Abstract
OBJECTIVE: To determine the strength of evidence supporting an accentuated bleeding risk when patients with CHADS2 risk factors (chronic heart failure, hypertension, advanced age, diabetes, and prior stroke/transient ischemic attack) receive warfarin.
METHODS: A systematic literature search of MEDLINE (January 1, 1950, through December 22, 2009) and Cochrane CENTRAL (through December 22, 2009) was conducted to identify studies that reported multivariate results on the association between CHADS2 covariates and risk of bleeding in patients receiving warfarin. Each covariate was evaluated for its association with a specific type of bleeding. Individual evaluations were rated as good, fair, or poor using methods consistent with those recommended by the Agency for Healthcare Research and Quality. The strength of the associations between each CHADS2 covariate and a specific type of bleeding was determined using Grading of Recommendations Assessment, Development and Evaluation criteria as insufficient, very low, low, moderate, or high for the entire body of evidence.
RESULTS: Forty-one studies were identified, reporting 127 multivariate evaluations of the association between a CHADS2 covariate and bleeding risk. No CHADS2 covariate had a high strength of evidence for association with any bleeding type. For the vast majority of evaluations, the strength of evidence between covariates and bleeding was low. Advanced age was the only covariate that had a moderate strength of evidence for association; this was the strongest independent positive predictor for major bleeding. Similar findings were observed regardless of whether all included studies, or only those evaluating patients with atrial fibrillation, were assessed.
CONCLUSION: The associations between CHADS2 covariates and increased bleeding risk were weak, with the exception of age. Given the known association of the CHADS2 score and stroke risk, the decision to prescribe warfarin should be driven more by patients' risk of stroke than by the risk of bleeding.
The associations between CHADS2 covariates and increased bleeding risk were weak, with the exception of age; given the known association of CHADS2 score and stroke risk, the decision to prescribe warfarin should be driven more by the patient's risk of stroke than by risk of bleeding.
AF = atrial fibrillation; AHR = adjusted hazard ratio; AOR = adjusted odds ratio; CHADS2 = chronic heart failure, hypertension, advanced age, diabetes, and prior stroke/transient ischemic attack; CHF = chronic heart failure; CI = confidence interval; CVD = cerebrovascular disease; HTN = hypertension; TIA = transient ischemic attack
Approximately 5% of patients with atrial fibrillation (AF) will develop an embolic stroke each year.1,2 However, this risk is not equally distributed among patients. The CHADS2 scoring system (in which 1 point is assigned for chronic heart failure [CHF], hypertension [HTN], advanced age, and diabetes and 2 points for prior stroke/transient ischemic attack [TIA]) was derived in an effort to predict cardioembolic stroke risk based on the presence of 1 or more risk factors in a large population of untreated patients with AF. In large clinical trials, warfarin therapy reduced the risk of embolic stroke vs aspirin or placebo in patients with AF and 2 or more CHADS2 risk factors.1-6 However, a recent meta-analysis found that less than half of patients with AF and an indication for therapy received warfarin (48%; 95% confidence interval [CI], 43%-54%).7
Several studies suggest that physicians withhold warfarin therapy for many patients because of a perceived increased risk of bleeding.8-10 Like the risk of embolic stroke, the occurrence of bleeding may be related to individual patient characteristics. Because the CHADS2 risk factors for the development of embolic stroke in patients with AF are also listed in the approved warfarin-prescribing information as risk factors for bleeding, clinicians are caught in a quandary.11 A direct evaluation of the evidence supporting an association between CHADS2 risk factors and bleeding would assist clinicians in weighing the potential benefit and detriment of anticoagulant prophylaxis for their patients with AF.
Therefore, we conducted a systematic review of evidence to determine the strength of the association between the components of CHADS2 and bleeding risk in patients treated with warfarin, first irrespective of indication and then focusing on only those with AF.
METHODS
Literature Search
Two investigators conducted a systematic literature search independently using MEDLINE (January 1, 1950, through December 22, 2009) and Cochrane CENTRAL (through December 22, 2009). The complete search strategy is available in the Supporting Online Material (a link to which is provided at the end of this article). A manual review of references from each pertinent article, identified review articles, and treatment guidelines was also conducted to identify additional articles.
Study Selection
Studies were eligible for inclusion in the systematic review if they: (1) reported on a population of patients receiving warfarin, (2) reported on the association between CHADS2 characteristics and the risk of any bleeding event, (3) conducted multivariate analysis to determine the association between patient characteristics and risk of bleeding and reported results on at least 1 covariate of interest, and (4) were published in the English language. Two investigators (W.T.C. and V.T.) determined study eligibility independently, with disagreements resolved by discussion or by a third investigator (C.I.C.).
Data Abstraction and Synthesis
For each included study, 2 independent investigators (W.T.C. and V.T.) abstracted data on the following information: author, year, study design, population included, indication for warfarin, duration of follow-up in patient-years, previous warfarin use, country, “major” or “minor” or “any” bleeding definition, event rate (as bleeding events per 100 patient-years), P value for the univariate association between a patient characteristic (covariate) and bleeding, and effect size and P value for the multivariate association between a covariate and bleeding. Covariates of interest included CHF, HTN, advanced age, diabetes, and prior stroke/TIA (cerebrovascular disease [CVD]) (or the CHADS2 risk factors). Qualitative synthesis of data is reported using descriptive statistics.
Quality Assessment
In this systematic review, an evaluation was defined as an assessment of a covariate for its association with a specific type of bleeding. Each evaluation was rated for its validity using methods consistent with those recommended by the Agency for Healthcare Research and Quality.12 More specifically, hierarchy of study design, objectivity of bleeding definition, sample size, overall event rate, and magnitude of effect size were considered. Individual evaluations were then given an overall ranking of good, fair, or poor.
We used the criteria and methods of GRADE (Grading of Recommendations Assessment, Development, and Evaluation) to assess the strength of the body of evidence for each CHADS2 covariate. This system uses 4 required domains: risk of bias, consistency, directness, and precision.13 Two investigators (O.J.P. and D.M.S.) made all assessments (with disagreements resolved through discussion). The evidence pertaining to each of the CHADS2 covariates was classified into 5 broad categories: high, moderate, low, very low, or insufficient. The features that determined the strength of evidence for the different outcomes evaluated in this review are described in more detail in Table S1 (Supporting Online Material).
Subgroup Analysis
Although our base-case analyses evaluated patients with any indication for oral anticoagulation receiving warfarin therapy, in subgroup analysis we limited eligible studies to those enrolling patients who had AF and were receiving warfarin therapy.
RESULTS
Study Identification and Characteristics
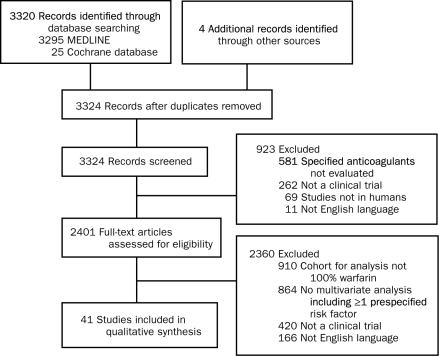
Our literature search revealed 3324 nonduplicate citations. On title and abstract review, 923 citations were excluded, leaving 2401 citations for full-text review. On full-text review, 2360 were excluded (Figure 1). A total of 41 studies published between 1988 and 2009 from 9 different countries were included in this systematic review.14-55 These studies included 2 post hoc analyses of randomized controlled trials, 12 prospective observational studies, 22 retrospective observational studies, 4 mixed prospective and retrospective studies, and 1 study of unclear study design.
FIGURE 1.
Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) flow diagram of study identification, inclusion, and exclusion of studies evaluating predictors of bleeding in patients treated with warfarin.
Key characteristics of included studies can be found in Table 1. The total sample size was 166,871, with the number of patients enrolled in a study ranging from 66 to 6988. Total duration of follow-up ranged from 66 to 133,976 patient-years (median, 1163 patient-years). Indications for warfarin therapy consisted of AF, venous thromboembolism prophylaxis and treatment, CVD, myocardial infarction, valve replacement, and antiphospholipid syndrome. Forty-one percent of studies did not report previous warfarin use (an inception cohort), and the remaining studies included patients who began receiving warfarin therapy during the study or had some previous warfarin use.
TABLE 1.
Characteristics of Included Studies Evaluating the Association Between Select Covariates and Warfarin-Associated Bleeding Riska
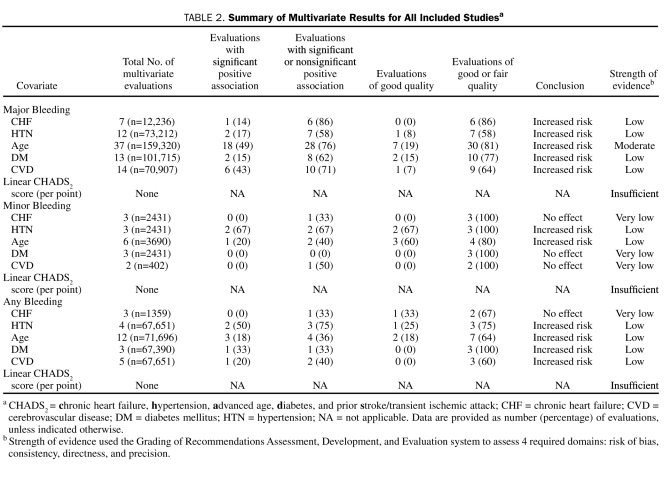
All 41 studies reported results of multivariate analysis as either adjusted odds ratios (AORs) or adjusted hazard ratios (AHRs). Among the 41 studies, 168 evaluations assessed the association between a CHADS2 covariate and bleeding (either univariate or multivariate), with all studies reporting at least one multivariate result and 127 evaluations (76%) providing multivariate results. Although 39 (31%) of the 127 multivariate evaluations reported a significant association between the CHADS2 covariates and any types of bleeding, the remaining 88 (69%) found no significant association (Table 2). Of the evaluations, 72% were for major bleeding, with the remaining split between minor (11%) and any bleeding (17%) (see Tables S2-S7 in Supporting Online Material).
TABLE 2.
Summary of Multivariate Results for All Included Studiesa
Bleeding Risk Among Patients Treated With Warfarin for any Indication: Evidence of Association Between CHF and Warfarin-Associated Bleeding Risk
Thirteen studies (n=16,333) reported 22 evaluations of the association between CHF and bleeding.14-26 Of the evaluations, 5%, 59%, and 36% were rated as good, fair, or poor, respectively. The prevalence of CHF in the studies ranged from 5% to 76%. Major bleeding events ranged from 0.8 to 10.8 events per 100 patient-years, whereas none reported the event rates for minor bleeding. Only 1 evaluation reported the event rate of any bleeding as 6.2 events per 100 patient-years.
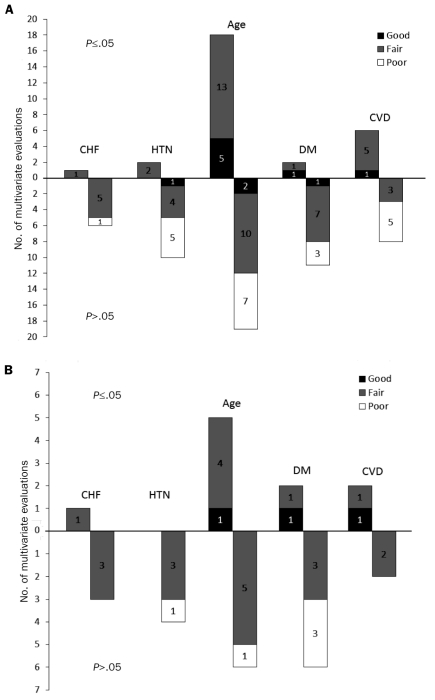
Thirteen (59%) of the 22 evaluations included multivariate results, of which 7 (54%) (6 fair, 1 poor quality), 3 (23%), and 3 (23%) were for major (Figure 2), minor, and any bleeding, respectively. Among these, only 1 evaluation found CHF to be an independent positive predictor of major bleeding (Figure 2 and Table S2 in Supporting Online Material).25 No significant associations between CHF and minor or any bleeding were seen (Table 2). We graded the strength of the body of evidence for this association as low for major, minor, and any bleeding.
FIGURE 2.
Quality rating of multivariate associations between the covariates and major bleeding for (A) all included studies and (B) studies involving only patients with atrial fibrillation. The upright bars represent the evaluations that showed statistically significant independent associations between the covariate and major bleeding, whereas the inverted bars represent evaluations that did not show statistically significant independent associations between the covariate and major bleeding. The black, grey, and white shaded bars represent good, fair, and poor quality ratings of the evaluations, respectively. CHF = chronic heart failure; CVD = cerebrovascular disease; DM = diabetes mellitus; HTN = hypertension.
Evidence of Association Between HTN and Warfarin-Associated Bleeding Risk
Twenty-one studies (n=88,151) reported 33 evaluations of the association between HTN and bleeding.14-25,27-35 Of the evaluations, 12%, 46%, and 42% were rated as good, fair, and poor, respectively. The prevalence of HTN in studies ranged from 4% to 79%. The event rates of major and any bleeding ranged from 0.8 to 10.8 and from 3.7 to 12.2 events per 100 patient-years, respectively; event rates for minor bleeding were not reported.
Of the 33 evaluations, 19 (58%) included multivariate results, of which 12 (63%) (1 good, 6 fair, 5 poor quality), 3 (16%), and 4 (21%) were for major (Figure 2), minor, and any bleeding, respectively. Six evaluations (32%), 2 from each bleeding type (for 1 minor bleeding association, statistical significance was suggested because the 95% CIs did not cross the line of unity; P=.06), found HTN to be an independent positive predictor of any type of bleeding, with an AOR of bleeding ranging from 2.3 to 3.6 and an AHR of 1.10 to 1.25 (see Table S3 in Supporting Online Material). The remaining evaluations found no significant association between HTN and either minor or any bleeding. We graded the strength of the body of evidence for this association as low for major, minor, and any bleeding (Table 2).
Evidence of an Association Between Age and Warfarin-Associated Bleeding Risk
Thirty-eight studies (n=165,226) reported 59 evaluations of the association between age and bleeding.14-17,19-22,24,25,27-55 Of the evaluations, 20%, 51%, and 29% were rated as good, fair, and poor, respectively. Age was evaluated as both a continuous variable (linear age per year, per 5 years or per decade) and categorical variable (with the most common cutoffs at 65 years, 75 years, 80 years, or 90 years). Major, minor, and any bleeding event rates ranged from 0.5 to 10.8, 2.7 to 21.8, and 3.7 to 13.0 events per 100 patient-years, respectively.
Of the 59 evaluations, 55 (93%) included multivariate results, of which 37 (67%) (7 good, 23 fair, 7 poor quality), 6 (11%), and 12 (22%) were for major (Figure 2), minor, and any bleeding, respectively. Of the 22 evaluations (40%), all rated as good or fair quality, that found age to be an independent positive predictor of bleeding, 18 (82%) were for major bleeding. Effect sizes for significant major bleeding risks were as high as an AOR of 2.5 when evaluating age as a linear variable per 5 years17 and as high as an AHR of 2.75 when evaluating patients older than 75 years vs those younger than 75 years (see Table S4 in Supporting Online Material).21 Thus, evidence of moderate strength suggests that age increases major bleeding risk in those receiving warfarin. Because less than 40% of the multivariate evaluations found either a significant or nonsignificant positive association between advanced age and minor or any bleeding risk in those receiving warfarin, the strength of evidence suggesting that age increases these bleeding types was low (Table 2).
Evidence of Association Between Diabetes and Warfarin-Associated Bleeding Risk
Eighteen studies (n=106,336) reported 29 evaluations of the association between diabetes and bleeding.14-19,21-25,27,29,31,32,34-36 Of the 29 evaluations, 7%, 55%, and 38% were rated as good, fair, and poor, respectively. The prevalence of diabetes in the studies ranged from 4% to 53%. Major bleeding events ranged from 0.8 to 10.8 events per 100 patient-years; event rates for minor bleeding were not reported. Only 1 evaluation reported the event rate of any bleeding as 7.7 events per 100 patient-years.
Of the 29 evaluations, 19 (66%) reported multivariate results, of which 13 (68%) (2 good, 8 fair, 3 poor quality), 3 (16%), and 3 (16%) were for major (Figure 2), minor, and any bleeding, respectively. Only 3 multivariate evaluations of good or fair quality (16%), 2 for major bleeding and 1 for any bleeding, found diabetes mellitus to be an independent positive predictor of bleeding. In 1 study, the AOR for major bleeding in patients with diabetes was 4.4 (see Table S5 in Supporting Online Material).23 No significant associations between diabetes and minor bleeding were seen. We graded the strength of evidence for this association as low for major or any bleeding and very low for minor bleeding (Table 2).
Evidence of Association Between CVD and Warfarin-Associated Bleeding Risk
Thirteen studies (n=93,020) reported 25 evaluations of the association between CVD and bleeding.15-17,19,20,23,24,27,30,32,33,51,55 Of the evaluations, 4%, 60%, and 36% were rated as good, fair, and poor, respectively. The definition of CVD varied by study, ranging from history of stroke, TIA, or cerebrovascular stroke. The prevalence of CVD in studies ranged from 1% to 58%. The event rates of major and any bleeding ranged from 0.6 to 8.1 and from 3.7 to 12.2 events per 100 patient-years; the event rates for minor bleeding were not reported.
Of the 25 evaluations, 21 (84%) included multivariate results, of which 14 (67%) (1 good, 8 fair, 5 poor), 2 (10%), and 5 (24%) were for major (Figure 2), minor, and any bleeding, respectively. A total of 7 multivariate evaluations (33%) found CVD to be an independent predictor of bleeding (6 for major bleeding and 1 for any bleeding). The AOR of major bleeding for those with CVD was as high as 3.6, and the AHR of any bleeding was 1.12 in patients with previous stroke (see Table S6 in Supporting Online Material).23,27 We graded the strength of evidence for this association as low for major and any bleeding and very low for minor bleeding (Table 2).
CHADS2 Score
Only 1 study of fair quality (n=783) evaluated the association between CHADS2 score, reported as a linear variable, and any bleeding.33 The bleeding event rate in the study was 3.7 events per 100 patient-years. Multivariate results were not reported. Thus, data are insufficient to grade the strength of evidence for the association between the CHADS2 score and any bleeding risk (Table 2).
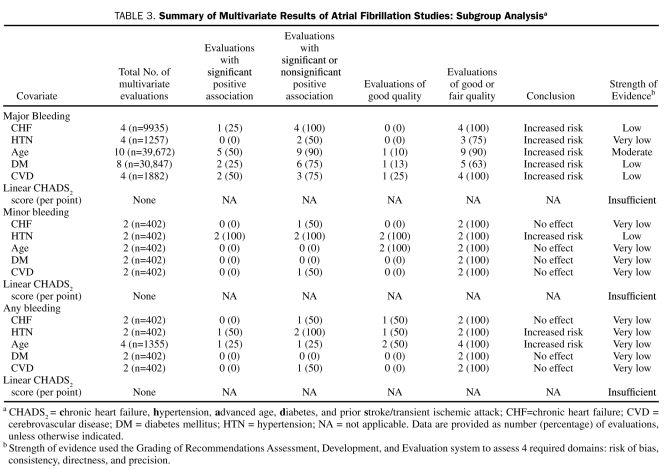
Bleeding Risk Among Patients With AF
Thirteen studies (n=50,448) evaluated an association between a CHADS2 covariate in a population in which all patients had AF and were receiving warfarin therapy.15,17,18,23-25,33,34,36,38-40,43,54 Follow-up ranged from 355 to 18,946 patient-years (median, 814 patient-years). A total of 69 evaluations reported an association between a covariate and bleeding, of which 52 (75%) were multivariate (30, 10, and 12 evaluations for major, minor, and any bleeding, respectively).
Among the 30 multivariate evaluations for major bleeding, more evaluations (n=10) assessed the association between age and major bleeding in patients with AF who were receiving warfarin, as compared with those with CHF (n=4), HTN (n=4), diabetes (n=8), or CVD (n=4). Of the 10 evaluations (n=39,672) reporting a multivariate association between age and major bleeding, 9 (90%) found a positive association with major bleeding, 5 (50%) of which were significant. We graded the strength of evidence for this association to be moderate. Less than half of the multivariate results showed CHF, diabetes, and CVD to be independent positive predictors for major bleeding, with a low strength of evidence. No significant association between HTN and major bleeding was noted, with the strength of evidence graded as very low (Table 3).
TABLE 3.
Summary of Multivariate Results of Atrial Fibrillation Studies: Subgroup Analysisa
Of the 10 multivariate evaluations for minor bleeding, 2 (20%) showed HTN to have a significant positive association with minor bleeding. None of the other covariates (CHF, age, diabetes, or CVD) were found to be significant predictors of minor bleeding. We graded the strength of evidence for HTN to be low, with the other covariates being very low.
Of the 12 multivariate evaluations for any bleeding, one found HTN and another found age to be independent positive predictors of any bleeding. None of the other evaluations found CHF, advanced age, diabetes, or CVD to have any effect on any bleeding. We graded the strength of evidence for each of these factors to be very low.
Overall, associations identified from studies enrolling only patients with AF were rated to be of similar quality and provided comparable conclusions regarding strength of evidence as those of the overall analysis (Figure 2, Tables 2 and 3).
DISCUSSION
The number of patients and patient evaluations in our study was generally greater for major bleeding than minor or any bleeding. None of the CHADS2 covariates had a high strength of association with warfarin-related bleeding risk. The strength of evidence of most covariates was low to very low, with the exception of advanced age and major bleeding. The moderate strength of association between age and major bleeding risk was the strongest among all covariates, whereas the very low strength of association between diabetes and minor bleeding was the weakest. The subgroup analysis in our study revealed that advanced age was also the strongest independent positive predictor of bleeding risk among all CHADS2 covariates in patients specifically with AF. As in the full analysis, the strength of associations between the other covariates and bleeding risk was very low.
Diener et al38 and Lip et al56 published an evaluation comparing the predictive value of 5 different contemporary bleeding risk stratification schemas using combined SPORTIF III and V warfarin data (N=3665). Of the schemas evaluated, the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score appeared to be the best predictor of bleeding in patients taking warfarin (C statistic, 0.66; 95% CI, 0.61-0.70); however, all schemas provided only moderate predictive ability (C statistic, 0.52-0.66; a C statistic of 0.50 implies that a schema is no better at predicting a bleeding event than random guessing). The fact that no one schema was vastly superior to another despite each using different risk factors for bleeding is not surprising given the current results of our systematic literature review. The preponderance of the medical literature suggests that many risk factors have either conflicting or scant data supporting their association with bleeding of any severity, clarifying why developing a highly predictive schema has proved difficult. Of the 5 schemas, none used heart failure as a risk factor, 2 (40%) used HTN, 3 (60%) used stroke, and 1 used diabetes by itself (20%). In contrast, age was used as a risk factor in all 5 schemas, albeit in different forms (continuous and categorical formats with different cutoffs for advancing age). The use of advancing age to predict bleeding is very much supported by the findings of our systematic review, which suggest that age is a relatively reliable risk factor for bleeding.
In the evaluation of CHADS2 covariates, it is important to recognize that one covariate may be serving as a surrogate for a different but related risk factor. Therefore, we caution that results must not be taken at face value. For example, the association seen with increasing age and bleeding risk may also be attributed to a decline in renal function, multiple comorbid conditions, or concomitant use of many other drugs. Furthermore, why these risk factors are associated with bleeding risk is often unknown.
Unlike the weak association with bleeding risk, the use of CHADS2 covariates in predicting stroke risk is relatively well established.56,57 Previous studies found that the risk of stroke in patients with AF increased as the number of CHADS2 risk factors increased, with an increase of up to 1.5 times for each 1-point increase in the CHADS2 score when no anticoagulant therapy was given. Warfarin therapy significantly reduced stroke risk, by approximately two-thirds, from 4.5% to 1.4%, when compared with placebo or no treatment, and by two-fifths when compared with aspirin.2-5,56 The Anticoagulation and Risk Factors in Atrial Fibrillation cohort study showed that the highest net benefit of warfarin was among patients with moderate to high risk for stroke because the absolute increase in risk for intracranial hemorrhage due to warfarin remains fairly stable across CHADS2 risk categories.58
Several limitations must be considered when interpreting the results of our systematic review. Included studies defined bleeding end points in various ways. Although we limited ourselves to descriptive analysis only (no pooling of results through meta-analysis) and stratified our analyses by bleeding severity (major, minor, or any), important residual heterogeneity may still have been present.
Our systematic review included mostly observational studies, which have inherent selection and information biases that may result in an erroneous estimate of association. Selection bias may have occurred if patients were included in the selected studies because risk factors were presumed to be associated with bleeding. Furthermore, information bias could have occurred if patients were misclassified as having or not having a potential risk factor (ie, HTN, diabetes) or as having or not having a bleeding event. Observational studies can only reveal potential associations between covariates and the risk of bleeding; they cannot prove causality.
Negative reporting bias is a potential limitation of any systematic review. Authors commonly omit insignificant results from their publications, resulting in conclusions biased toward a covariate being a risk factor for bleeding. Of the 41 studies included in our systematic review, 27 (66%) reported insignificant results, suggesting that this is of some potential concern in this systematic review. However, this bias would tend to further weaken the associations we have found. In addition, many of the studies included in this systematic review were underpowered (type 2 error) because the presence and/or occurrence of covariate or bleeding events was low in the patient samples. For example, in the study by Landefeld and Goldman,20 the failure to find CVD as a risk factor for major bleeding may be explained by the low baseline presence of CVD (only 4 of 375 patients) in the overall population. We devised an analysis protocol to accommodate for this, a description of which follows.
Given these cautions, limitations, and caveats, we used a strength-of-evidence rating scale that incorporated many features, allowing decision makers to draw conclusions on the basis of the preponderance of the evidence and reducing the influence of any 1 potentially biased study on the process. First, we evaluated the number of evaluations and patients included because many evaluations allow for the assessment of consistency, and larger evaluations are less likely to be underpowered. We then evaluated both the number and corresponding percentage of the total number of evaluations for a covariate when there was a significant positive association or a number of evaluations with a positive direction of effect regardless of significance. This gave us insight into the effect of a covariate on bleeding and the consistency of the effect across studies. Finally, we evaluated the quality of the evaluations because higher-quality evidence increases confidence in the results.
In light of their expected efficacy, safety, and ease of use, the newer oral anticoagulants (eg, Factor Xa inhibitors, direct thrombin inhibitors) are likely to replace warfarin therapy in many patients. Because the risk of bleeding associated with various patient characteristics is not yet known specifically with these agents, new data will be required. Fortunately, detailed analysis of the large-scale phase 3 clinical trials of these agents should provide much stronger evidence than is available for warfarin.
CONCLUSION
This systematic review found that the strength of evidence supporting the association between CHADS2 covariates and bleeding risk is low to very low, with the exception of advanced age, for which the strength of evidence is moderate. This appeared true regardless of whether the assessment included all studies or only those evaluating patients with AF. It is important to note that these associations were not as strong as those between the CHADS2 covariates and stroke risk. Thus, these findings support the recommendation that the decision to prescribe oral anticoagulant prophylaxis to patients with AF should be driven more by patients' risk of stroke than by the risk of bleeding due to these factors.
Supplementary Material
Acknowledgments
We would like to thank Dr Kearon and colleagues for providing additional data pertaining to their trial (Kearon et al47); Erica L. Baker, PharmD, and Devi Mathur, MD, for their help with study procurement and data collection; and Ruth Sussman, PhD, for her editorial assistance.
Footnotes
REFERENCES
- 1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-2375 [DOI] [PubMed] [Google Scholar]
- 2. Atrial Fibrillations Investigations Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449-1457 [PubMed] [Google Scholar]
- 3. Stroke Prevention in Atrial Fibrillation Investigators Predictors of thromboembolism in atrial fibrillation: I, Clinical features of patients at risk. Ann Intern Med. 1992;116(1):1-5 [DOI] [PubMed] [Google Scholar]
- 4. Stroke Prevention in Atrial Fibrillation Investigators Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomized clinical trial. Lancet. 1996;348(9028):633-638 [PubMed] [Google Scholar]
- 5. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification scheme for predicting stroke: results from National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864-2870 [DOI] [PubMed] [Google Scholar]
- 6. EAFT (European Atrial Fibrillation Trial) Study Group Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993;342(8882):1255-1262 [PubMed] [Google Scholar]
- 7. Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm. 2009;15(3):244-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160(1):41-46 [DOI] [PubMed] [Google Scholar]
- 9. Monette J, Gurwitz JH, Rochon PA, Avorn J. Physician attitudes concerning warfarin for stroke prevention in atrial fibrillation: results of a survey of long-term care practitioners. J Am Geriatr Soc. 1997;45(9):1060-1065 [DOI] [PubMed] [Google Scholar]
- 10. McCormick D, Gurwitz JH, Goldberg RJ. Prevalence and quality of warfarin use for patients with atrial fibrillation in the long-term care setting. Arch Intern Med. 2001;161(20):2458-2463 [DOI] [PubMed] [Google Scholar]
- 11. Coumadin [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2009. http://packageinserts.bms.com/pi/pi_coumadin.pdf [Google Scholar]
- 12. Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3, suppl):21-35 [DOI] [PubMed] [Google Scholar]
- 13. Schünemann HJ, Oxman AD, Brozek J, et al. GRADE Working Group Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stroke Prevention in Atrial Fibrillation Investigators Bleeding during antithrombotic therapy in patients with atrial fibrillation. Arch Intern Med. 1996;156(4):409-416 [PubMed] [Google Scholar]
- 15. Abdelhafiz AH, Wheeldon NM. Risk factors for bleeding during anticoagulation of atrial fibrillation in older and younger patients in clinical practice. Am J Geriatr Pharmacother. 2008;6(1):1-11 [DOI] [PubMed] [Google Scholar]
- 16. Berwaerts J, Webster J. Analysis of risk factors involved in oral-anticoagulant-related intracranial haemorrhages. QJM. 2000;93(8):513-521 [DOI] [PubMed] [Google Scholar]
- 17. Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med. 2004;141(10):745-752 [DOI] [PubMed] [Google Scholar]
- 18. Gasse C, Hollowell J, Meier CR, Haefeli WE. Drug interactions and risk of acute bleeding leading to hospitalisation or death in patients with chronic atrial fibrillation treated with warfarin. Thromb Haemost. 2005;94(3):537-543 [DOI] [PubMed] [Google Scholar]
- 19. Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120(11):897-902 [DOI] [PubMed] [Google Scholar]
- 20. Landefeld CS, Goldman L. Major bleeding in outpatients treated with warfarin: incidence and prediction by factors known at the start of outpatient therapy. Am J Med. 1989;87(2):144-152 [DOI] [PubMed] [Google Scholar]
- 21. Manzano-Fernández S, Marin F, Pastor-Perez FJ, et al. Impact of chronic kidney disease on major bleeding complications and mortality in patients with indication for oral anticoagulation undergoing coronary stenting. Chest. 2009;135(4):983-990 [DOI] [PubMed] [Google Scholar]
- 22. Petitti DB, Strom BL, Melmon KL. Prothrombin time ratio and other factors associated with bleeding in patients treated with warfarin. J Clin Epidemiol. 1989;42(8):759-764 [DOI] [PubMed] [Google Scholar]
- 23. Poli D, Antonucci E, Marcucci R, et al. Risk of bleeding in very old atrial fibrillation patients on warfarin: relationship with ageing and CHADS2 score. Thromb Res. 2007;121(3):347-352 [DOI] [PubMed] [Google Scholar]
- 24. Sam C, Massaro JM, D'Agostino RB, Sr, et al. Warfarin and aspirin use and the predictors of major bleeding complications in atrial fibrillation (the Framingham Heart Study). Am J Cardiol. 2004;94(7):947-951 [DOI] [PubMed] [Google Scholar]
- 25. Schauer DP, Moomaw CJ, Wess M, Webb T, Eckman MH. Psychosocial risk factors for adverse outcomes in patients with nonvalvular atrial fibrillation receiving warfarin. J Gen Intern Med. 2005;20(12):1114-1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wandell PE. Anticoagulant treatment of patients in Swedish primary health care: safety aspects. Eur J Clin Pharmacol. 2001;57(1):61-64 [DOI] [PubMed] [Google Scholar]
- 27. Berlowitz DR, Miller DR, Oliveria SA, Cunningham F, Gomez-Caminero A, Rothendler JA. Differential associations of beta-blockers with hemorrhagic events for chronic heart failure patients on warfarin. Pharmacoepidemiol Drug Saf. 2006;15(11):799-807 [DOI] [PubMed] [Google Scholar]
- 28. Cortelazzo S, Finazzi G, Viero P, et al. Thrombotic and hemorrhagic complications in patients with mechanical heart valve prosthesis attending an anticoagulation clinic. Thromb Haemost. 1993;69(4):316-320 [PubMed] [Google Scholar]
- 29. Fihn SD, McDonell M, Martin D, et al. Warfarin Optimized Outpatient Follow-up Study Group Risk factors for complications of chronic anticoagulation: a multicenter study. Ann Intern Med. 1993;118(7):511-520 [DOI] [PubMed] [Google Scholar]
- 30. Gitter MJ, Jaeger TM, Petterson TM, Gersh BJ, Silverstein MD. Bleeding and thromboembolism during anticoagulant therapy: a population-based study in Rochester, Minnesota. Mayo Clin Proc. 1995;70(8):725-733 [DOI] [PubMed] [Google Scholar]
- 31. Lind M, Boman K, Johansson L, et al. Thrombomodulin as a marker for bleeding complications during warfarin treatment. Arch Intern Med. 2009;169(13):1210-1215 [DOI] [PubMed] [Google Scholar]
- 32. McMahan DA, Smith DM, Carey MA, Zhou XH. Risk of major hemorrhage for outpatients treated with warfarin. J Gen Intern Med. 1998;13(5):311-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poli D, Antonucci E, Grifoni E, Abbate R, Gensini GF, Prisco D. Bleeding risk during oral anticoagulation in atrial fibrillation patients older than 80 years. J Am Coll Cardiol. 2009;54(11):999-1002 [DOI] [PubMed] [Google Scholar]
- 34. Suzuki S, Yamashita T, Kato T, et al. Incidence of major bleeding complication of warfarin therapy in Japanese patients with atrial fibrillation. Circ J. 2007;71(5):761-765 [DOI] [PubMed] [Google Scholar]
- 35. Wallvik J, Själander A, Johansson L, Bjuhr O, Jansson JH. Bleeding complications during warfarin treatment in primary healthcare centres compared with anticoagulation clinics. Scand J Prim Health Care. 2007;25(2):123-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shireman TI, Mahnken JD, Howard PA, Kresowik TF, Hou Q, Ellerbeck EF. Development of a contemporary bleeding risk model for elderly warfarin recipients. Chest. 2006;130(5):1390-1396 [DOI] [PubMed] [Google Scholar]
- 37. Bini EJ, Rajapaksa RC, Weinshel EH. Positive predictive value of fecal occult blood testing in persons taking warfarin. Am J Gastroenterol. 2005;100(7):1586-1592 [DOI] [PubMed] [Google Scholar]
- 38. Diener HC, Executive Steering Committee of the SPORTIF III and V Investigators Stroke prevention using the oral direct thrombin inhibitor ximelagatran in patients with non-valvular atrial fibrillation: pooled analysis from the SPORTIF III and V studies. Cerebrovasc Dis. 2006;21(4):279-293 [DOI] [PubMed] [Google Scholar]
- 39. Douketis JD, Arneklev K, Goldhaber SZ, Spandorfer J, Halperin F, Horrow J. Comparison of bleeding in patients with nonvalvular atrial fibrillation treated with ximelagatran or warfarin: assessment of incidence, case-fatality rate, time course and sites of bleeding, and risk factors for bleeding. Arch Intern Med. 2006;166(8):853-859 [DOI] [PubMed] [Google Scholar]
- 40. Fang MC, Go AS, Hylek EM, et al. Age and the risk of warfarin-associated hemorrhage: the anticoagulation and risk factors in atrial fibrillation study. J Am Geriatr Soc. 2006;54(8):1231-1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fihn SD, Callahan CM, Martin DC, McDonell MB, Henikoff JG, White RH, National Consortium of Anticoagulation Clinics The risk for and severity of bleeding complications in elderly patients treated with warfarin. Ann Intern Med. 1996;124(11):970-979 [DOI] [PubMed] [Google Scholar]
- 42. Goudie BM, Donnan PT, Fairfield G, Al-Agilly SS, Cachia PG. Dependency rather than old age increases the risk of warfarin-related bleeding. Br J Gen Pract. 2004;54(506):690-692 [PMC free article] [PubMed] [Google Scholar]
- 43. Gulløv AL, Koefoed BG, Petersen P. Bleeding during warfarin and aspirin therapy in patients with atrial fibrillation: the AFASAK 2 study. Arch Intern Med. 1999;159(12):1322-1328 [DOI] [PubMed] [Google Scholar]
- 44. Gurwitz JH, Goldberg RJ, Holden A, Knapic N, Ansell J. Age-related risks of long-term oral anticoagulant therapy. Arch Intern Med. 1988;148(8):1733-1736 [PubMed] [Google Scholar]
- 45. Isaacs C, Paltiel O, Blake G, Beaudet M, Conochie L, Leclerc J. Age-associated risks of prophylactic anticoagulation in the setting of hip fracture. Am J Med. 1994;96(6):487-491 [DOI] [PubMed] [Google Scholar]
- 46. Kagansky N, Knobler H, Rimon E, Ozer Z, Levy S. Safety of anticoagulation therapy in well-informed older patients. Arch Intern Med. 2004;164(18):2044-2050 [DOI] [PubMed] [Google Scholar]
- 47. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349(7):631-639 [DOI] [PubMed] [Google Scholar]
- 48. Lindh JD, Holm L, Dahl ML, Alfredsson L, Rane A. Incidence and predictors of severe bleeding during warfarin treatment. J Thromb Thrombolysis. 2008;25(2):151-159 [DOI] [PubMed] [Google Scholar]
- 49. Metlay JP, Hennessy S, Localio AR, et al. Patient reported receipt of medication instructions for warfarin is associated with reduced risk of serious bleeding events. J Gen Intern Med. 2008;23(10):1589-1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ogendo SW. Warfarin-related bleeding following open heart surgery in Nairobi. East Afr Med J. 2001;78(3):139-143 [DOI] [PubMed] [Google Scholar]
- 51. Ruiz-Irastorza G, Khamashta MA, Hunt BJ, Escudero A, Cuadrado MJ, Hughes GR. Bleeding and recurrent thrombosis in definite antiphospholipid syndrome: analysis of a series of 66 patients treated with oral anticoagulation to a target international normalized ratio of 3.5. Arch Intern Med. 2002;162(10):1164-1169 [DOI] [PubMed] [Google Scholar]
- 52. Shalansky S, Lynd L, Richardson K, Ingaszewski A, Kerr C. Risk of warfarin-related bleeding events and supratherapeutic international normalized ratios associated with complementary and alternative medicine: a longitudinal analysis. Pharmacotherapy. 2007;27(9):1237-1247 [DOI] [PubMed] [Google Scholar]
- 53. Shireman TI, Howard PA, Kresowik TF, Ellerbeck EF. Combined anticoagulant-antiplatelet use and major bleeding events in elderly atrial fibrillation patients. Stroke. 2004;35(10):2362-2367 [DOI] [PubMed] [Google Scholar]
- 54. Wallerstedt SM, Gleerup H, Sundstrom A, Stigendal L, Ny L. Risk of clinically relevant bleeding in warfarin-treated patients—influence of SSRI treatment. Pharmacoepidemiol Drug Saf. 2009;18(5):412-416 [DOI] [PubMed] [Google Scholar]
- 55. White RH, Beyth RJ, Zhou H, Romano PS. Major bleeding after hospitalization for deep-venous thrombosis. Am J Med. 1999;107(5):414-424 [DOI] [PubMed] [Google Scholar]
- 56. Lip GY, Frison L, Halperin JL, Lane D. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score [published online ahead of print November 24, 2010]. J Am Coll Cardiol. doi:10.1016/j.jacc.2010.09.024 [DOI] [PubMed] [Google Scholar]
- 57. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857-867 [DOI] [PubMed] [Google Scholar]
- 58. Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151:297-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.