared fluoroquinolones to TMP-SMX for empirical therapy and concluded that fluoroquinolones were cost-minimizing when the rate of TMP-SMX resistance exceeded 22% in the community.10 A subsequent model of fluoroquinolones and TMP-SMX constructed by independent investigators showed similar conclusions.11 No similar economic analyses have examined how nitrofurantoin compares to the IDSA-recommended empirical therapy for uncomplicated UTIs. Therefore, we performed a cost-minimization and sensitivity analysis comparing TMP-SMX, fluoroquinolones, and nitrofurantoin to determine the threshold level of antimicrobial resistance for which each of these antibiotics becomes cost-minimizing.
PATIENTS AND METHODS
To determine the threshold level of TMP-SMX and fluoroquinolone resistance among community uropathogens that would make nitrofurantoin cost-minimizing, we employed a decision tree model using the DATA software (version 4.0, TreeAge Software, Williamstown, MA). The model is based on previous models of uncomplicated UTI treatment caused by Escherichia coli in women older than 18 years.10-12 All the models used a payer (program) perspective for the study. To obtain information on clinical outcomes and cost of uncomplicated UTIs, a systematic review of the literature was performed. We searched MEDLINE articles from 1996 through July 27, 2010, with the keywords urinary, urine, or cystitis and combined these words with cost, cure, success, response, or efficacy. Similar systematic searches were conducted using Embase and Cochrane Library databases. Three reviewers (J.A.M., C.W.J., L.G.M.) assessed the abstracts, and if an abstract suggested that the article contained data on clinical cure rates of uncomplicated UTIs based on antimicrobial susceptibility, the article was reviewed. Reference lists of retrieved articles were reviewed to find additional studies. For articles published in non-English languages, we reviewed only the abstracts.
The cost-minimization model of UTI treatment on scenarios was derived from the review of the literature and published models.10-20 In the model, it was assumed that clinicians would choose to empirically treat uncomplicated UTIs with a 3-day course of generic TMP-SMX, a 3-day course of a generic fluoroquinolone (ciprofloxacin), or a 5-day course of generic nitrofurantoin macrocrystals. The 5-day course of nitrofurantoin was based on studies suggesting that a 3-day nitrofurantoin course may not be equivalent to that of TMP-SMX or fluoroquinolones.8,13 It was also assumed that empirical treatment involved a clinic visit and urinalysis to confirm the presence of UTI and that urine cultures were not performed initially, as recommended by prior IDSA guidelines.21
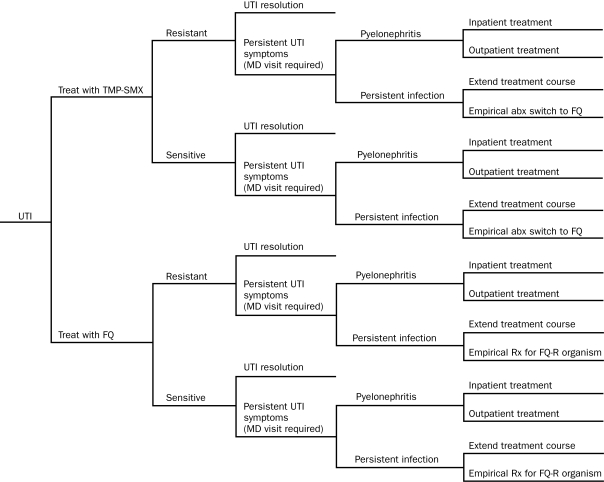
In the model, infection would resolve with initial therapy or would fail to respond to initial therapy (Figure 1). In the latter case, the UTI would resolve after continuation with that treatment or the original antibiotic treatment would be changed. Potential complications of clinical nonresponse included persistence of cystitis symptoms, outpatient treatment of pyelonephritis, or hospitalization for pyelonephritis. Persistence of cystitis symptoms would result in a return visit to the physician, performance of a urine culture, and either an extended course of the same antibiotic or a change to a different antibiotic.
FIGURE 1.
Decision tree diagram that shows intermediate and final outcomes for an uncomplicated Escherichia coli urinary tract infection (UTI) treated with either trimethoprim-sulfamethoxazole (TMP-SMX) or a fluoroquinolone (FQ). Similar trees were constructed comparing nitrofurantoin with FQ, TMP-SMX with nitrofurantoin, and a 3-way model comparing nitrofurantoin, TMP-SMX, and FQ. After each outcome on the right side of the tree, the probability of developing a vaginal yeast infection was considered (not shown; see text). abx = antibiotic; FQ-R = FQ resistant; MD = medical doctor; Rx = treatment.
The decision analysis model for the comparison of TMP-SMX and fluoroquinolones is shown in Figure 1. Two similar models were constructed comparing nitrofurantoin with fluoroquinolones and TMP-SMX with nitrofurantoin. For simplicity, the Figure does not include the probability of vaginal yeast infection, which was calculated for each outcome on the basis of published probabilities.7,22-25 Other potential antibiotic effects or complications (eg, Stevens-Johnson syndrome) were considered rare and not included in our model, given that a previous investigation of uncomplicated UTIs found that expensive but uncommon events had almost no effect on overall treatment costs.26
Finally, a model was constructed that compared all 3 treatments: nitrofurantoin, TMP-SMX, and fluoroquinolones. This model was used to evaluate the most cost-effective empirical therapy for uncomplicated UTI at varying levels of fluoroquinolone and TMP-SMX resistance. Because nitrofurantoin resistance has remained relatively stable in the United States and Europe, nitrofurantoin resistance was held constant at 1.2% for the model.4,5,8
On the basis of our model, 6 major scenarios arose: (1) treatment of a TMP-SMX–resistant infection with TMP-SMX; (2) treatment of a TMP-SMX–sensitive infection with TMP-SMX; (3) treatment of a nitrofurantoin-resistant infection with nitrofurantoin; (4) treatment of a nitrofurantoin-sensitive infection with nitrofurantoin; (5) treatment of a fluoroquinolone-resistant infection with ciprofloxacin; and (6) treatment of a fluoroquinolone-sensitive infection with ciprofloxacin. Ciprofloxacin was used in the model because it is widely considered by clinicians to be the fluoroquinolone of choice in the treatment of uncomplicated UTIs due to its efficacy, tolerability, and low cost.27,28
Costs
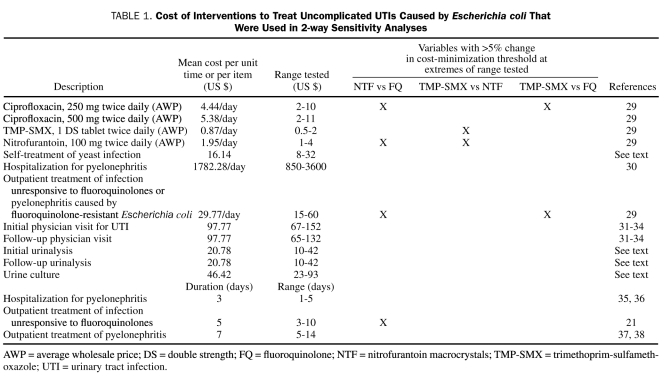
Costs used in this model were based on the systematic review of the literature and a survey of costs derived from national and local sources (Table 1). Antibiotic cost was defined as the median of all generic manufacturers' average wholesale price (median AWP) listed in the 2010 Red Book.9 Costs of hospitalization were compiled from a national survey and incorporated the average cost of a day of hospitalization from the American Hospital Association.39 Costs of physician visits were derived from the literature31-33 and from physician payment schedules available from the Centers for Medicare and Medicaid Services.34 For the cost of outpatient pyelonephritis treatment, it was assumed that high-dose fluoroquinolones were used as therapy. For outpatient treatment of a fluoroquinolone-resistant infection, the cost of therapy with intramuscular ceftriaxone or gentamicin was used.29 Costs of urinalysis and urine culture were calculated as an average of costs obtained from 3 different commercial laboratories (Diagnostic Laboratories, San Pedro, CA; Quest Diagnostics, Madison, NJ; American Bio-Clinical Laboratories, Los Angeles, CA). The cost of a course of self-treatment of vaginal yeast infection was calculated from a survey of prices for over-the-counter antifungal products available from 3 different national pharmacies (CVS, Walgreens, and Rite Aid).
TABLE 1.
Cost of Interventions to Treat Uncomplicated UTIs Caused by Escherichia coli That Were Used in 2-way Sensitivity Analyses
Probabilities
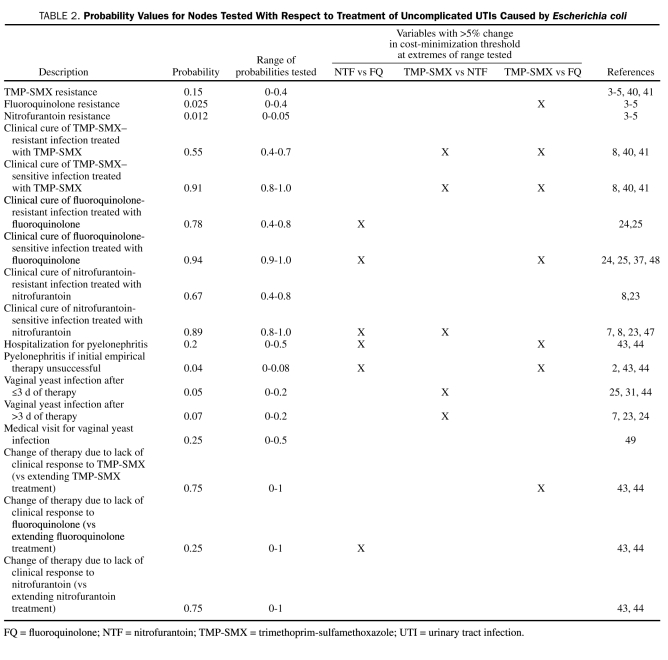
Probabilities for clinical events were obtained from published estimates,3-5,7,8,23-25,31,37,40-49 with use of the mean value as the point estimate in the model (Table 2). These estimates were derived from previous models and the systematic review of the literature.
TABLE 2.
Probability Values for Nodes Tested With Respect to Treatment of Uncomplicated UTIs Caused by Escherichia coli
Sensitivity analyses
One-way sensitivity analyses were performed for the following comparisons: mean cost of empirical treatment with nitrofurantoin and fluoroquinolones vs fluoroquinolone resistance, mean cost of empirical treatment with TMP-SMX and nitrofurantoin vs TMP-SMX resistance, and mean cost of empirical treatment with TMP-SMX and fluoroquinolones vs TMP-SMX resistance. In addition, a 2-way sensitivity analysis was performed to determine how changing the values of each cost and probability in the model would affect the cost-minimization threshold. For costs, a range incorporating 50% to 200% of the point estimate was used (Table 1). For probabilities, the range of probabilities for clinical events found in the literature survey was used (Table 2). To minimize authors' bias, if the literature was scarce (only 1 source) describing the probability of an event, a range incorporating values from at least 50% to 200% of the point estimate was used (Table 2).
RESULTS
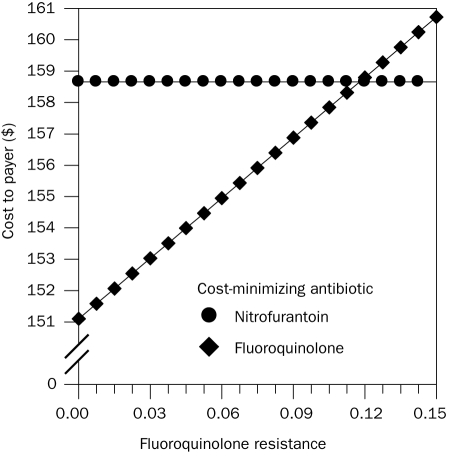
For the comparison of nitrofurantoin and fluoroquinolones, the sensitivity analysis demonstrated that, when the percentage of fluoroquinolone-resistant E coli exceeds 12%, empirical treatment with nitrofurantoin becomes cost-minimizing; below that level, fluoroquinolones are cost minimizing compared to nitrofurantoin (Figure 2). At the 12% fluoroquinolone resistance breakpoint, the mean total cost of empirical treatment of UTI with fluoroquinolones and nitrofurantoin was $159. When comparing nitrofurantoin and TMP-SMX, nitrofurantoin was cost-minimizing when the percentage of TMP-SMX–resistant E coli exceeded 17%. When TMP-SMX resistance was below 17%, TMP-SMX was cost-minimizing compared to nitrofurantoin. When comparing fluoroquinolones and TMP-SMX, fluoroquinolones were cost-minimizing when the percentage of TMP-SMX–resistant E coli exceeded 10%.
FIGURE 2.
Comparison of the mean cost of treatment of uncomplicated Escherichia coli urinary tract infection with nitrofurantoin and a fluoroquinolone vs the community prevalence of fluoroquinolone-resistant E coli. The threshold for fluoroquinolone resistance above which nitrofurantoin becomes less expensive was 12% with an estimated cost of $159 to the payer.
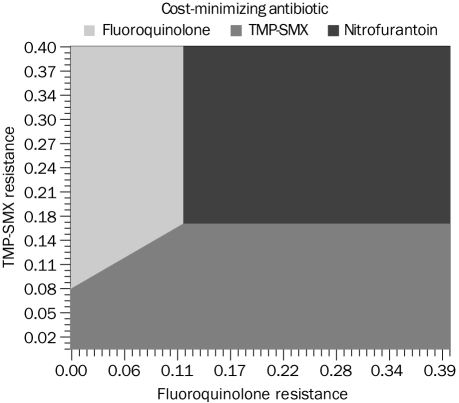
For the model comparing all 3 treatments, nitrofurantoin was cost-minimizing when the percentage of TMP-SMX resistance exceeded 17% and fluoroquinolone resistance exceeded 12%. With lower percentages of TMP-SMX and fluoroquinolone resistance, TMP-SMX or a fluoroquinolone was cost-minimizing depending on prevalence of resistance to these 2 agents (Figure 3).
Figure 3.
Three-way strategy analysis demonstrating the threshold level of fluoroquinolone and trimethoprim-sulfamethoxazole (TMP-SMX) resistance among Escherichia coli above which empirical treatment of urinary tract infection with nitrofurantoin is cost-minimizing. This Figure assumes a constant prevalence of nitrofurantoin resistance at 1.2% (see text for details).
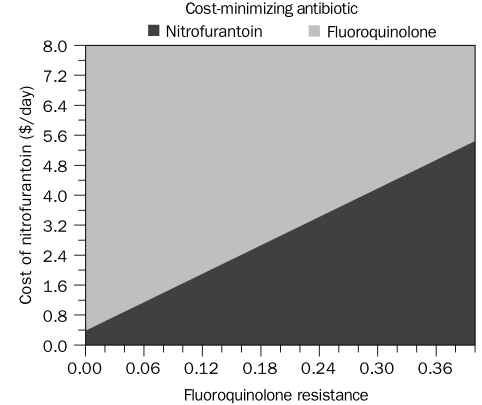
In 2-way sensitivity analyses of all comparison models, the cost of antibiotics had a significant effect on the cost-minimization thresholds. In particular, the resistance threshold of fluoroquinolones that makes nitrofurantoin cost-minimizing was sensitive to the cost of nitrofurantoin (Figure 4). Cost of outpatient therapy for a UTI that was not responsive to a fluoroquinolone had a moderate effect on the threshold (Table 1). At the extreme values of the range for cost of outpatient therapy after fluoroquinolone failure, the 12% breakpoint for fluoroquinolone resistance varied from 6% to 18%. The costs of most other interventions, including physician visits, urinalyses, and treatment of yeast infections, had a negligible effect on the cost-minimization thresholds (Table 1). Two-way sensitivity analyses with greater than 5% (absolute) difference in the threshold at extreme values of the range are noted in Table 1 and Table 2.
FIGURE 4.
Two-way sensitivity analysis showing the effect of varying the cost of nitrofurantoin on the threshold of fluoroquinolone-resistant Escherichia coli needed to make nitrofurantoin cost-minimizing.
DISCUSSION
Using a cost-minimization model of empirical treatment of uncomplicated UTIs, we have performed, to our knowledge, the first decision analysis to explore the role of nitrofurantoin in empirical therapy for uncomplicated UTIs. In comparison with fluoroquinolones, nitrofurantoin was found to be cost-minimizing when the prevalence of fluoroquinolone-resistant E coli in the community reached 12%. In comparison with TMP-SMX, nitrofurantoin was cost-minimizing when E coli resistance to TMP-SMX was 17%. The 3-way model that considered nitrofurantoin, fluoroquinolones, and TMP-SMX demonstrated that, within ranges of uropathogen resistance found in many communities, nitrofurantoin can be cost-minimizing. Our data support the 2011 IDSA recommendations that nitrofurantoin be considered as first-line empirical therapy for uncomplicated UTIs.6
To date, nitrofurantoin has been a less commonly used treatment of uncomplicated UTIs. Older monohydrate formulations of nitrofurantoin required dosing 4 times per day and were more cumbersome than twice-daily ciprofloxacin or TMP-SMX. In addition, data from the literature suggested that a 3-day course of nitrofurantoin was not as effective as a 3-day course of TMP-SMX or fluoroquinolones.8,13 Until recently, nitrofurantoin was considered an inferior agent for the treatment of uncomplicated UTIs.50 Currently available macrocrystal formulations of nitrofurantoin allow for twice-daily dosing. Furthermore, a recent clinical trial published after 1999 IDSA guidelines data suggests that nitrofurantoin for 5 days is as effective as TMP-SMX for 3 days in treating acute UTIs in women.8 Supported by these newer data, the 2011 IDSA guidelines have elevated nitrofurantoin from an alternative agent to first-line therapy for uncomplicated UTIs.6
In contrast, the new IDSA guidelines demote fluoroquinolones from first-line therapy to an alternative agent for uncomplicated UTIs.6 A major impetus for limiting the use of fluoroquinolones has been to delay the progression of resistance to these drugs. Fluoroquinolones are among the most commonly used antibiotics, and the development of widespread resistance to these agents may have grave implications for the treatment of more serious infections, such as pneumonia and complicated UTIs. By providing an effective alternative for the treatment of uncomplicated UTIs, nitrofurantoin may contribute to a reduction in overall fluoroquinolone use and thus help to reduce selection pressure for increased resistance to fluoroquinolones.51-53
Despite availability and use of nitrofurantoin for more than 5 decades, the prevalence of nitrofurantoin resistance among common uropathogens remains low. In the United States and worldwide, the prevalence of nitrofurantoin resistance among uropathogens is 1% to 2%.4,5,8,54 For TMP-SMX, the prevalence of resistance among uropathogens is greater than 15% in every area of the United States and as high as 42% in the West South Central region.4 For fluoroquinolones in the United States, the prevalence of resistance among uropathogenic E coli in some regions has been reported as high as 20%.4 The prevalence of fluoroquinolone resistance in other regions of the world is similarly high.54-57 Of note, these measures of resistance prevalence may overestimate resistance found in uncomplicated UTIs because surveillance of resistance of cystitis is rarely performed.50 However, the last nationwide survey of antibiotic resistance among uropathogens causing outpatient UTIs was conducted in 2005,58 and the prevalence of resistance may be higher. Our model suggests that nitrofurantoin use for uncomplicated UTIs may be cost-minimizing compared with TMP-SMX in many areas of the United States and cost-competitive compared with generic ciprofloxacin.
Our investigation has limitations. One limitation was the sensitivity of the model to several variables for which testing extreme values of the range caused a greater than 5% change in the cost-minimization threshold. This was especially evident for antibiotic cost. In the comparison of nitrofurantoin and fluoroquinolones, a small change in the cost of either drug was associated with substantial changes in the cost-minimization threshold. Comparing TMP-SMX with fluoroquinolones, fluoroquinolones were cost-minimizing when TMP-SMX resistance was 10%. Our breakpoint of TMP-SMX resistance is lower than previously published breakpoints (19%-21%) because generic fluoroquinolones that are less costly have become available since the publication of those analyses.10,11
Other variables that had a significant effect on the cost-minimization threshold included the probability of clinical cure with antibiotics. This finding is likely a result of our very conservative approach and the wide ranges in probability that were used for these clinical events. For example, the point estimate for the probability of clinical cure of an infection with a nitrofurantoin-resistant organism treated with nitrofurantoin was 67%. The range used for the sensitivity analysis was between 40% and 80%. However, this range is probably overly broad. The cure rate of uncomplicated UTIs with placebo is reported to be more than 50%.59 Therefore, cure rates of nitrofurantoin treatment of nitrofurantoin-resistant uropathogens are unlikely to be as low as 40%.
Another limitation of our study is the paucity of data on clinical cure rates of uncomplicated UTIs when the causative organism is resistant to the antibiotic selected for treatment. We found only 2 studies that reported clinical cure rates of uncomplicated UTIs due to nitrofurantoin-resistant organisms treated with nitrofurantoin. In these studies, a total of only 14 patients were infected with such organisms.8,23 There was a similar lack of data for fluoroquinolones24,25 but relatively more data for TMP-SMX.8,40,41 More research is needed to better determine the probability of curing UTIs when the causative organism is resistant to the antibiotic used, especially given how increasingly common this scenario is in primary care. A final limitation is that our model does not contain data on the wide range of adverse events that can occur with each agent. However, precise rates of these rare events in the population being treated are not available, and previous UTI models found that, in sensitivity analyses, rare and expensive events have almost no effect on study results.26
Our investigation has several strengths. First, the decision analysis is the first to compare nitrofurantoin against IDSA recommended antibiotics for uncomplicated UTI treatment. Second, the model is based on previously published decision analysis models developed independently by 2 different investigative groups. In addition, the model is the first to address cost-minimization of empirical treatment of uncomplicated UTIs since generic fluoroquinolones have become available. Finally, the 3-way comparison of nitrofurantoin, TMP-SMX, and fluoroquinolones evaluates the cost-effectiveness of the most popular antibiotics used in the treatment of uncomplicated UTIs at current rates of antibiotic resistance among uropathogens. The 3-way model may help policy makers, hospital administrators, and antimicrobial stewardship committees customize their recommendations for first-line therapy for uncomplicated UTIs based on local resistance patterns.
CONCLUSION
Nitrofurantoin is an important treatment option for uncomplicated UTIs in the current era of increasing fluoroquinolone and TMP-SMX resistance among uropathogens. Nitrofurantoin is likely cost-minimizing compared with TMP-SMX in most areas of the United States and cost-competitive compared with generic ciprofloxacin. Our findings complement recent data that demonstrate that the efficacy and tolerability of nitrofurantoin are comparable with the efficacy and tolerability of TMP-SMX for treating uncomplicated UTIs. On the basis of the efficacy, cost, and relatively low effect of nitrofurantoin on promoting further antimicrobial resistance, clinicians, local guidelines, and payers should support its use as a first-line option for empirical treatment of uncomplicated UTIs.
Acknowledgments
We thank Kalpana Gupta, MD, for her assistance with obtaining resistance data for nitrofurantoin and Samantha Eells, MPH, for her critical review of the submitted manuscript.
Footnotes
We acknowledge logistic support from the M01 RR 00425 grant to the General Clinic Research Center at Harbor-UCLA Medical Center.
An earlier version of this article appeared Online First.
REFERENCES
- 1. Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel J. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509-515 [DOI] [PubMed] [Google Scholar]
- 2. Johnson JR, Stamm W. Diagnosis and treatment of acute urinary tract infections [published correction appears in Infect Dis Clin North Am.1990;4(2):following xii] Infect Dis Clin North Am. 1987;1(4):773-791 [PubMed] [Google Scholar]
- 3. Gupta K, Scholes D, Stamm W. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736-738 [DOI] [PubMed] [Google Scholar]
- 4. Karlowsky JA, Kelly LJ, Thornsberry C, Jones ME, Sahm DF. Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. Antimicrob Agents Chemother. 2002;46(8):2540-2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kahlmeter G. An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO.SENS Project. J Antimicrob Chemother. 2003;51:69-76 [DOI] [PubMed] [Google Scholar]
- 6. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120 [DOI] [PubMed] [Google Scholar]
- 7. Spencer RC, Moseley DJ, Greensmith MJ. Nitrofurantoin modified release versus trimethoprim or co-trimoxazole in the treatment of uncomplicated urinary tract infection in general practice. J Antimicrob Chemother. 1994;33(suppl A):121-129 [DOI] [PubMed] [Google Scholar]
- 8. Gupta K, Hooton T, Roberts P, Stamm W. Short-course nitrofurantoin for the treatment of acute uncomplicated cystitis in women. Arch Intern Med. 2007;167:2207-2212 [DOI] [PubMed] [Google Scholar]
- 9. 2010 Red Book: Pharmacy's Fundamental Reference. 114th ed. New York, NY: Thomson Reuters (Healthcare); 2010. [Google Scholar]
- 10. Le TP, Miller LG. Empirical therapy for uncomplicated urinary tract infections in an era of increasing antimicrobial resistance: a decision and cost analysis. Clin Infect Dis. 2001;33:615-621 [DOI] [PubMed] [Google Scholar]
- 11. Perfetto EM, Gondek K. Escherichia coli resistance in uncomplicated urinary tract infection: a model for determining when to change first-line empirical antibiotic choice. Manag Care Interface. 2002;15:35-42 [PubMed] [Google Scholar]
- 12. Perfetto EM, Keating K, Merchant S, Nichols BR. Acute uncomplicated UTI and E. coli resistance: implications for first-line empirical antibiotic therapy. J Manag Care Pharm. 2004;10:17-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE, Infectious Diseases Society of America (IDSA) Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Clin Infect Dis. 1999;29:745-758 [DOI] [PubMed] [Google Scholar]
- 14. Anderson RU. Management of lower urinary tract infections and cystitis. Urol Clin North Am. 1999;26:729-735 [DOI] [PubMed] [Google Scholar]
- 15. Hooton TM. Practice guidelines for urinary tract infection in the era of managed care. Int J Antimicrob Agents. 1999;11:241-245 [DOI] [PubMed] [Google Scholar]
- 16. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(suppl 1):1-7 [PubMed] [Google Scholar]
- 17. Carlson KJ, Mulley AG. Management of acute dysuria: a decision-analysis model of alternative strategies. Ann Intern Med. 1985;102:244-249 [DOI] [PubMed] [Google Scholar]
- 18. Iravani A. Advances in the understanding and treatment of urinary tract infections in young women. Urology. 1991;37:503-511 [DOI] [PubMed] [Google Scholar]
- 19. Echols RM, Tosiello RL, Haverstock DC, Tice AD. Demographic, clinical, and treatment parameters influencing the outcome of acute cystitis. Clin Infect Dis. 1999;29:113-119 [DOI] [PubMed] [Google Scholar]
- 20. Johnson JR, Stamm WE. Urinary tract infections in women: diagnosis and treatment. Ann Intern Med. 1989;111(11):906-917 [DOI] [PubMed] [Google Scholar]
- 21. Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for the antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Clin Infect Dis. 1999;29:745-758 [DOI] [PubMed] [Google Scholar]
- 22. Barry HC, Ebell MH, Hickner J. Evaluation of suspected urinary tract infection in ambulatory women: a cost-utility analysis of office-based strategies. J Fam Pract. 1997;44:49-60 [PubMed] [Google Scholar]
- 23. Stein GE. Comparison of single-dose fosfomycin and a 7-day course of nitrofurantoin in female patients with uncomplicated urinary tract infection. Clin Ther. 1999;21(11):1864-1872 [DOI] [PubMed] [Google Scholar]
- 24. Iravani A, Klimberg I, Briefer C, Munera C, Kowalsky SF, Echols RM. A trial comparing low-dose, short-course ciprofloxacin and standard 7 day therapy with co-trimoxazole or nitrofurantoin in the treatment of uncomplicated urinary tract infection. J Antimicrob Chemother. 1999;43(suppl A):67-75 [PubMed] [Google Scholar]
- 25. Henry DC, Jr, Bettis RB, Riffer E, et al. Comparison of once-daily extended-release ciprofloxacin and conventional twice-daily ciprofloxacin for the treatment of uncomplicated urinary tract infection in women. Clin Ther. 2002;24:2088-2103 [DOI] [PubMed] [Google Scholar]
- 26. Le TP, Miller LG. Empirical therapy for uncomplicated urinary tract infections in an era of increasing antimicrobial resistance: a decision and cost analysis. Clin Infect Dis. 2001;33:615-621 [DOI] [PubMed] [Google Scholar]
- 27. Talan DA, Naber KG, Palou J, Elkharrat D. Extended-release ciprofloxacin (Cipro XR) for treatment of urinary tract infections. Int J Antimicrob Agents. 2004;23S1:S54-S66 [DOI] [PubMed] [Google Scholar]
- 28. Kallen AJ, Welch HG, Sirovich BE. Current antibiotic therapy for isolated urinary tract infections in women. Arch Intern Med. 2006;166:635-639 [DOI] [PubMed] [Google Scholar]
- 29. 2007 Red Book: Pharmacy's Fundamental Reference. 111th ed. Fleming T, ed. Montvale, NJ: Medical Economics Company; 2007. [Google Scholar]
- 30. AHA Hospital Statistics. 2007 edition Chicago, IL: American Hospital Association; 2007. [Google Scholar]
- 31. Hooton TM, Winter C, Tiu F, Stamm WE. Randomized comparative trial and cost analysis of 3-day antimicrobial regimens for treatment of acute cystitis in women. JAMA. 1995;273:41-45 [PubMed] [Google Scholar]
- 32. Neuner JM, Hamel MB, Phillips RS, Bona K, Aronson MD. Diagnosis and management of adults with pharyngitis: a cost-effectiveness analysis. Ann Intern Med. 2003;139:113-122 [DOI] [PubMed] [Google Scholar]
- 33. Rubin N, Foxman B. The cost-effectiveness of placing urinary tract infection treatment over the counter. J Clin Epidemiol. 1996;49:1315-1321 [DOI] [PubMed] [Google Scholar]
- 34. Centers for Medicare and Medicaid Services US Department of Health and Human Services Web site. http://www.cms.gov/ Accessed February 23, 2011
- 35. Coley AL, Todd MW, Harrington P. Treatment of serious urinary tract infections at a university teaching hospital: a retrospective chart review. Hosp Formul. 1990;25(5):548-552 [PubMed] [Google Scholar]
- 36. Bailey RR, Lynn KL, Robson RA, Peddie BA, Smith A. Comparison of ciprofloxacin and netilmicin for the treatment of acute pyelonephritis. N Z Med J. 1992;105(930):102-103 [PubMed] [Google Scholar]
- 37. Talan DA, Stamm WE, Hooton TM, et al. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women: a randomized trial. JAMA. 2000;283(12):1583-1590 [DOI] [PubMed] [Google Scholar]
- 38. Takahashi S, Hirose T, Satoh T, et al. Efficacy of a 14-day course of oral ciprofloxacin therapy for acute uncomplicated pyelonephritis. J Infect Chemother. 2001;7:255-257 [DOI] [PubMed] [Google Scholar]
- 39. AHA Hospital Statistics. 2010 edition Chicago, IL: American Hospital Association; 2010. [Google Scholar]
- 40. Brown PD, Freeman A, Foxman B. Prevalence and predictors of trimethoprim-sulfamethoxazole resistance among uropathogenic Escherichia coli isolates in Michigan. Clin Infect Dis. 2002;34(8):1061-1066 [DOI] [PubMed] [Google Scholar]
- 41. Raz R, Chazan B, Kennes Y, et al. Empiric use of trimethoprim-sulfamethoxazole (TMP-SMX) in the treatment of women with uncomplicated urinary tract infections in a geographical area with a high prevalence of TMP-SMX-resistant uropathogens. Clin Infect Dis. 2002;34:1165-1169 [DOI] [PubMed] [Google Scholar]
- 42. Engel JD, Schaeffer AJ. Evaluation of and antimicrobial therapy for recurrent urinary tract infections in women. Urol Clin North Am. 1998;25:685-701 [DOI] [PubMed] [Google Scholar]
- 43. Rosenberg M. Pharmacoeconomics of treating uncomplicated urinary tract infections. Int J Antimicrob Agents. 1999;11:247-251 [DOI] [PubMed] [Google Scholar]
- 44. Barry HC, Ebell MH, Hickner J. Evaluation of suspected urinary tract infection in ambulatory women: a cost-utility analysis of office-based strategies. J Fam Pract. 1997;44(1):49-60 [PubMed] [Google Scholar]
- 45. Henry NK, Schultz HJ, Grubbs NC, Muller SM, Ilstrup DM, Wilson WR. Comparison of ciprofloxacin and co-trimoxazole in the treatment of uncomplicated urinary tract infection in women. J Antimicrob Chemother. 1986;18(suppl D):103-106 [DOI] [PubMed] [Google Scholar]
- 46. Henry DC, Nenad RC, Iravani A, et al. Comparison of sparfloxacin and ciprofloxacin in the treatment of community-acquired acute uncomplicated urinary tract infection in women. Clin Ther. 1999;21(6):966-981 [DOI] [PubMed] [Google Scholar]
- 47. Christiaens TC, De Meyere M, Verschraegen G, Peersman W, Heytens S, De Maeseneer J. Randomized controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. Br J Gen Pract. 2002;52(482):729-734 [PMC free article] [PubMed] [Google Scholar]
- 48. McCarty JM, Richard G, Huck W, et al. A randomized trial of short-course ciprofloxacin, ofloxacin, or trimethoprim/sulfamethoxazole for the treatment of acute urinary tract infection in women. Am J Med. 1999;106:292-299 [DOI] [PubMed] [Google Scholar]
- 49. Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidasis. N Engl J Med. 2004;351:876-883 [DOI] [PubMed] [Google Scholar]
- 50. Miller LG, Tang AW. Treatment of uncomplicated urinary tract infections in an era of increasing antimicrobial resistance. Mayo Clin Proc. 2004;79(8):1048-1053 [DOI] [PubMed] [Google Scholar]
- 51. Norrby SR, Nord CE, Finch R, European Society of Clinical Microbiology and Infectious Diseases Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect Dis. 2005;5(2):115-119 [DOI] [PubMed] [Google Scholar]
- 52. McDonald LC, Chen FJ, Lo HJ, et al. Emergence of reduced susceptibility and resistance to fluoroquinolones in Escherichia coli in Taiwan and contributions of distinct selective pressures. Antimicrob Agents Chemother. 2001;45(11):3084-3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cizman M, Orazem A, Krizan-Hergouth V, Kolman J. Correlation between increased consumption of fluoroquinolones in outpatients and resistance of Escherichia coli from urinary tract infections [letter]. J Antimicrob Chemother. 2001;47:502 [DOI] [PubMed] [Google Scholar]
- 54. Zhanel GG, Hisanaga TL, Laing NM, et al. Antibiotic resistance in Escherichia coli outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents. 2006;27:468-475 [DOI] [PubMed] [Google Scholar]
- 55. Arslan H, Azap O, Ergonul O, Timurkaynak F. Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J Antimicrob Chemother. 2005;56:914-918 [DOI] [PubMed] [Google Scholar]
- 56. Astal ZE. Increasing ciprofloxacin resistance among prevalent urinary tract bacterial isolates in the Gaza Strip. Singapore Med J. 2005;46(9):457-460 [PubMed] [Google Scholar]
- 57. Johnson L, Sabel A, Burman WJ, et al. Emergence of fluoroquinolone resistance in outpatient urinary Escherichia coli isolates. Am J Med. 2008;121:876-884 [DOI] [PubMed] [Google Scholar]
- 58. Karlowsky JA, Hoban DJ, Decorby MR, Laing NM, Zhanel GG. Fluoroquinolone-resistant urinary isolates of Escherichia coli from outpatients are frequently multidrug resistant: results from the North American Urinary Tract Infection Collaborative Alliance-Quinolone Resistance study. Antimicrob Agents Chemother. 2006;50:2251-2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Christiaens TC, De Meyere M, Verschraegen G, Peersman W, Heytens S, De Maeseneer JM. Randomised controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. Br J Gen Pract. 2002;52:729-734 [PMC free article] [PubMed] [Google Scholar]