Abstract
OBJECTIVE: To identify and describe the frequency, histologic features, and clinical outcome of colon polyposis and neoplasia in Cowden syndrome—a rare familial hamartoma tumor syndrome associated with mutations in the PTEN gene.
PATIENTS AND METHODS: Patients with a clinical diagnosis of PTEN hamartoma tumor syndrome-Cowden phenotype were retrospectively identified and studied. Only those who underwent colonoscopy or colon pathologic interpretation were included in the final analysis.
RESULTS: From 1994 to 2009, 13 patients met study inclusion criteria. Of the 10 patients who underwent colonoscopy, 9 (90%; 95% confidence interval [CI], 57%-100%) had polyps, and 7 (70%; 95% CI, 39%-90%) were estimated to have more than 50 polyps. Pathologic findings of the colon were reviewed in 11 patients, and the spectrum of tumors included hamartomatous, inflammatory, adenomatous, ganglioneuromatous, hyperplastic, and juvenile polyps. Of the 13 patients, 2 (15%; 95% CI, 3%-43%) had left-sided adenocarcinoma without microsatellite instability. Five (38%) of the 13 patients underwent colectomy secondary to polyp dysplasia.
CONCLUSION: Patients with Cowden syndrome have a heavy colon polyp burden with a wide pathologic spectrum, both benign and malignant. The colon polyposis results in a previously unreported morbidity with a high colectomy rate.
Patients with Cowden syndrome have a heavy colon polyp burden with a wide pathologic spectrum, both benign and malignant; the colon polyposis results in a previously unreported morbidity with a high colectomy rate.
AJCC = American Joint Committee on Cancer; CI = confidence interval; CS = Cowden syndrome; GI = gastrointestinal; NCCN = National Comprehensive Cancer Network; PHTS = PTEN hamartoma tumor syndrome
The PTEN hamartoma tumor syndrome (PHTS) is a spectrum of autosomal dominant clinical disorders associated with mutations of the PTEN or phosphatase and tensin homolog gene on chromosome 10.1,2 Cowden syndrome (CS) is the most common phenotype, with an estimated incidence of 1 in 200,000 to 250,000 people.3 The pathognomonic features of CS are mucocutaneous and include trichilemmomas, acral keratoses, and papillomatous lesions. Patients with CS also have a high risk of thyroid, breast, and endometrial neoplasms.4 Gastrointestinal (GI) polyposis is a common manifestation and can occur throughout the entire tract; however, the frequency of colon involvement in patients with CS is unclear.5
Cowden syndrome is classified as a hamartomatous polyposis syndrome—hamartomas being disorganized overgrowths of cells and tissue native to the anatomic location in which they occur. In the GI tract, these include both epithelial and stromal proliferations. Hamartomatous colon polyps, which can histologically resemble juvenile or Peutz-Jeghers polyps, are thought to predominate in CS. However, a wide variety of other polyp histologic findings have been described, including adenomatous, inflammatory, hyperplastic, lymphoid, ganglioneuromatous, and leiomyomatous polyps.6-8 Despite reports of multiple colon polyp histologic diagnoses from single patients,9,10 it is uncertain whether this is a common phenomenon.11
Traditionally, patients with CS have not been considered to have an increased risk of colorectal cancers.6,12 This impression has started to change. Several case reports have described colorectal cancer in CS, including one describing multiple colorectal cancers in a single patient.13-15 A recent study of a multicenter cohort of PTEN mutation carriers reported 13% with colorectal cancer; all affected patients were younger than age 50 years.16 Furthermore, a recent compilation of cases reported a 16% (95% confidence interval [CI], 8%-24%) lifetime colorectal cancer risk in patients with CS.17
The uncertainty of the prevalence and risks of colorectal polyposis in patients with CS is reflected in the lack of recommendations regarding colorectal screening or surveillance in the National Comprehensive Cancer Network (NCCN) treatment guidelines.18 The purpose of this study was to investigate the spectrum of colon disease in patients with CS, with special emphasis on polyp frequency, histopathologic findings, and clinical outcomes.
PATIENTS AND METHODS
The Mayo Clinic Institutional Review Board approved this study. Records coded for PHTS or the component syndromes at our institution were identified for retrospective review. Forty-six patients with clinical diagnoses of PHTS were identified. Patients who had undergone colon investigation, which was considered colonoscopy, colon pathologic interpretation, or both at our institution between 1994 and 2009 were selected. All records were initially accessed on December 1, 2009, and data collected after that point were not included.
Seventeen patients with a clinical diagnosis of PHTS (all classified as CS) had prior colon investigation and were further characterized. Demographic data, genetic testing results, colonoscopy reports, and colon pathologic reports were collected. Age at the time of the initial colonoscopy or pathologic review performed at our institution was recorded. The pathology reports included biopsy and resection specimens collected at our institution and specimens collected at other institutions and subsequently reviewed by pathologists at our institution. Colorectal cancer staging was performed using the 2010 American Joint Committee on Cancer (AJCC) staging system (7th edition).19 Estimation of colon polyp burden was obtained directly from the endoscopy reports. Polyps located in the cecum, ascending, and proximal transverse colon were considered right sided, and polyps from the distal transverse, descending, and sigmoid colon were considered left sided. Pancolonic was used to describe polyps noted in the right and left sides of the colon.
Patients were excluded if they were younger than 18 years at the time of initial colon investigation (n=1) or had insufficient documentation to meet the revised International Cowden Consortium (ICC) criteria for diagnosis (n=3).20
Age is reported as mean ± SD and number of colonoscopies as median (range). Other data are presented as proportions. For clinical findings associated with CS, 95% CIs were calculated using the modified Wald method.
RESULTS
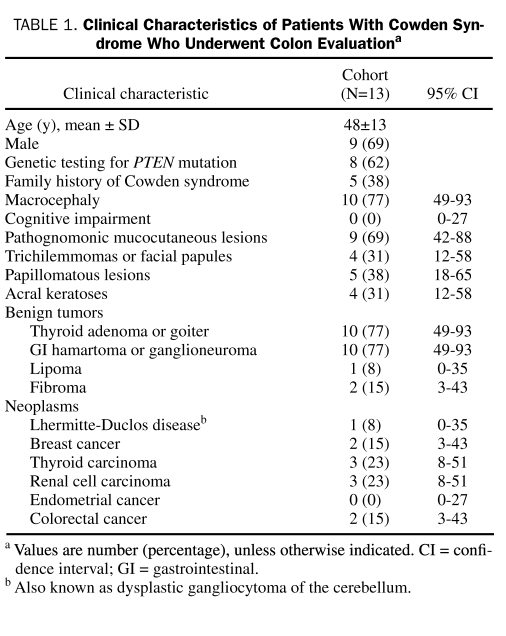
Thirteen patients met the diagnostic criteria for CS and had colon investigation. The mean ± SD age at initial colon evaluation was 48±13 years, and 9 (69%) of the 13 patients were men. Twelve (92%) of the 13 patients self-identified race as white; 1 patient (8%) did not disclose race. The clinical characteristics of the cohort are summarized in Table 1. Of the 8 patients who underwent genetic testing, 7 (88%) were confirmed to have PTEN mutations.
TABLE 1.
Clinical Characteristics of Patients With Cowden Syndrome Who Underwent Colon Evaluationa
Five (38%) of the 13 patients underwent colectomy. The mean age at surgery was 53±8 years. All colectomies were performed for dysplasia; this included 3 total colectomies and 1 subtotal colectomy with ileosigmoidal anastomosis for multiple polyps with low-grade dysplasia without neoplasm and 1 low anterior resection with partial sigmoidectomy for excision of adenocarcinoma.
Colonoscopic Findings
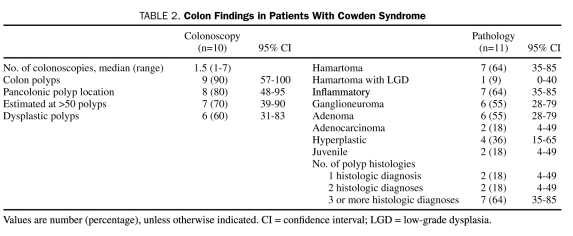
Of the 13 patients, 10 (77%) underwent colonoscopy at our institution. Five (50%) had 1 colonoscopy, 4 (40%) had 2 colonoscopies, and 1 patient (10%) had 7 colonoscopies (Table 2). Of these 10 patients, the initial colonoscopy was performed for screening purposes in 6 (60%) and for symptom investigation in 3 (30%). One patient (10%) did not have a clearly stated indication for the procedure. Of note, of the 10 patients who underwent endoscopy, 9 (90%; 95% CI, 57%-100%) had polyps, and 7 (70%; 95% CI, 39%-90%) were estimated to have more than 50 polyps. Numerous polyps on colonoscopy were the initial finding that led to the diagnosis of CS in 4 (40%) of the 10 patients.
TABLE 2.
Colon Findings in Patients With Cowden Syndrome
Colon Polyp Pathology
Of the 13 patients in the cohort, 11 (85%) had polyps examined histologically, and the findings are summarized in Table 2. The most common diagnoses were hamartomas and nonspecific inflammatory polyps.
Colorectal Cancer
Colorectal cancer was diagnosed in 2 of the 13 patients (15%; 95% CI, 3%-43%). One patient was a 55-year-old man with no known family history of colon cancer or genetic disease. At his initial screening colonoscopy, hundreds of polyps were evident throughout the colon, and multiple biopsy specimens were obtained. A large pedunculated tubular adenoma removed from the descending colon contained adenocarcinoma in the head of the polyp. Pathologic interpretation revealed a well-differentiated adenocarcinoma with invasion only into the lamina propria (AJCC stage 0 [TisN0M0]); it was completely resected with polypectomy. Molecular analysis revealed the absence of microsatellite instability. Biopsy findings on other polyps revealed adenomas and hamartomas.
A mutation in exon 2 of the PTEN gene was found in this patient; subsequently, other family members were diagnosed as having CS. He later underwent a complete colectomy due to the multiple adenomatous polyps and concern for further malignant transformation.
The other patient was a 62-year-old man with no family history of colon cancer or genetic disease. He underwent colonoscopy for evaluation of hematochezia; more than 50 small polyps were evident throughout the colon as well as a 2-cm friable rectosigmoid mass. Biopsy of the mass revealed a moderately differentiated adenocarcinoma. Other biopsy specimens were consistent with hamartomatous and inflammatory polyps.
The patient underwent surgical resection of the rectum and sigmoid colon as treatment for the neoplasm. Histologically, the adenocarcinoma showed focal invasion into but not through the muscularis propria, and multiple regional lymph nodes were negative for metastatic tumor (AJCC stage I [T2N0M0]). Molecular analysis was negative for microsatellite instability. He was later found to have a mutation in exon 5 of the PTEN gene.
DISCUSSION
Cowden syndrome is considered a hamartomatous polyposis syndrome, and colon polyps are a common manifestation. The prevailing dogma has been that these polyps do not confer an elevated risk of colorectal cancer; thus, no consensus recommendations are available for colorectal cancer screening.18 Evidence is growing that patients with CS are indeed at increased risk of colorectal neoplasm.16,17 In our series of 13 patients, 2 were diagnosed as having colorectal cancer. As data accumulate on the risk of colon neoplasia in this population, determining the appropriate age and frequency of screening colonoscopies will be necessary.
Current published recommendations for colon screening vary widely, with some advocating for a baseline examination as early as age 15 years and as frequently as every 2 years thereafter12 and some recommending no screening.21 Two recent studies suggesting an increased risk of colorectal cancer have taken the initiative of providing screening recommendations.16,17 Riegert-Johnson et al17 propose starting with colonoscopy at age 45 years and screening every 5 years thereafter. Heald et al16 propose a screening colonoscopy at age 35 years on the basis of their observation of colon neoplasms in patients with CS who are younger than 50 years. Prospective studies investigating the utility of early colorectal cancer screening in this population would be beneficial but are difficult because of the rarity of the disorder. Until multispecialty consensus guidelines are developed and endorsed by the NCCN, in our opinion it would be reasonable to perform a baseline screening colonoscopy at age 35 years or at the time of diagnosis (whichever is later) and then proceed with interval testing as needed.
Recent estimations of colon polyp prevalence in CS range from 65.6% to 93%.8,16,22 Our findings of 9 of 10 patients with colon polyps on endoscopy are in agreement with these results. Of note, this is much higher than the still frequently cited landmark study by Starink et al,23 which included patients without adequate GI evaluation and reported a 29% incidence of colon polyps. Furthermore, 7 of our 10 patients had more than 50 polyps. This provides evidence that patients with CS have numerous colon polyps, clearly an underreported feature.24
Our patients had a wide range of histologic diagnoses, with hamartoma and inflammatory polyp being the most common. This finding highlights that colorectal polyps of CS lack pathognomonic histologic features. However, the presence of multiple polyps of varied pathologic features should prompt consideration of CS, particularly when hamartomatous, ganglioneuromatous, or inflammatory polyps are present. We also found a high prevalence of adenomatous polyps, suggesting that adenomas may indeed be a manifestation of CS, rather than a coincidental finding as currently considered.5
A novel finding from this series is the higher than expected morbidity associated with colon polyposis. The high rate of colectomy has not previously been reported in CS. There are no specifications of clinical outcomes in the literature review by Marra et al8 evaluating published cases with GI work-up or in the studies suggesting the increased risk of colorectal cancer.16,17 Case reports have described patients treated with colectomy in the setting of adenocarcinoma,13,15 but to our knowledge no prior descriptions have been published of this treatment solely for dysplasia without malignancy.
When our finding of a high frequency of colectomy in patients with multiple polyps is considered, recommendations for increased colorectal screening should be seen in a new light. Colonoscopy at a younger age may decrease invasive colorectal cancer, but for many patients with a high polyp burden, it may lead to an increase in definitive treatment strategies such as colectomy. If colon screening is incorporated into NCCN treatment guidelines, the likely increase in morbidity should be considered and discussed with patients.
The major limitations of the current study are its retrospective nature and the small cohort size. However, when evaluating the literature on CS, this can be considered a large single-center series. There might be referral bias because patients with no abnormal findings on a previous colonoscopy would be less likely to undergo the procedure again at our institution. However, we think this is limited by the presumed early referral to tertiary centers for treatment of patients with this rare syndrome.
CONCLUSION
Patients with CS who underwent a colon evaluation had a high polyp burden with varying pathologic findings, including frequent adenomas and adenocarcinomas. Thus, colorectal cancer screening should be incorporated into treatment guidelines and clinical practice for this population. The high rate of dysplastic polyps resulted in frequent colectomy, and patients should be counseled regarding this possible outcome before undergoing colonoscopy.
REFERENCES
- 1. Nelen MR, Padberg GW, Peeters EA, et al. Localization of the gene for Cowden disease to chromosome 10q22-23. Nat Genet. 1996;13(1):114-116 [DOI] [PubMed] [Google Scholar]
- 2. Hobert JA, Eng C. PTEN hamartoma tumor syndrome: an overview. Genet Med. 2009;11(10):687-694 [DOI] [PubMed] [Google Scholar]
- 3. Nelen MR, Kremer H, Konings IB, et al. Novel PTEN mutations in patients with Cowden disease: absence of clear genotype-phenotype correlations. Eur J Hum Genet. 1999;7(3):267-273 [DOI] [PubMed] [Google Scholar]
- 4. Patnaik MM, Raza SS, Khambatta S, Stanich PP, Goetz MP. Oncophenotypic review and clinical correlates of phosphatase and tensin homolog on chromosome 10 hamartoma tumor syndrome. J Clin Oncol. 2010;28(36):e767-e768 [DOI] [PubMed] [Google Scholar]
- 5. Pilarski R. Cowden syndrome: a critical review of the clinical literature. J Genet Couns. 2009;18(1):13-27 [DOI] [PubMed] [Google Scholar]
- 6. Carlson GJ, Nivatvongs S, Snover DC. Colorectal polyps in Cowden's disease (multiple hamartoma syndrome). Am J Surg Pathol. 1984;8(10):763-770 [DOI] [PubMed] [Google Scholar]
- 7. Hizawa K, Iida M, Matsumoto T, et al. Gastrointestinal manifestations of Cowden's disease: report of four cases. J Clin Gastroenterol. 1994;18(1):13-18 [DOI] [PubMed] [Google Scholar]
- 8. Marra G, Armelao F, Vecchio FM, Percesepe A, Anti M. Cowden's disease with extensive gastrointestinal polyposis. J Clin Gastroenterol. 1994;18(1):42-47 [DOI] [PubMed] [Google Scholar]
- 9. Chen YM, Ott DJ, Wu WC, Gelfand DW. Cowden's disease: a case report and literature review. Gastrointest Radiol. 1987;12(4):325-329 [DOI] [PubMed] [Google Scholar]
- 10. Campos FG, Habr-Gama A, Kiss DR, et al. Cowden syndrome: report of two cases and review of clinical presentation and management of a rare colorectal polyposis. Curr Surg. 2006;63(1):15-19 [DOI] [PubMed] [Google Scholar]
- 11. Zbuk KM, Eng C. Hamartomatous polyposis syndromes. Nat Clin Pract Gastroenterol Hepatol. 2007;4(9):492-502 [DOI] [PubMed] [Google Scholar]
- 12. Schreibman IR, Baker M, Amos C, McGarrity TJ. The hamartomatous polyposis syndromes: a clinical and molecular review. Am J Gastroenterol. 2005;100(2):476-490 [DOI] [PubMed] [Google Scholar]
- 13. Hover AR, Cawthern T, McDanial W. Cowden disease: a hereditary polyposis syndrome diagnosable by mucocutaneous inspection. J Clin Gastroenterol. 1986;8(5):576-579 [DOI] [PubMed] [Google Scholar]
- 14. Taylor AJ, Dodds WJ, Stewart ET. Alimentary tract lesions in Cowden's disease. Br J Radiol. 1989;62(742):890-892 [DOI] [PubMed] [Google Scholar]
- 15. Bosserhoff AK, Grussendorf-Conen EI, Rubben A, et al. Multiple colon carcinomas in a patient with Cowden syndrome. Int J Mol Med. 2006;18(4):643-647 [PubMed] [Google Scholar]
- 16. Heald B, Mester J, Rybicki L, Orloff MS, Burke CA, Eng C. Frequent gastrointestinal polyps and colorectal adenocarcinomas in a prospective series of PTEN mutation carriers. Gastroenterology. 2010;139(6):1927-1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Riegert-Johnson DL, Gleeson FC, Roberts M, et al. Cancer and Lhermitte-Duclos disease are common in Cowden syndrome patients. Hered Cancer Clin Pract. 2010;8(1):6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Comprehensive Cancer Network Genetic/familial high-risk assessment: breast and ovarian. NCCN Clinical Practice Guidelines in Oncology. Published March 8, 2010. http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf Accessed May 14. 2010
- 19. Edge SB, Byrd DR, Compton CC, Green FL, Trotti A. Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 20. Eng C. Will the real Cowden syndrome please stand up: revised diagnostic criteria. J Med Genet. 2000;37(11):828-830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strate LL, Syngal S. Hereditary colorectal cancer syndromes. Cancer Causes Control. 2005;16(3):201-213 [DOI] [PubMed] [Google Scholar]
- 22. Kato M, Mizuki A, Hayashi T, et al. Cowden's disease diagnosed through mucocutaneous lesions and gastrointestinal polyposis with recurrent hematochezia, unrevealed by initial diagnosis. Intern Med. 2000;39(7):559-563 [DOI] [PubMed] [Google Scholar]
- 23. Starink TM, van der Veen JP, Arwert F, et al. The Cowden syndrome: a clinical and genetic study in 21 patients. Clin Genet. 1986;29(3):222-233 [DOI] [PubMed] [Google Scholar]
- 24. Weber HC, Marsh D, Lubensky I, Lin A, Eng C. Germline PTEN/MMAC1/TEP1 mutations and association with gastrointestinal manifestations in Cowden disease [abstract G2902]. Gastroenterology. 1998;114(suppl 1):A702 [Google Scholar]