Abstract
OBJECTIVE: To assess the benefit of proactive palliative medicine consultation for delineation of goals of care and quality-of-life preferences before implantation of left ventricular assist devices as destination therapy (DT).
PATIENTS AND METHODS: We retrospectively reviewed the cases of patients who received DT between January 15, 2009, and January 1, 2010.
RESULTS: Of 19 patients identified, 13 (68%) received proactive palliative medicine consultation. Median time of palliative medicine consultation was 1 day before DT implantation (range, 5 days before to 16 days after). Thirteen patients (68%) completed advance directives. The DT implantation team and families reported that preimplantation discussions and goals of care planning made postoperative care more clear and that adverse events were handled more effectively. Currently, palliative medicine involvement in patients receiving DT is viewed as routine by cardiac care specialists.
CONCLUSION: Proactive palliative medicine consultation for patients being considered for or being treated with DT improves advance care planning and thus contributes to better overall care of these patients. Our experience highlights focused advance care planning, thorough exploration of goals of care, and expert symptom management and end-of-life care when appropriate.
The authors found that proactive palliative medicine consultation for patients being considered for or being treated with destination therapy improves advance care planning and thus contributes to better overall care of these patients.
DT = destination therapy; ICD = implantable cardioverter-defibrillator; LVAD = left ventricular assist device; PM = palliative medicine; QOL = quality of life
You matter because you are you. You matter to the last moment of your life, and we will do all we can, not only to help you die peacefully, but also to live until you die.
Dame Cicely Saunders
The left ventricular assist device (LVAD) is a promising technology that supports circulation in patients with advanced heart failure. Originally developed to bridge patients to heart transplant, the LVAD is being used as destination therapy (DT) for patients who are not candidates for heart transplant. Compared with medical therapy, use of DT improves survival, quality of life (QOL), and functional status in appropriately selected patients with advanced heart failure.1-5 In our experience at Mayo Clinic, the 2-year survival rate of patients receiving DT is 75%.6 However, as a result of occasional adverse events (eg, stroke, infection, or multiorgan failure), some patients or their surrogate decision-makers may request withdrawal of LVAD support.4,7,8 In other situations, patients or caregivers may become overwhelmed with financial and psychosocial issues related to DT8-11 and are at risk of burnout and isolation because of outpatient needs and limited community support.11-14
Several analyses7,15,16 have concluded that goals of care of patients receiving DT are often undefined. Many patients either have inadequate advance directives that do not address potential problems (such as worsening comorbid conditions, complications, and worsening QOL) or simply lack advance care documents.7,16,17 Without clearly defined goals and/or explicit advance directives, DT may merely maintain circulation in a moribund patient, a situation referred to as “destination nowhere.”18 Also, protocols and processes regarding LVAD management and comfort at the end of life are often lacking7,17; hence, ethical quandaries (eg, withdrawal of device support) may arise.19,20
To avoid situations in which advance care wishes are unclear or unknown, palliative medicine (PM) consultation has been suggested8,17,21-23 to address end-of-life preferences, facilitate advance care planning, manage symptoms, and maximize QOL. Several authors have called for PM involvement in patients with advanced heart disease to improve health status and QOL,24-29 and a recent randomized study of early palliative care vs standard care in advanced lung cancer has demonstrated improved QOL, improved mood, and survival benefit.30 Herein, we describe our initial experience with proactive PM consultation in patients receiving DT.
PATIENTS AND METHODS
In January 2009, a multidisciplinary conference was held at our institution to discuss ethical dilemmas and longitudinal care issues regarding DT. This forum concluded that patients may benefit from proactive PM consultation because only a few DT patients had engaged in advance care planning. We have sought to provide PM consultation to patients undergoing DT. In addition, we have sought to clarify impressions about PM as a means to promote life-affirming and patient-centered care and to provide education regarding potential ethical dilemmas and their resolution.
From January 15, 2009, to January 1, 2010, 19 patients with heart failure underwent DT at our institution (Table 1). Demographic and clinical data were abstracted from each patient's medical records. The Mayo Clinic Institutional Review Board approved the study in accordance with federal regulations.
TABLE 1.
Characteristics and Outcomes of 19 Patients With Advanced Heart Failure Receiving LVAD-DTa
Initial Intervention
Patients who consented to DT were offered PM consultation as part of standard multidisciplinary care. The PM consultations were performed by an interdisciplinary team of allied health practitioners and physicians with board certification in PM. Consultations were requested by cardiovascular care specialists before DT or shortly thereafter. Psychosocial assessment by a dedicated social worker occurred in parallel. Social worker assessment included completion of a caregiving plan and a screening assessment for mental health (eg, depression), chemical dependency, cognitive functioning, and educational level of patients and caregivers. The PM consultants (physician and/or allied health care practitioner, henceforth referred to as the PM team) reviewed the goals of care and advance care plans with patients and/or families, unless the patients or families refused.
Accounting for unique challenges regarding DT, the PM team worked to devise a preparedness plan. We define preparedness planning as specific advance care planning that assisted patients and families in thinking about psychosocial and financial considerations, caregiving concerns, QOL determinants, and ethical issues that may affect clinical DT outcomes. Although advance directives often address patient preferences if the patient is in a persistent vegetative state or terminal state, preparedness planning focuses on situations unique to DT (Table 2).
TABLE 2.
Common Differences Between Traditional Advance Directives and Preparedness Plans in Patients Receiving LVAD as Destination Therapy
With preparedness planning, attention was paid to time-limited trials of intensive care measures, potential clinical pitfalls, and advance care preferences. Examples included (but were not limited to) the roles of blood transfusions, antibiotics, hemodialysis, artificial nutrition and hydration, and long-term mechanical ventilation. Possible scenarios, such as device failure and complications that might adversely affect QOL but not necessarily threaten survival (eg, acute or chronic stroke, intracranial hemorrhage, incurable infection, or permanent renal failure), were discussed. Patients' social situations and their spiritual and religious beliefs were explored. Finally, the PM team assisted with expectation setting. The aggregate documentation from the PM and social worker consultations, as well as previous or new advance directive documents incorporated into the patients' electronic medical records, is what we collectively refer to as preparedness planning (Table 2 and Figure 1).
FIGURE 1.
Major components of the preparedness plan for patients receiving a left ventricular assist device (LVAD) as destination therapy. Decisions are made on the basis of a detailed discussion with patients and their loved ones regarding care preferences in the event of the development of poor quality of life, acute device failure, catastrophic complication (eg, sepsis or stroke), or a progressive comorbid condition (eg, pulmonary disease or malignant tumor).
Follow-up Intervention
The PM team worked with patients after DT. When adverse events occurred, the PM team assisted with preparedness plan implementation, symptom management, and family- and patient-centered support. This intervention often included psychosocial support to family, assistance with interpretation of advance directives and surrogacy issues, and assurance of ethically appropriate comfort care if DT was withdrawn. In patients doing well clinically, the PM team focused on enhancement of QOL. This intervention often included ongoing symptom and QOL assessment and assertion that DT met the patients' goals of care. The establishment of relationships during periods of relative wellness was aimed at building rapport to facilitate decision making should patients experience clinical deterioration or adverse events.
RESULTS
Summary of the Patient Group
The characteristics of 19 patients (16 men and 3 women) and outcomes data are summarized in Table 1. The median (range) age of patients at the time of initial DT was 70.9 (55.3-78.1) years. All 19 patients had New York Heart Association class IIIB/IV symptoms, and the mean ± SD pre-LVAD left ventricular ejection fraction was 17%±5%. Ischemic cardiomyopathy was the most common cause of heart failure (12 patients [63%]). Median duration of heart failure was 60 months (mean ± SD, 71±51 months); 12 patients (63%) underwent prior sternotomy before LVAD. No patient required renal replacement therapy before DT, but 2 patients did after DT. Seventeen patients (89%) had implantable cardioverter-defibrillators (ICDs) before DT; 14 ICDs also had biventricular resynchronization function. All patients received a continuous flow LVAD (HeartMate II, Thoratec, Pleasanton, CA).
Thirteen patients (68%) received proactive PM consultation, with 6 being unable to complete consultation because of clinical factors (eg, emergency surgery or patient and/or family not available or agreeable to consultation). Median (range) time to PM consultation was 1 day before LVAD implantation (5 days before to 16 days after LVAD implantation). Five patients died after DT: 2 deaths were due to early DT-related adverse events, 1 due to cardiac arrhythmia, and 2 due to posttraumatic intracranial hemorrhage. Median (range) time from LVAD implantation to death in these 5 patients was 76 days (range, 1-161 days). During the intervention period, new or revised advance directive availability in the medical record was 68% (14/19). Of these 14 patients, 11 received PM consultation.
Comparison of the 13 patients who completed PM consultation with the 6 who did not revealed no significant differences in age (70.2±6.1 vs 67.6±7.4 years; P=.43), sex (85% vs 83% male; P>.99), ejection fraction (16.5%±3.8% vs 17.5%±7.7%; P=.70), or history of sternotomy (62% vs 67%; P>.99). Duration of heart failure demonstrated a trend toward longer duration (84±50 vs 42±43 months; P=.09) in the intervention group. Finally, 11 patients (85%) in the intervention group had documented advance directives compared with 3 patients (50%) in the group that did not undergo a PM consultation (P=.26). Considering the small sample size, the significance of this is unknown, yet the trend is noteworthy.
Illustrative Case Reports
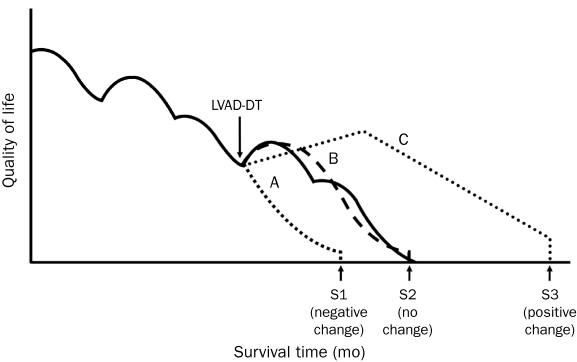
The case histories of 6 patients who were part of the study group are discussed subsequently. These 6 cases are described in detail because they exemplify unique aspects of preparedness planning or illustrate how palliative care could potentially contribute to improved outcomes. These cases are grouped into 4 care trajectories. The first 3 are end-of-life trajectories for patients who have died, as suggested by Rizzieri et al8: (1) patients with improved functionality, survival, and QOL with no complications occurring before 90 days (Figure 2; line C); (2) patients with suboptimal or delayed recovery or debilitative comorbid conditions and poor QOL (no net survival or QOL benefit) (Figure 2; line B); and (3) patients with postoperative complications (within 90 days of procedure) and with relatively shortened survival (Figure 2; line A). The fourth group, line C+ (not shown on the graph), comprises patients who have improved QOL and functionality but have not had DT-related complications negatively affecting survival. They remain on the upswing of line C. The selected 6 cases (corresponding to patient case numbers listed in Table 1) are illustrative of case complexity.
FIGURE 2.
End-of-life trajectory and quality-of-life adjusted survival with a left ventricular assist device as destination therapy (LVAD-DT). A, B, and C represent potential care trajectories after DT, each relative to the natural history of end-stage heart failure. Trajectory A relates to a patient with a suboptimal outcome who has a shortened survival (S1). Trajectory B represents a chronic course of complications and suboptimal outcome in which there is no change in survival (S2). Trajectory C shows a relative survival benefit from DT that is associated with prolonged survival (S3) with improvement in quality of life.
From Rizzieri et al,8 with permission from BioMed Central.
Line A Case: Case 3. A 55-year-old woman with biventricular heart failure and left ventricular thrombus underwent DT evaluation. As a single woman with no children and limited family resources, she received social work assessment focusing on adequate support. The PM team worked with the patient on her written advance directive and helped her discuss potential complications with her mother and siblings. She underwent DT the next day, complicated by intraoperative coagulopathy, hemorrhagic shock, and multiorgan system failure. Despite heroic efforts, including transfusions and renal replacement therapy, the patient was moribund. The family was well informed regarding the patient's wishes as outlined in her preparedness plan. The family felt supported because rapport was already built with the LVAD and PM teams, contributing to a smooth transition to comfort care only. The patient died on postoperative day 2 after life-sustaining measures, including LVAD support, were discontinued.
Line B Case: Case 5. A 73-year-old man had a complex medical history, including coronary artery disease with previous coronary artery bypass grafting and an embolic stroke. He later was diagnosed as having lymphoma, and anthracycline-based chemotherapy superimposed on ischemic heart disease led to inotropic-dependent heart failure. With refractory symptoms, advanced age, and comorbid conditions, DT was suggested. During preimplantation consultation with the social worker and PM team, the patient stated, “My wife knows what I want and she can make the decisions for me.” The patient exercised only a durable health care power of attorney and did not wish to further discuss preparedness planning. After DT, biliary sepsis developed, with progressive hepatic and renal dysfunction. The patient remained ventilator and vasopressor dependent with worsening cytopenias, but the LVAD otherwise functioned appropriately. Multidisciplinary meetings were held, without consensus regarding prognosis or care plan. Prognostic estimates ranged from extremely poor to 50-50. Although the family mentioned being discouraged by the patient's clinical situation, they did not feel strongly about altering his care trajectory.
The PM team helped the patient's family better understand his complex situation and how it portended poor QOL. The team encouraged the family to articulate their understanding of the patient's wishes in lieu of a specific advance directive. A decision was made to not escalate care and to attempt to remove obtrusive interventions in which burden was greater than benefit. After continuous hemodialysis was switched to intermittent hemodialysis, the patient's hemodynamics worsened. The family chose not to reescalate care, and the patient died in the presence of his family later that day after life-sustaining measures were stopped.
Line C Cases. Case 1. A 64-year-old man with advanced ischemic cardiomyopathy underwent peripheral artery bypass for critical leg ischemia. Postoperatively, fluid overload and renal failure developed despite diuresis, inotropes, vasopressors, intra-aortic balloon pump, and ultrafiltration. The patient was referred for DT.
The patient had good family support, which allowed for detailed advance care planning with social work and PM teams. However, the patient became depressed and displayed behavioral dyscontrol and nonadherence with a medical plan of care (thought to be due to multifactorial delirium and underlying personality traits). Given his dire prognosis, he underwent DT, with informed consent obtained from his surrogate.
Postoperatively, the patient's behavioral dyscontrol escalated, and the patient displayed nonadherence in attempting to disconnect his LVAD and in injuring nurses. The PM team intervened with the psychiatric team to stabilize the patient's clinical course. The patient's behavior improved in the following weeks, and he was discharged home 2 months after DT, with his wife noting he displayed a “more positive demeanor than it had been in 25 years.” The LVAD and a clear plan for acceptable QOL helped the patient to enjoy a renewed life. Unfortunately, 1 month later the patient fell and sustained an intracranial hemorrhage; he died 2 days later after LVAD support was withdrawn because it was no longer consistent with his previously stated goals of care. The family's ultimate decision to withdraw LVAD support reportedly helped with his preparedness planning, and rapport was established with the PM team before DT.
Case 16. A 73-year-old man with valvular cardiomyopathy developed heart failure with effort intolerance and worsening left ventricular indices; he received DT without complication. Although the PM team was consulted postoperatively, the patient underwent preparedness planning focusing on longitudinal care provision. He asserted his wish to not continue life support measures if he “was completely dependent on others for care.” Initial recovery was uneventful, and he completed rehabilitation without incident. The patient was a determined, self-sufficient man who performed all LVAD-related care alone because his wife was disabled; his functional status improved during the next 3 months.
Three months after implantation, the patient fell and sustained a skull fracture with subarachnoid bleeding. His clinical status was precarious, but the patient survived without operative intervention. His LVAD and mechanical values functioned well despite anticoagulation withdrawal. However, the patient's neurologic and cognitive status did not improve, and he exhibited progressive agitation, noncooperation with care, and loss of inhibition due to frontal lobe injury.
Prognosis was discussed during multidisciplinary care conferences, and behavioral dyscontrol escalated such that sedation with midazolam and fentanyl was required to prevent harm to himself, the staff, or his LVAD. On the basis of the patient's wishes in his preparedness plan, a collective decision was made by the patient's family to withdraw LVAD support. Staff and family were “extremely appreciative of the presence and comfort offered to them and the patient” by the PM team, particularly in empowering the patient's wife to make difficult decisions on her husband's behalf and ensuring that the patient was comfortable and treated with dignity in his final days.
Line C+ Cases. Case 8. A 69-year-old man with ischemic cardiomyopathy and colon cancer (in remission) developed worsening dyspnea, fatigue, and frequent ICD firings, prompting referral for DT. The patient's adult children and wife (whom he recently married) were supportive, although the couple held different religious beliefs. In his preparedness plan, the patient (a Roman Catholic) made his spiritual preferences known, including wishes regarding time-limited trials of advanced measures, such as hemodialysis and blood transfusion. This intervention allowed his wife (a Jehovah's Witness) to avoid situations that could compromise her conscience. Since implantation, the patient has made an excellent recovery and reports substantially improved QOL and capacity for activity. Because of his gratitude for his new lease on life, the patient counsels other patients considering DT before implantation.
Case 18. A 77-year-old man with ischemic heart failure had progressive effort intolerance, dyspnea, and low output symptoms for 2 years before presentation. He worked actively as a barber, except when limited by his heart failure, and planned to go back to work after the procedure. He lived with his wife of 50 years, who was the primary caregiver. He underwent DT, and his postoperative course was complicated by bilateral pneumothoraces, limiting his ability to participate in physical therapy because of pain and dyspnea. These symptoms were effectively managed by the PM team, and he was eventually discharged to inpatient rehabilitation and then home. Two weeks later, the patient was readmitted for dehydration and failure to thrive due to dizziness and persistent nausea that limited oral intake. The patient's wife was overwhelmed with the care the patient required and felt burned out. In this case, the social work and PM team worked together to address both the patient's symptoms and his caregiver's needs. Fortunately, the patient went from “wondering if he should have [agreed to the DT]” to feeling much better and looking forward to rehabilitation at home. His QOL improved with LVAD functioning but also with aggressive symptom and adverse effects management by the PM team.
DISCUSSION
In this report, we describe our initial experience with proactive PM consultation for patients receiving DT. To our knowledge, this is the first such report of a standardized and integrated PM approach to advance care preparedness planning for patients receiving DT. Despite impressive survival outcomes, some patients receiving DT have serious adverse events that may compromise patient and caregiver QOL. Our approach has encouraged development of preparedness plans congruent with patients' goals. Financial and safety issues are proactively evaluated by the social worker if they could negatively affect post-DT care.
Advance directives are requested of patients receiving DT and are finalized with PM assistance. As reported elsewhere, our intervention has led to improved advance directive completion and availability in the electronic medical record compared with previous rates.16 This is a key outcome because advance directives can enhance patient autonomy and provide physicians and surrogates with insights into patients' health care–related goals, values, and preferences.
Consultation with a PM specialist has facilitated the honing of advance directives into personalized preparedness plans. The preparedness plan is an expanded advance directive for patients with LVADs. It specifically addresses circumstances that arise when the circulatory system is mechanically supported, but medical conditions or functional limitations are such that significant long-term morbidity or impaired QOL persist. Consultation with a PM specialist inherently involves advance care plan clarification; documents now contain preparedness plan details regarding patient goals if DT does not improve outcomes as expected. This clarity has been valuable to the DT team, particularly if outcome is suboptimal.
Attempts at reverting to team-based social workers who are not familiar with the nuances of DT or omitting PM consultation have been suboptimal in our experience. Although no differences in survival or patient-reported satisfaction have been reported, a lack of clarity among caregivers regarding goals of care and disposition has been noted by health care professionals at our institution.
With preparedness planning, if adverse events or QOL issues arise, PM personnel are able to promptly intervene and do not have to focus on building rapport with the patient and family during times of crisis. This key outcome of our intervention has assisted with patient-centered outcomes consistent with a patient's goals of care. Families, as well as primary care team members, have expressed gratitude that the PM consultant is able to assimilate into the patient's care when adverse events or suboptimal outcomes occur. This positive feedback has generally taken on the form of thank you letters or verbal praise but has not been analyzed systematically to this point.
We recognize several limitations to the current study. This is a small, nonrandomized, observational pilot study at a single tertiary care center; hence, results may not be readily generalizable to other populations. We believe the findings demonstrate value-added and present available supporting data; however, our observations are lacking well-defined outcome measures or other metrics.
Several challenges arose during this initial experience with proactive PM consultation in patients receiving DT. Not all patients or caregivers understood what PM was and often confused it with comfort care, hospice, or not proceeding with aggressive care measures. This challenge, coupled with clinical factors (eg, emergency procedures), meant that not all DT patients at our institution received PM consultation. Nevertheless, during our first year, 13 (68%) of 19 received PM consultation, which is an improvement compared with our prior experience of 0 of 36 patients receiving proactive PM consultation.21 Before this endeavor, only 5 (14%) of 36 DT patients received PM consultation at any point in their care.21 This finding is despite the fact that half of these 36 patients had died since the first implantation, with a median survival of 172 days (range, 1-1030 days) for all deceased DT patients at our institution from March 2003 to January 2009.21 We believe that proactive PM consultation before DT or soon postoperatively is valuable in expectation setting and avoidance of possible ethical dilemmas. Ideally, preparedness planning would occur sooner, but late outside referrals and occasional emergent need for DT have sometimes interfered with a consistently early PM consultant presence.
Although the line A and B cases have completed outcomes through death, there are opportunities to intervene with the line C and C+ patients. We are continuing to define the intermediate- and long-term role of PM in working with the DT team. Optimum timing of PM follow-up (whether regular, routine visits or episodic follow-up visits when clinical changes occur) has yet to be ascertained. Currently, PM relies heavily on cardiologists to refer back to PM specialists if clinical or QOL changes are noted. We believe there may be ongoing benefit of longitudinal PM follow-up if goals of care change because of an LVAD-related complication or if secondary comorbid conditions occur or progress.
We recognize that a finite amount of work can be done shortly before DT when patients are critically ill. Even several months after DT, some patients only vaguely recall their preparedness planning. Patients tend to be seriously ill when DT is being discussed, and patient engagement with the PM team has been variable. Nonetheless, caregivers have articulated their appreciation (in the form of verbal feedback) for balanced information, have valued hearing about risks and benefits associated with DT, and did not negatively construe discussing these issues up front. Family members have reported that their perception of the postoperative course was less difficult by having information before or around the time of implantation.
Our DT team has consistently worked to articulate a primary goal of PM: symptom management and promotion of QOL in patients with life-limiting, life-threatening illness. Often, DT is used as a permanent means of palliation, with the goal of improving QOL when cure cannot be achieved. For patients with successful post-DT outcomes, the PM team has helped patients and caregivers cope with the transition from a terminal prognosis in the setting of end-stage heart failure to living well after DT implantation. The multidisciplinary DT team, including the PM team, continues to refine its role in promoting positive outcomes and improving survivorship by helping patients adapt to life after implantation.
Consultation with a PM specialist for patients receiving DT is now regarded as routine and integral by our cardiovascular teams. The DT multidisciplinary team is supportive of PM specialist involvement and looks to PM consultants for guidance and care suggestions regarding complex situations. Patients admitted to the heart failure hospital service before DT are cared for by heart failure cardiologists who recognize that PM consultation is adjunctive medical care, rather than resignation of optimism for these patients. Most notably, the PM team has the full support of the cardiothoracic surgeons who implant the devices. The teams work together collegially and provide input in terms of complex decision making to promote patient-centered outcomes.
CONCLUSION
Destination therapy is a lifesaving but life-altering therapy. Guidelines have been developed to address optimal clinical management of LVADs. Given medical complexities with DT, we conclude that improvements in QOL may be amplified with PM consultation throughout the patient's course—both before DT for rapport building and after DT for longitudinal care provision and assertion of acceptable QOL. We view DT as a permanent palliative therapy for incurable heart failure—one that improves QOL and reduces symptom burden. Our experience with PM and DT highlights higher rates of advance directive completion, improved establishment of preparedness plans, and better working relations with cardiac care specialists. In tandem, these factors interplay to affect patients, families, and caregivers regardless of DT outcome.
Supplementary Material
REFERENCES
- 1. Lietz K, Long JW, Kfoury AG, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007;116(5):497-505 [DOI] [PubMed] [Google Scholar]
- 2. Long JW, Healy AH, Rasmusson BY, et al. Improving outcomes with long-term “destination” therapy using left ventricular assist devices. J Thorac Cardiovasc Surg. 2008;135(6):1353-1360 [DOI] [PubMed] [Google Scholar]
- 3. Park SJ, Tector A, Piccioni W, et al. Left ventricular assist devices as destination therapy: a new look at survival. J Thorac Cardiovasc Surg. 2005;129(1):9-17 [DOI] [PubMed] [Google Scholar]
- 4. Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435-1443 [DOI] [PubMed] [Google Scholar]
- 5. Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241-2251 [DOI] [PubMed] [Google Scholar]
- 6. Boilson BA, Schirger JA, Durham JA, III, et al. Transformation of LVAD destination therapy, “good to great”: ever increasing survival benefit [abstract 3662]. Circulation 2009;120:S844 [Google Scholar]
- 7. MacIver J, Ross HJ. Withdrawal of ventricular assist device support. J Palliat Care. 2005;21(3):151-156 [PubMed] [Google Scholar]
- 8. Rizzieri AG, Verheijde JL, Rady MY, McGregor JL. Ethical challenges with the left ventricular assist device as a destination therapy. Philos Ethics Humanit Med. 2008;3(1):20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bieniarz MC, Delgado R. The financial burden of destination left ventricular assist device therapy: who and when? Curr Cardiol Rep. 2007;9(3):194-199 [DOI] [PubMed] [Google Scholar]
- 10. Zambroski CH. Managing beyond an uncertain illness trajectory: palliative care in advanced heart failure. Int J Palliat Nurs. 2006;12(12):566-573 [DOI] [PubMed] [Google Scholar]
- 11. Zambroski CH, Combs P, Cronin SN, Pfeffer C, Edgar Allan Poe, “the pit and the pendulum,” and ventricular assist devices. Crit Care Nurse. 2009;29(6):29-39 [DOI] [PubMed] [Google Scholar]
- 12. Casida JM, Peters RM, Magnan MA. Self-care demands of persons living with an implantable left-ventricular assist device. Res Theory Nurs Pract. 2009;23(4):279-293 [DOI] [PubMed] [Google Scholar]
- 13. MacIver J, Ross HJ, Delgado DH, et al. Community support of patients with a left ventricular assist device: the Toronto General Hospital experience. Can J Cardiol. 2009;25(11):e377-e381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson SR, Givertz MM, Stewart GC, Mudge GH., Jr Ventricular assist devices the challenges of outpatient management. J Am Coll Cardiol. 2009;54(18):1647-1659 [DOI] [PubMed] [Google Scholar]
- 15. Dudzinski DM. Ethics guidelines for destination therapy. Ann Thorac Surg. 2006;81(4):1185-1188 [DOI] [PubMed] [Google Scholar]
- 16. Swetz KM, Mueller PS, Ottenberg AL, Dib C, Freeman MR, Sulmasy DP. The use of advance directives among patients with left ventricular assist devices. Hosp Pract (Minneap). 2011;39(1):78-84 [DOI] [PubMed] [Google Scholar]
- 17. Landzaat LH, Sinclair CT, Rosielle DA. Continuous-flow left ventricular assist device [letter]. N Engl J Med. 2010;362(12):1149 [DOI] [PubMed] [Google Scholar]
- 18. Bramstedt KA. Destination nowhere: a potential dilemma with ventricular assist devices. ASAIO J. 2008;54(1):1-2 [DOI] [PubMed] [Google Scholar]
- 19. Bramstedt KA. Elective inactivation of total artificial heart technology in non-futile situations: inpatients, outpatients and research participants. Death Stud. 2004;28(5):423-433 [DOI] [PubMed] [Google Scholar]
- 20. Bramstedt KA, Wenger NS. When withdrawal of life-sustaining care does more than allow death to take its course: the dilemma of left ventricular assist devices. J Heart Lung Transplant. 2001;20(5):544-548 [DOI] [PubMed] [Google Scholar]
- 21. Mueller PS, Swetz KM, Freeman MR, et al. Ethical analysis of withdrawing ventricular assist device support. Mayo Clin Proc. 2010;85(9):791-797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferris H, Hunt S. Destination ventricular assist devices for heart failure #205. J Palliat Med. 2009;12(10):956-957 [DOI] [PubMed] [Google Scholar]
- 23. Schwarz ER, Philip KJ, Simsir SA, et al. Maximal care considerations when treating patients with end-stage heart failure: ethical and procedural quandaries in management of the very sick [published online ahead of print February 27, 2010] J Relig Health. Doi: 10.1007/s10943-010-9326-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boyd KJ, Worth A, Kendall M, et al. Making sure services deliver for people with advanced heart failure: a longitudinal qualitative study of patients, family carers, and health professionals. Palliat Med. 2009;23(8):767-776 [DOI] [PubMed] [Google Scholar]
- 25. Goodlin SJ. Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54(5):386-396 [DOI] [PubMed] [Google Scholar]
- 26. Jaarsma T, Beattie JM, Ryder M, et al. Palliative care in heart failure: a position statement from the palliative care workshop of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009;11(5):433-443 [DOI] [PubMed] [Google Scholar]
- 27. O'Leary N, Murphy NF, O'Loughlin C, Tiernan E, McDonald K. A comparative study of the palliative care needs of heart failure and cancer patients. Eur J Heart Fail. 2009;11(4):406-412 [DOI] [PubMed] [Google Scholar]
- 28. Pantilat SZ, Steimle AE. Palliative care for patients with heart failure. JAMA. 2004;291(20):2476-2482 [DOI] [PubMed] [Google Scholar]
- 29. Widera E, Pantilat S. Hospitalization as an opportunity to integrate palliative care in heart failure management. Curr Opin Support Palliat Care. 2009;3(4):247-251 [DOI] [PubMed] [Google Scholar]
- 30. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.