Abstract
Heart failure with preserved ejection fraction (HF-PEF) is the clinical syndrome of heart failure associated with normal or near-normal systolic function. Because inhibition of the adrenergic and renin-angiotensin-aldosterone systems has been so effective in the treatment of systolic heart failure, these same therapies have been the subject of recent clinical trials of HF-PEF. In this review, we examine the current evidence about treatment of HF-PEF, with particular emphasis on reviewing the literature for large-scale randomized clinical studies. The lack of significant benefit with neurohormonal antagonism in HF-PEF suggests that this condition might not involve neurohormonal activation as a critical pathophysiologic mechanism. Perhaps heart failure as we traditionally think of it is the wrong paradigm to pursue as we try to understand this condition of volume overload known as HF-PEF.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CAD = coronary artery disease; CI = confidence interval; EF = ejection fraction; HF = heart failure; HF-PEF = heart failure with preserved ejection fraction; HR = hazard ratio; HTN = hypertension; LV = left ventricular; LVH = LV hypertrophy; NYHA = New York Heart Association; RAAS = renin-angiotensin-aldosterone system
Heart failure with preserved ejection fraction (HF-PEF), historically known as diastolic heart failure, refers to the clinical syndrome of heart failure (HF) associated with normal or near-normal systolic function. Its prevalence has been increasing during the past 2 decades and accounts for approximately half the cases of HF.1,2 Heart failure with preserved ejection fraction increases with age and especially affects those older than 70 years.3 A review of the ADHERE (Acute Decompensated Heart Failure National Registry) database4 showed that, compared with patients who have HF with systolic dysfunction, patients with HF-PEF are older, more likely to be women, and more likely to have hypertension (HTN) but less likely to have had a myocardial infarction. Other cardiovascular comorbidities are also common in these patients, including obesity, coronary artery disease (CAD), diabetes mellitus, atrial fibrillation, and hyperlipidemia.5 Patients with HF-PEF were previously assumed to have better prognosis than patients with depressed systolic function.6 More recent data suggest that mortality rates and rates of rehospitalization are not significantly different between the 2 groups.7-9 However, in contrast to the improvements in survival with systolic HF, mortality from HF-PEF has remained the same.1 This dissociation may in large part be due to the clinical and pathophysiologic heterogeneity of HF-PEF and the consequent varied and noncardiovascular causes of death in patients with this syndrome10 (Figure 1).
FIGURE 1.
Mode of death of patients in trials of heart failure with preserved ejection fraction. Left, I-PRESERVE = Irbesartan in Heart Failure with Preserved Ejection Fraction Study. Right, CHARM-Preserved = Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity.
Data from Circulation.10
PATHOPHYSIOLOGY
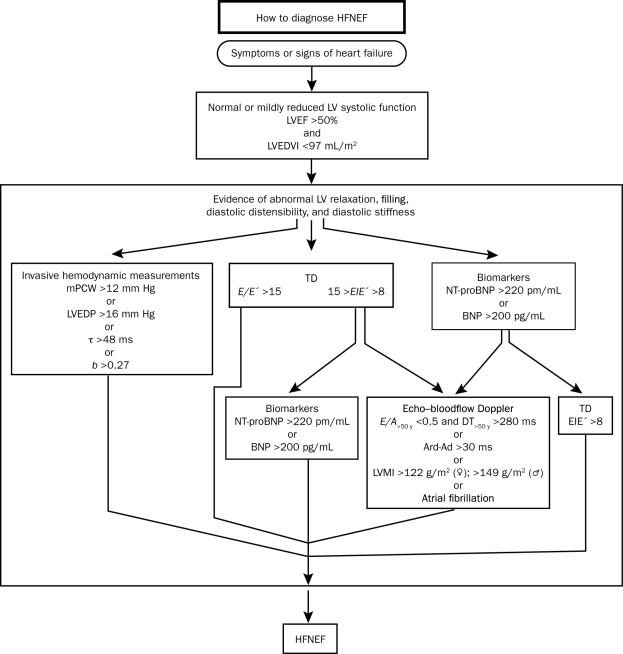
Heart failure with preserved ejection fraction is usually diagnosed when the clinical syndrome of HF (volume overload, dyspnea, exertional intolerance) is associated with a preserved systolic function and some evidence of diastolic dysfunction (by cardiac catheterization, echocardiography, or cardiac biomarkers) (Figure 2).11 However, this clinical syndrome may be mimicked by restrictive cardiomyopathy, pericardial disease, dynamic mitral regurgitation, ischemic heart disease, and exercise-induced pulmonary HTN.12 There are no universally agreed on criteria for the diagnosis of HF-PEF, which is particularly apparent in the variable enrollment criteria for patients in clinical trials of HF-PEF (Table 1). Even an ejection fraction (EF) cutoff for “preserved” or normal was not uniform in these trials. This lack of consensus is emblematic of an uncertain pathophysiologic construct for this condition. Kass16 suggests that HF be divided into 3 categories: (1) systolic (depressed systolic function as a predominant feature), (2) diastolic (resulting from abnormal diastolic properties including fibrosis and infiltrative disorders that restrict filling and cause elevated pressures), and (3) a potpourri of conditions that involve multiple features of the renal and cardiovascular systems.
FIGURE 2.
Criteria to diagnose heart failure with normal left ventricular ejection fraction (HFNEF), referred to as heart failure with preserved ejection fraction in the current study, as per the consensus statement by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Ad = duration of mitral valve atrial wave flow; Ard = duration of reverse pulmonary vein atrial systole flow; b = constant of left ventricular chamber stiffness; BNP = brain natriuretic peptide; DT = deceleration time; E = early mitral valve flow velocity; E′= early TD lengthening velocity; E/A = ratio of early (E) to late (A) mitral valve flow velocity; LAVI = left atrial volume index; LVEDP = left ventricular end-diastolic pressure; Echo = echocardiography; LVEDVI = left ventricular end-diastolic volume index; LVEF = left ventricular ejection fraction; LVMI = left ventricular mass index; mPCW = mean pulmonary capillary wedge pressure; NT-proBNP = N-terminal pro-brain natriuretic peptide; τ = time constant of left ventricular relaxation; TD = tissue Doppler.
From Eur Heart J,11 with permission.
TABLE 1.
Inclusion Criteria for Patients Enrolled in Trials of HF-PEF
Heart failure with preserved ejection fraction appears to be a heterogeneous disorder with multiple pathophysiologic processes. Traditionally, HF-PEF has been attributed to a primary cardiac disorder of myocardial stiffening from hypertrophy and fibrosis,17,18 abnormal calcium handling, and even venous turgor. Hypertrophy and fibrosis are particularly central to the traditional paradigm of HF-PEF. The renin-angiotensin-aldosterone system (RAAS) contributes to the fibrosis of hypertensive heart disease,17 and treatment with lisinopril decreases myocardial fibrosis in left ventricular
Article Highlights
Heart failure with preserved ejection fraction (HF-PEF) refers to the clinical syndrome of heart failure associated with normal or near-normal systolic function
HF-PEF increases with age and is more likely to affect women
Morbidity and mortality of HF-PEF are similar to those of heart failure with systolic dysfunction
There is no clear pathophysiologic mechanism to HF-PEF, but many elements of the cardiac, vascular, and renal systems have been implicated
Treatment to date has focused on the renin-angiotensin-aldosterone system and the adrenergic nervous system, but clinical trials have failed to show any significant benefit to their blockade
Better understanding of the underlying pathophysiology of HF-PEF will help establish effective therapies
Treatment of HF-PEF remains empirical and centered around the control of blood pressure and volume status
hypertrophy (LVH) with a concomitant improvement in left ventricular (LV) diastolic function.19 The stiff heart then requires increased pressure to fill the left ventricle to a normal end-diastolic volume.20
However, confirming these hypotheses in human studies using contemporary community-based cohorts has been difficult. Other mechanisms have been proposed, including abnormal ventricular-vascular coupling related to decreased vascular compliance,21,22 impaired renal handling of salt and fluid, chronotropic incompetence,23 lack of vasodilator reserve, precapillary pulmonary HTN,24 anemia, and sleep disturbances. Sex-related differences in vascular function and LV remodeling may also help to explain the increased susceptibility of women to HF-PEF. Finally, indirect evidence25 suggests that nitric oxide bioavailability may be important in modulating peripheral and pulmonary vascular tone and LV compliance, paving the way for phosphodiesterase-5 inhibition as a therapeutic target.
Surprisingly, in contrast to the well-established role of neurohormonal activation in systolic HF, evidence supporting such a mechanism in HF-PEF is scarce.26 However, most clinicians think that neurohormonal antagonism, eg, RAAS and adrenergic blockers, is effective in HF-PEF despite a lack of evidence to support this paradigm. Importantly, the primary stimulus for salt and water retention in this condition is unclear. A unifying hypothesis for HF-PEF remains elusive and represents the primary challenge to finding effective therapies for HF-PEF. We have reviewed the literature (PubMed) for large randomized clinical trials (>100 patients) that evaluated the role of different pharmacological interventions in patients with HF-PEF. As outlined subsequently, these randomized clinical treatment trials of HF-PEF suggest that, although HF-PEF shares the clinical syndrome of volume overload with the concept of HF, it appears to be distinct from HF from a pathophysiologic perspective.
TREATMENT
The 2009 focused American College of Cardiology/American Heart Association HF update27 concluded that treatment of patients with HF-PEF should aim at achieving the following goals: (1) aggressive control of systolic and diastolic HTN because ventricular hypertrophy leads to increased chamber stiffness; (2) coronary revascularization in patient with CAD because myocardial ischemia can impair ventricular relaxation; (3) control of ventricular rate especially in the setting of atrial arrhythmias because tachycardia shortens the time for ventricular filling and coronary perfusion; and (4) control of fluid overload with diuretics while being cautious with the dose because patients with HF-PEF may be sensitive to preload reduction, which could lead to hypotension. However, these recommendations were based primarily on consensus opinion. Furthermore, these guidelines do not provide information on how to specifically achieve these goals. In this review, we examine the evidence to support the use of commonly prescribed agents to achieve these goals in the management of HF-PEF, with particular emphasis on the few large-scale randomized clinical trials completed to date in this field.
RAAS Inhibition
The RAAS is involved in processes associated with HF, such as HTN, LVH, myocardial fibrosis, and vascular dysfunction. Furthermore, inhibition of the RAAS has been shown to be beneficial in the treatment of patients with systolic HF. Whether this antagonism is achieved by inhibiting angiotensin-converting enzyme (ACE) or through blockade of the angiotensin or aldosterone receptor, the improvements in morbidity and mortality have been robust and reproducible.28-33 Because ACE inhibitors and angiotensin receptor blockers (ARBs) have physiologic benefits such as the regression of LVH in patients with HTN,33 it was widely believed that RAAS blockade would also be of benefit in the prevention and/or treatment of HF-PEF.
ACE inhibitors and ARBs are commonly used in many patients with HTN, diabetes, and CAD. However, proving that they specifically prevent HF-PEF has been difficult. In the ALLHAT study (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial),34 lisinopril was inferior to chlorthalidone in preventing new-onset HF-PEF. Subsequent prospective randomized trials of RAAS blockade in the treatment of HF-PEF have been similarly disappointing. For example, in a trial of 150 patients with HF-PEF35 who were randomized to diuretics alone, diuretics plus irbesartan, or diuretics plus ramipril, the regimen of diuretics alone was effective in reducing symptoms of HF and improving quality of life; the addition of ramipril or irbesartan did not improve symptoms further. In fact, all 3 groups had slight improvement in exercise capacity as assessed by the 6-minute walk test.
PEP-CHF. The PEP-CHF (Perindopril in Elderly People with Chronic Heart Failure) study15 was a randomized, double-blind, international, multicenter trial that compared perindopril (target dosage, 4 mg/d) to placebo in patients 70 years of age or older who had evidence of clinical HF with relatively preserved EF (≥40%) and echocardiographic evidence of diastolic dysfunction. The study randomized 850 patients, and the mean follow-up was 26 months. The primary end point was the composite of all-cause mortality or unplanned HF-related hospitalization. The primary outcome was not different between the 2 groups (25.1% in the placebo group and 23.6% in the perindopril group; hazard ratio [HR], 0.92; 95% confidence interval [CI], 0.70-1.21; P=.54). However, the power of the study was limited by a lower than expected event rate despite a longer than planned follow-up period. A post hoc analysis using only the first year of follow-up (to account for the large crossover to open-label ACE inhibitor use) suggested a nonsignificant trend toward a reduction in primary outcome and in hospitalization for HF. Perindopril did improve the secondary end points of 6-minute walk distance and New York Heart Association (NYHA) class. In contrast, a smaller randomized trial showed that use of the ACE inhibitor enalapril for HF-PEF (n=71) failed to improve exercise capacity or aortic distensibility.36
CHARM-Preserved. In CHARM-Preserved (Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity), a multicenter, double-blind, international trial, the role of the ARB candesartan was studied in 3023 patients with HF-PEF (EF, ≥40%; mean EF, 54%) who were randomized to candesartan (target dose, 32 mg) or placebo and were followed up for a mean duration of 36 months.13 The primary outcome of cardiovascular death or admission to hospital for HF was not significantly different between the 2 groups (22% in the candesartan group, 24% in the placebo group; HR, 0.89; 95% CI, 0.77-1.03; P=.118). Similar to the findings in PEP-CHF, candesartan appeared to decrease hospital admissions for HF, but this outcome was a secondary end point of the study.
I-PRESERVE. In I-PRESERVE (Irbesartan in Heart Failure with Preserved Ejection Fraction Study), the ARB irbesartan was analyzed in the largest study of HF-PEF to date.14 This trial randomized patients with an EF of at least 45% to irbesartan (target dosage, 300 mg/d) or placebo. A total of 4128 patients were randomized and followed up for a mean duration of 49 months. In addition to the size of the study, a particular strength of this trial was the baseline patient demographics characteristic of community-based HF-PEF cohorts. The primary outcome was a composite of death from any cause or hospitalization for cardiovascular causes. Again, there was no difference for the primary end point between the groups (100.4 events per 1000 patient-years in the irbesartan group and 105.4 events per 1000 patient-years in the placebo group; HR, 0.95; 95% CI, 0.86-1.05; P=.35). In contrast to the other HF-PEF trials, a review of secondary outcomes showed no significant differences in all-cause mortality, cardiovascular hospitalization, or a composite of HF outcomes (death due to worsening HF, sudden death, or hospitalization due to worsening of HF). Furthermore, at 6 months, the improvement in the Minnesota Living With Heart Failure scale and the change in N-terminal pro-brain natriuretic peptide (NT-proBNP) were not significantly different between the 2 groups.
Ultimately, RAAS antagonism may have failed in the treatment of HF-PEF because blood pressure reduction itself may be more important than mode of blood pressure reduction in these patients.37 In addition, the enrollment of patients with alternative pathophysiologic mechanisms or non–HF-PEF conditions, eg, pulmonary HTN, may have obscured any benefit from RAAS blockade.
Aldosterone Antagonism
Because aldosterone has been implicated in both vascular and myocardial fibrosis, it has become a focus of interest in the treatment of HF-PEF. Mottram et al38 randomized 30 patients with treated HTN, EF greater than 50%, exertional dyspnea, and diastolic dysfunction to spironolactone or placebo for 6 months. Spironolactone treatment resulted in reduction in posterior wall thickness, long-axis strain rate, peak systolic strain, and cyclic variation of integrated back-scatter. The increase in strain was independent of changes in blood pressure in the spironolactone group. Outcome trials are under way to evaluate the role of aldosterone antagonism in large-scale clinical trials.
TOPCAT. Treatment Of Preserved Cardiac function heart failure with an Aldosterone anTagonist (TOPCAT) is an ongoing National Institutes of Health–sponsored, double-blind, randomized, placebo-controlled, multicenter, international trial. Patients with HF-PEF are being randomized to spironolactone (up-titrated as tolerated to a maximal dosage of 45 mg/d) or placebo.
Aldo-DHF. Aldosterone Receptor Blockade in Diastolic Heart Failure (Aldo-DHF) will include 420 patients to be treated with spironolactone for 1 year in a placebo-controlled study.39 The primary end points are physical performance (quantified by spiroergometry) and Doppler-echocardiographic parameters for diastolic dysfunction. This study will look at secondary end points of quality of life and morbidity.
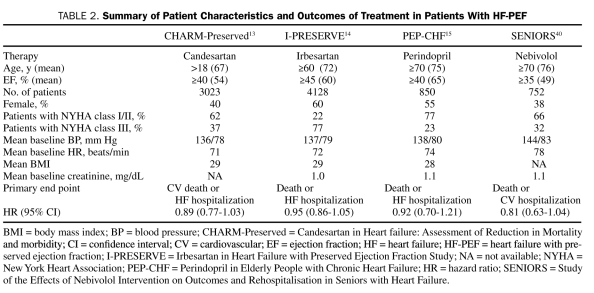
In Summary. Studies to date (Table 2) have failed to show improvement in mortality with the use of RAAS antagonists for HF-PEF. With respect to morbidity, the evidence is conflicting: perindopril showed improvement in functional capacity, and candesartan decreased hospitalizations for HF; however, irbesartan showed no benefit in either improving quality of life or decreasing hospitalizations for HF. These conflicting findings may be a consequence of trial conduct. In their review of 21 trials of HF-PEF, Paulus et al41 emphasized that nonadherence to diagnostic guidelines may have led to excessive enrollment of HF patients with eccentric LV remodeling and ischemic heart disease. Although differences in study design, qualifying entry criteria (eg, EF), and drug-specific effects may explain these disparate findings, it is reasonable to conclude that no compelling evidence exists to recommend RAAS antagonists as first-line therapy for HF-PEF without a specific comorbidity such as HTN, diabetes mellitus, or CAD. A recent meta-analysis of these trials also notes the limitations of this evidence to date.42
TABLE 2.
Summary of Patient Characteristics and Outcomes of Treatment in Patients With HF-PEF
Sympathetic Nervous System Inhibition
Similar to RAAS inhibition, sympathetic nervous system inhibition with β-blockers has shown significant benefits in the treatment of HF with systolic dysfunction. The potential benefits of β-blockers in patients with HF-PEF include changes in adrenergic receptor profiles, improved ventricular remodeling, decreased arrhythmic risk, and improved metabolic efficiency.43 This hypothesis has been explored in several small surrogate end point trials, such as the Swedish Doppler-Echocardiographic study (SWEDIC).44 Patients were randomized in a double-blind design to carvedilol or placebo, and indices of diastolic function were monitored with echocardiography after 6 months of therapy. At the end of the study, a significant improvement was noted in the E:A ratio (ratio of early [E] to late [A] mitral valve flow velocity), although no significant improvement occurred in other diastolic function variables such as deceleration time, isovolumic relaxation time, or ratio of systolic to diastolic pulmonary venous flow velocity. In a study from Japan, carvedilol decreased levels of brain natriuretic peptide, improved NYHA class, and increased exercise capacity in 40 patients randomized to carvedilol or placebo.45 Similarly, the use of nebivolol in patients with HTN and LV diastolic dysfunction led to an increase in E:A ratio and improvement in hemodynamics, including pulmonary artery pressure and pulmonary wedge pressure.46 In contrast, Borlaug et al23 have suggested that exercise intolerance in patients with HF-PEF is related to chronotropic incompetence, which would be expected to worsen with β-blockade.
SENIORS. In SENIORS (Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure), the effect of nebivolol, a β1-selective blocker with vasodilating properties related to nitric oxide modulation, on HF morbidity and mortality was evaluated in a study of elderly patients (≥70 years) with clinical HF regardless of EF.40 In this multicenter, international, double-blind study, 2135 patients were randomized to nebivolol (target dosage, 10 mg/d) or placebo and were followed up for 21 months. The primary composite outcome of all-cause mortality or hospital admission for cardiovascular disease was significantly reduced with nebivolol (31%) vs placebo (35%) (HR, 0.86; 95% CI, 0.74-0.99; P =.039). A post hoc subgroup analysis showed no significant influence of EF on the reduction in primary outcome. However, when patients with an EF greater than 40% were examined, the primary outcome for all-cause mortality or hospital admission for cardiovascular disease was not statistically significant (HR, 0.83; 95% CI, 0.62-1.11; P=.203). In a subgroup of patients who underwent echocardiography at 12-month follow-up (n=1500), significant improvements in EF due to nebivolol therapy were seen in both depressed and preserved systolic function. However, the study was not powered to examine the interaction between EF and drug benefit.
Additional data about the role of β-blockers in patients with HF-PEF are from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry.9 In this registry, 4153 patients with HF-PEF were naïve to β-blockers at initial hospital admission. At discharge, 39% of these patients were receiving β-blocker therapy, and this therapy did not have a statistically significant effect on mortality, rehospitalization, or the combined end point. The COHERE (Carvedilol Heart Failure Registry) examined the benefit of carvedilol therapy in HF management.47 After treatment with carvedilol, patients with an EF greater than 40% had improved functional status and lower rates of hospitalization, but the magnitude of improvement with carvedilol therapy was less than that seen in patients with systolic dysfunction.
Whether β-blockers with different ancillary properties (ie, vasodilation, antioxidant activity, cardiometabolic effects) will benefit patients with HF-PEF remains largely unknown. More large-scale clinical trials are still evaluating the role of β-blockers in HF-PEF: (1) β-PRESERVE (β-blocker in heart failure with normal left ventricular ejection failure) is investigating the role of metoprolol succinate in HF-PEF,48 and (2) the J-DHF (Japanese Diastolic Heart Failure Study)49 will examine the role of carvedilol in patients with HF-PEF. Although the results from these studies are pending, the current clinical trial evidence does not support a primary role for β-blockers in the treatment of HF-PEF.
Calcium Channel Blockers
Little evidence is available about the benefits of verapamil in treating diastolic dysfunction. In a small randomized trial (n=20) with a 5-week crossover design, Setaro et al50 showed that treatment with verapamil improved exercise capacity by 33% and peak diastolic filling by 30%. Patients treated with verapamil also had a clinicoradiographic score improvement on a HF scale. Verapamil was also studied in 15 elderly patients with NYHA class II-III and normal LV systolic function in a placebo crossover design.51 After 3 months of therapy, verapamil significantly improved congestive HF score, increased exercise time, and improved indices of diastolic function. Calcium channel blockers have not been studied in large-scale outcome trials for the treatment of HF-PEF.
Digitalis
Because systolic function is preserved in patients with HF-PEF, the role of digitalis has been questioned in their treatment. A theoretical benefit could potentially be expected through a reduction in heart rate at rest and improved baroreceptor and parasympathetic function. The ancillary Digitalis Investigation Group (DIG) trial52 randomized 988 patients with HF-PEF (EF >45%) to digoxin or placebo to study the role of digoxin in HF-PEF. Treatment with digoxin had no effect on hospitalization for HR, mortality due to HF, or on all-cause mortality or hospitalization.
Statins
Statins have failed to show benefit in the treatment of systolic HF,53,54 and no large-scale trial has evaluated their role in HF-PEF. However, in an observational single-center study of 137 consecutive patients,55 statins were associated with significant reduction in mortality (adjusted relative risk, 0.22; 95% CI, 0.07-0.64; P=.005), whereas ACE inhibitors, β-blockers, ARBs, and calcium channel blockers had no effect on survival. Such survival benefits have not been observed in other HF studies or statin trials in other populations, raising important questions about confounding factors in this small observational study.
Exercise Training
One of the major symptoms in patients with HF-PEF is exertional dyspnea and exercise intolerance. In healthy individuals, diastolic and vascular function is enhanced with exercise; thus, LV input parallels LV output despite a shorter diastolic period associated with the exertional tachycardia. In diastolic dysfunction, the left ventricle is unable to rapidly decrease the intraventricular pressure during early diastole, leading to an increase in left atrial pressure and a subsequent increase in pulmonary capillary wedge pressure.
Grewal et al56 showed that, in patients with preserved systolic function and no evidence of exercise-induced ischemia, diastolic dysfunction was strongly and inversely associated with exercise capacity. Conversely, exercise training may improve diastolic dysfunction. Takemoto et al57 compared exercise-trained athletes with matched sedentary individuals and found that echocardiographic indices of diastolic function were significantly different between the 2 groups and suggested that LV diastolic dysfunction associated with aging is less pronounced in exercise-trained individuals. Furthermore, in a cohort of diabetic patients with varying degrees of diastolic dysfunction, exercise training led to an improvement in exercise capacity and had the potential to reverse diastolic dysfunction.58 In one evaluation of exercise, elderly women with NYHA class II or III and EF greater than 45% were randomized to a 12-week home-based exercise program or no intervention.59 Women assigned to the exercise program had significant improvement in the 6-minute walk test and in quality of life as measured by the Living With Heart Failure Questionnaire and the Geriatric Depression Scale. Large-scale trials have not evaluated the effect of exercise on outcomes in patients with HF-PEF; however, aerobic exercise could be beneficial in this population in terms of improved functional capacity, weight loss, and benefits through risk reduction such as improvement in HTN and diabetes control.
CONCLUSION
Ideally, the treatment of HF-PEF should relieve symptoms and increase longevity. Unfortunately, to date, studies of neurohormonal blockade in patients with HF-PEF have failed to show a mortality benefit or a clear improvement in quality of life. Although inhibitors of the RAAS and sympathetic nervous system should continue to be used in the population of patients with HF-PEF who have other comorbidities such as HTN, diabetes mellitus, or CAD, the use of these drugs for the primary treatment of HF-PEF remains unsupported by the available evidence.
Discordance in the treatment benefits of neurohormonal antagonism in HF-PEF vs systolic HF suggests that, despite these 2 conditions sharing a common clinical picture of volume overload, exercise intolerance, and significant mortality, HF-PEF does not appear to involve neurohormonal activation as a critical pathophysiologic mechanism. Clearly, a better understanding of the underlying causes of HF-PEF will help in planning clinical trials to investigate future therapies. Such examples include novel strategies like phosphodiesterase type 5 inhibition with sildenafil in the National Institutes of Health–sponsored RELAX (PhosphodiesteRasE-5 Inhibition to Improve Quality of Life And EXercise Capacity in Diastolic Heart Failure) trial, pacing in the RESET study, and vagal stimulation in the HOPE (Heart Outcomes Prevention Evaluation) investigation. Perhaps heart failure as we traditionally think of it is the wrong paradigm to pursue as we try to understand “heart failure” with preserved ejection fraction.
Footnotes
An earlier version of this article appeared Online First.
REFERENCES
- 1. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251-259 [DOI] [PubMed] [Google Scholar]
- 2. Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194-202 [DOI] [PubMed] [Google Scholar]
- 3. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure; Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387-1393 [DOI] [PubMed] [Google Scholar]
- 4. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (AD-HERE) Database. J Am Coll Cardiol. 2006;47(1):76-84 [DOI] [PubMed] [Google Scholar]
- 5. Lam CSP, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(1):18-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohn JN, Johnson G, Veterans Administration Cooperative Study Group Heart failure with normal ejection fraction: the V-HeFT Study. Circulation. 1990;81(2)(suppl):III48-III53 [PubMed] [Google Scholar]
- 7. Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355(3):260-269 [DOI] [PubMed] [Google Scholar]
- 8. Lenzen MJ, Scholte op Reimer WJM, Boersma E, et al. Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey. Eur Heart J. 2004;25(14):1214-1220 [DOI] [PubMed] [Google Scholar]
- 9. Hernandez AF, Hammill BG, O'Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53(2):184-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zile MR, Gaasch WH, Anand IS, et al. I-Preserve Investigators Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121(12):1393-1405 [DOI] [PubMed] [Google Scholar]
- 11. Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure? A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction (HFNEF) by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28(20):2539-2550 [DOI] [PubMed] [Google Scholar]
- 12. Tolle JJ, Waxman AB, Van Horn TL, Pappagianopoulos PP, Systrom DM. Exercise-induced pulmonary arterial hypertension. Circulation. 2008;118(21):2183-2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yusuf S, Pfeffer MA, Swedberg K, et al. CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777-781 [DOI] [PubMed] [Google Scholar]
- 14. Massie BM, Carson PE, McMurray JJ, et al. I-PRESERVE Investigators Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456-2467 [DOI] [PubMed] [Google Scholar]
- 15. Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J, PEP-CHF Investigators The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27(19):2338-2345 [DOI] [PubMed] [Google Scholar]
- 16. Kass DA. Is heart failure with decent systole due to bad diastole? J Card Fail. 2005;11(3):188-190 [DOI] [PubMed] [Google Scholar]
- 17. González A, López B, Díez J. Fibrosis in hypertensive heart disease: role of the renin-angiotensin-aldosterone system. Med Clin North Am. 2004;88(1):83-97 [DOI] [PubMed] [Google Scholar]
- 18. Mitchell JA, Ventura HO, Mehra MR. Early recognition and treatment of hypertensive heart disease. Curr Opin Cardiol. 2005;20(4):282-289 [DOI] [PubMed] [Google Scholar]
- 19. Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102(12):1388-1393 [DOI] [PubMed] [Google Scholar]
- 20. Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure: abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350(19):1953-1959 [DOI] [PubMed] [Google Scholar]
- 21. Desai AS, Mitchell GF, Fang JC, Creager MA. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail. 2009;15(8):658-664 [DOI] [PubMed] [Google Scholar]
- 22. Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107(5):714-720 [DOI] [PubMed] [Google Scholar]
- 23. Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114(20):2138-2147 [DOI] [PubMed] [Google Scholar]
- 24. Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119-1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takimoto E, Champion HC, Li M, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11(2):214-222 [DOI] [PubMed] [Google Scholar]
- 26. Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288(17):2144-2150 [DOI] [PubMed] [Google Scholar]
- 27. Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119(14):e391-e479 [DOI] [PubMed] [Google Scholar]
- 28. SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293-302 [DOI] [PubMed] [Google Scholar]
- 29. CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435 [DOI] [PubMed] [Google Scholar]
- 30. Granger CB, McMurray JJ, Yusuf S, et al. CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776 [DOI] [PubMed] [Google Scholar]
- 31. Young JB, Dunlap ME, Pfeffer MA, et al. Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) Investigators and Committees. Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation. 2004;110(17):2618-2626 [DOI] [PubMed] [Google Scholar]
- 32. Pitt B, Zannad F, Remme WJ, et al. Randomized Aldactone Evaluation Study Investigators The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709-717 [DOI] [PubMed] [Google Scholar]
- 33. Pitt B, Williams G, Remme W, et al. The EPHESUS trial: eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction: Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc Drugs Ther. 2001;15:79-87 [DOI] [PubMed] [Google Scholar]
- 34. Davis BR, Kostis JB, Simpson LM, et al. ALLHAT Collaborative Research Group Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118(22):2259-2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yip GW, Wang M, Wang T, et al. The Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart. 2008;94(5):573-580 [DOI] [PubMed] [Google Scholar]
- 36. Kitzman DW, Hundley WG, Brubaker PH, et al. A randomized, double-blinded trial of enalapril in older patients with heart failure and preserved ejection fraction: effects on exercise tolerance and arterial distensibility. Circ Heart Fail. 2010;3(4):477-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Solomon SD, Janardhanan R, Verma A, et al. Valsartan In Diastolic Dysfunction (VALIDD) Investigators Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369(9579):2079-2087 [DOI] [PubMed] [Google Scholar]
- 38. Mottram PM, Haluska B, Leano R, Cowley D, Stowasser M, Marwick TH. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;110(5):558-565 [DOI] [PubMed] [Google Scholar]
- 39. Aldosterone Receptor Blockade in Diastolic Heart Failure (ALDO-DHF): project description. Competence Network Web site http://www.knhi.de/en/Research/AP-ALDO-DHF/index.jsp Accessed February 17, 2011
- 40. Flather MD, Shibata MC, Coats AJ, et al. SENIORS Investigators Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26(3):215-225 [DOI] [PubMed] [Google Scholar]
- 41. Paulus WJ, Van Ballegoij JM. Treatment of heart failure with normal ejection fraction: an inconvenient truth. J Am Coll Cardiol. 2010;55(6):526-537 [DOI] [PubMed] [Google Scholar]
- 42. Shah RV, Desai AS, Givertz MM. The effect of renin-angiotensin system inhibitors on mortality and heart failure hospitalization in patients with heart failure and preserved ejection fraction: a systematic review and meta-analysis. J Card Fail. 2010;16(3):260-267 [DOI] [PubMed] [Google Scholar]
- 43. Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54(19):1747-1762 [DOI] [PubMed] [Google Scholar]
- 44. Bergström A, Andersson B, Edner M, Nylander E, Persson H, Dahlström U. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function: results of the Swedish Doppler-echocardiographic study (SWEDIC). Eur J Heart Fail. 2004;6(4):453-461 [DOI] [PubMed] [Google Scholar]
- 45. Takeda Y, Fukutomi T, Suzuki S, et al. Effects of carvedilol on plasma B-type natriuretic peptide concentration and symptoms in patients with heart failure and preserved ejection fraction. Am J Cardiol. 2004;94(4):448-453 [DOI] [PubMed] [Google Scholar]
- 46. Nodari S, Metra M, Dei Cas L. Beta-blocker treatment of patients with diastolic heart failure and arterial hypertension: a prospective, randomized, comparison of the long-term effects of atenolol vs. nebivolol. Eur J Heart Fail. 2003;5(5):621-627 [DOI] [PubMed] [Google Scholar]
- 47. Massie BM, Nelson JJ, Lukas MA, et al. COHERE Participant Physicians Comparison of outcomes and usefulness of carvedilol across a spectrum of left ventricular ejection fractions in patients with heart failure in clinical practice. Am J Cardiol. 2007;99(9):1263-1268 [DOI] [PubMed] [Google Scholar]
- 48. Zhou J, Shi H, Zhang J, Lu Y, Fu M, Ge J, β-PRESERVE Study Investigators Rationale and design of the β-blocker in heart failure with normal left ventricular ejection fraction (β-PRESERVE) study. Eur J Heart Fail. 2010;12:181-185 [DOI] [PubMed] [Google Scholar]
- 49. Hori M, Kitabatake A, Tsutsui H, et al. J-DHF Program Committee Rationale and design of a randomized trial to assess the effects of β-blocker in diastolic heart failure: Japanese Diastolic Heart Failure Study (J-DHF). J Card Fail. 2005;11(7):542-547 [DOI] [PubMed] [Google Scholar]
- 50. Setaro JF, Zaret BL, Schulman DS, Black HR, Soufer R. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66(12):981-986 [DOI] [PubMed] [Google Scholar]
- 51. Hung MJ, Cherng WJ, Kuo LT, Wang CH. Effect of verapamil in elderly patients with left ventricular diastolic dysfunction as a cause of congestive heart failure. Int J Clin Pract. 2002;56(1):57-62 [PubMed] [Google Scholar]
- 52. Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114(5):397-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kjekshus J, Apetrei E, Barrios V, et al. CORONA Group Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357(22):2248-2261 [DOI] [PubMed] [Google Scholar]
- 54. Gissi-HF Investigators Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1231-1239 [DOI] [PubMed] [Google Scholar]
- 55. Fukuta H, Sane DC, Brucks S, Little WC. Statin therapy may be associated with lower mortality in patients with diastolic heart failure: a preliminary report. Circulation. 2005;112(3):357-363 [DOI] [PubMed] [Google Scholar]
- 56. Grewal J, McCully R, Kane GC, Lam C, Pellikka PA. Left ventricular function and exercise capacity. JAMA. 2009;301(3):286-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Takemoto KA, Bernstein L, Lopez JF, Marshak D, Rahimtoola SH, Chandraratna PA. Abnormalities of diastolic filling of the left ventricle associated with aging are less pronounced in exercise-trained individuals. Am Heart J. 1992;124(1):143-148 [DOI] [PubMed] [Google Scholar]
- 58. Brassard P, Legault S, Garneau C, Bogaty P, Dumesnil JG, Poirier P. Normalization of diastolic dysfunction in type 2 diabetics after exercise training. Med Sci Sports Exerc. 2007;39(11):1896-1901 [DOI] [PubMed] [Google Scholar]
- 59. Gary RA, Sueta CA, Dougherty M, et al. Home-based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung. 2004;33(4):210-218 [DOI] [PubMed] [Google Scholar]