Abstract
Background
Blood pressure (BP) is a variable physiological parameter in health and disease. Increased BP variability over time in adults is associated with severity of end-organ damage and a higher rate of cardiovascular events, even after adjusting for the mean levels. This study tested the hypothesis that childhood BP variability, besides the mean levels, is also predictive of adulthood hypertension.
Methods
The study cohort consisted of 1,797 subjects (1,091 whites and 706 blacks; age = 21–48 years) enrolled in the Bogalusa Heart Study since childhood. BP variability was depicted as s.d. of 4–8 serial measurements in childhood.
Results
Blacks showed significantly greater childhood systolic BP (SBP) variability than whites. In multivariable logistic regression analyses, adjusting for race, sex, mean childhood age, s.d. of childhood body mass index (BMI), mean childhood BP levels, adulthood age and BMI, adult hypertension was significantly associated with s.d. of childhood SBP (odds ratio (OR) (95% confidence intervals) = 1.28 (1.09, 1.51), P = 0.002) and s.d. of childhood diastolic BP (DBP; 1.36 (1.16, 1.58), P < 0.001). When using adulthood BP levels as continuous dependent variables in linear regression models, adjusting for the same covariates, adulthood SBP and DBP levels were significantly associated with s.d. of childhood SBP (standardized regression coefficient β = 0.086, P < 0.001) and s.d. of childhood DBP (β = 0.105, P < 0.001), respectively.
Conclusions
Increases in BP variations as well as levels in early life are predictive of adult hypertension, which underscore the childhood origin of the natural history of essential hypertension.
Keywords: black–white, blood pressure, blood pressure variability, childhood, hypertension
Blood pressure (BP) is a highly variable physiological trait. In addition to different levels among individuals, the total variation of BP in a population has another important component, variations within the same individual at different time points (within-individual variability). There are several different ways of expressing the within-individual variability of BP over time, ranging from beat-to-beat changes1 to long-term changes between office visits.2–9 A long-term BP variability can be observed in a cohort with serial BP measurements over a long period of follow-up, as noted from childhood to adulthood in the Bogalusa Heart Study;10,11 a short-term BP variability can be measured by 24-h ambulatory BP monitoring.12–14
Excessive variability of BP in the early stages of hypertension was demonstrated as early as in 1921, and temporary elevation of BP above the usual normal values has been regarded as evidence of a possible prehypertensive state.15–17 In the past two decades, studies have shown that the wide variability of BP over a longer period2–8 or 24h12–14 is associated with severity of end-organ damage and an increase of subsequent cardiovascular and cerebrovascular events, even after adjusting for the mean BP levels. Large-scale clinical trials in Europe have demonstrated that the visit-to-visit BP variability is not random, but significantly reproducible over a long period of follow-up.9
Recently, the importance of long-term BP variability for stroke was highlighted in multiple articles appearing in the 13 March 2010 issue of Lancet,7–8,18 indicating that visit-tovisit variability of clinic systolic BP (SBP) was more predictive of stroke and coronary events than the variability measured by ambulatory BP monitoring.8 Of note, BP variability is receiving increasing attention because of its practical implications in the prevention and treatment of hypertension.18–20
Essential hypertension is considered a growth-related disorder with its origin in childhood.21,22 BP levels tend to “track” or “persist” over time and increases in childhood BP levels are a strong predictor of adulthood hypertension.23–25 However, information is scant regarding the influence of BP variability during childhood on the development of hypertension in adulthood. The Bogalusa Heart Study, a biracial (black–white) community-based long-term investigation of the early natural history of cardiovascular disease since childhood, provides a valuable longitudinal database on BP measurements over 36 years.26 Utilizing this database, the present report examines the independent predictive value of the within-individual variability of BP during childhood for adult hypertension.
Methods
Study cohort
In the community of Bogalusa, Louisiana, nine cross-sectional surveys of children aged 4–19 years were conducted between 1973 and1994 for cardiovascular risk factors. Linking these repeated cross-sectional examinations conducted every 2–3 years has resulted in serial observations during childhood. Two surveys of adults aged 21–48 years, who had been previously examined as children, were conducted as longitudinal studies in 2004–2007 and 2007–2009. A total of 1,797 adult subjects (1,091 whites and 706 blacks; 44.4% males; age range = 21–48 years; mean age = 31.5 years) who have been examined 4–8 times during childhood (age range = 4–19 years; mean age = 12.4 years), with 9,027 observations of BP, formed the study cohort for this report. The number of individuals who had been examined four, five, six, seven, and eight times during childhood was 726, 588, 269, 143, and 71, respectively; the average number of BP measurements was 5.0 times/person. All subjects in this study gave informed consent at each examination, and for those under 18 years of age, consent of a parent/guardian was obtained. Study protocols were approved by the Institutional Review Board of the Tulane University Health Sciences Center.
Body mass and BP measurements
Identical protocols were used by trained examiners across all surveys since 1973.26 Replicate measurements of height and weight were made, and the mean values were used for analysis. Body mass index (BMI, weight in kilograms divided by the square of the height in meters) was used as a measure of obesity. In all surveys, BP levels were measured between 8:00 am and 10:00 am on the right arm of subjects in a relaxed, sitting position by two trained observers (three replicates each). The mean values of the six were used for analysis. SBP and diastolic BP (DBP) were recorded using a mercury sphygmomanometer. The fourth Korotkoff phase was used for DBP for children and adults because the fourth phase is more reliably measured in childhood and more predictive of adult hypertension.27 Hypertension in adults was defined as SBP ≥140 mm Hg or DBP ≥90 mm Hg or taking antihypertensive medications at the time of any examination as adults.
Statistical methods
Standard deviation of multiple serial measurements of BP during childhood was calculated for each individual and used as an index of long-term variability of SBP and DBP. In order to examine the independent effect of BP variability on adult hypertension, mean levels of multiple measurements of BP in childhood were included in all the regression models for adjustment. Long-term variability of BMI in childhood was calculated as s.d. The s.d. of BMI was adjusted for mean childhood BMI by regression residual analyses before the association analyses.
Analyses of covariance were performed using general linear models to test differences in study variables between blacks and whites and between males and females. The relationships of BP variability in childhood to hypertension in adulthood were examined by multivariable logistic regression models using adulthood hypertension as a dichotomous dependent variable, adjusting for race, sex, adulthood BMI, adulthood age, mean childhood age, mean value-adjusted s.d. of childhood BMI, and mean childhood levels of BP. The above covariates except for race and sex were standardized into Z-scores with mean = 0 and variance = 1 before logistic regression analyses to make odds ratios (ORs) comparable, particularly for BP levels and variability. The logistic regression analyses were also performed by race and sex groups. Race–BP and sex–BP interactions were tested by including respective interaction terms in the logistic regression models. In addition, multivariable linear regression models were performed using adulthood BP levels as continuous dependent variables, and s.d.s of BP as predictor variables, adjusting for the above covariates. Standardized regression coefficients were estimated to facilitate comparisons of effects of mean childhood BP levels against BP variability. All P values presented are two sided.
Results
Table 1 shows the mean levels of study variables in childhood and adulthood by race and sex. In general, sex and race differences in BP levels became greater with increasing age. The mean number of BP measurements was 5.0 (white males), 4.9 (white females), 5.2 (black males), and 5.1 (black females). The s.d. of SBP showed significant race (blacks > whites) and sex (males > females) differences in blacks; s.d. of DBP did not show significant sex and race differences. Adulthood SBP and DBP levels were significantly higher in males and blacks than females and whites, respectively, after excluding 137 individuals who were taking antihypertensive medications at the time of examination. The overall prevalence of hypertension was 14.1% in adulthood (n = 253), with whites having a lower prevalence than blacks (10.5% vs. 19.6%, P < 0.001).
Table 1.
Means (s.d.) of study variables in childhood and adulthood by race and sex
| Male | Female | Sex Difference | ||||
|---|---|---|---|---|---|---|
| White (n = 497) | Black (n = 300) | White (n = 594) | Black (n = 406) | White | Black | |
| Childhood first | ||||||
| Age (year) | 9.1 (2.9) | 9.1 (2.9) | 8.9 (2.9) | 8.7 (2.8) | 0.264 | 0.069 |
| SBP (mm Hg) | 99.1 (9.5) | 98.3 (10.1) | 97.5 (9.6) | 96.7 (9.8) | 0.013 | 0.155 |
| DBP (mm Hg) | 60.6 (8.2) | 61.4 (7.8) | 60.1 (8.2) | 60.4 (8.1) | 0.608 | 0.290 |
| Childhood last | ||||||
| Age (year) | 17.9 (2.2) | 18.1 (2.0) | 17.9 (2.5) | 17.9 (2.0) | 0.830 | 0.154 |
| SBP (mm Hg) | 113.6 (10.0) | 115.9 (11.1)** | 108.5 (9.3) | 110.6 (9.2)** | <0.001 | <0.001 |
| DBP (mm Hg) | 69.8 (8.1) | 69.5 (8.5) | 69.3 (8.1) | 70.2 (8.4) | 0.243 | 0.165 |
| Childhood BP variability (mm Hg) | ||||||
| s.d. of SBP | 8.3 (3.6) | 9.7 (3.9)** | 7.4 (3.0) | 8.2 (3.5)** | <0.001 | <0.001 |
| s.d. of DBP | 6.9 (2.8) | 6.7 (2.7) | 7.1 (2.8) | 7.2 (3.0) | 0.230 | 0.076 |
| Adulthood | (n = 462)a | (n = 273)a | (n = 564)a | (n = 361)a | ||
| Age (year) | 32.4 (7.4) | 28.9 (7.3)** | 31.8 (7.4) | 30.2 (7.2)* | 0.130 | 0.024 |
| BMI (kg/m2) | 27.5 (5.5) | 26.8 (6.3) | 26.4 (6.9) | 29.1 (8.3)** | <0.001 | <0.001 |
| SBP (mm Hg) | 115.6 (10.3) | 118.7 (13.8) | 108.3 (10.3)** | 112.9 (13.7)** | <0.001 | <0.001 |
| DBP (mm Hg) | 76.8 (8.5) | 76.5 (10.9) | 72.0 (7.9)** | 74.1 (10.3)** | <0.001 | <0.001 |
BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Individuals (n = 137) taking antihypertensive medications were excluded. Racial difference within sex groups:
P < 0.05;
P < 0.01.
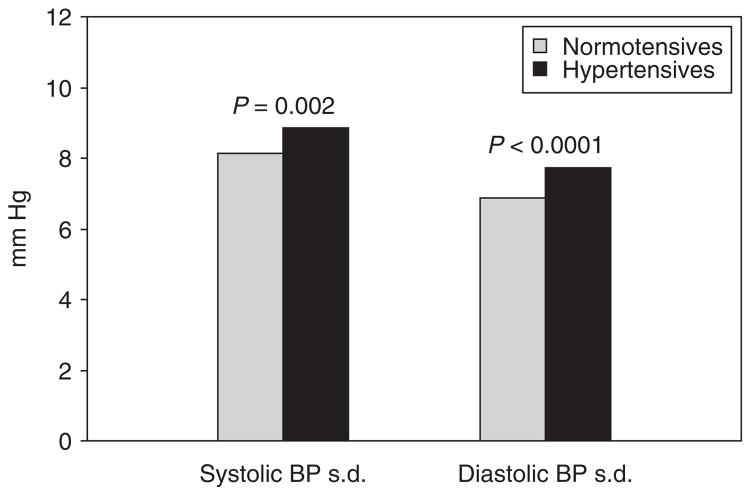
Figure 1 shows covariates-adjusted s.d. of SBP and DBP in childhood by hypertension status in adulthood. The covariates included race, sex, adulthood BMI, adulthood age, mean childhood age, childhood BMI variability, and mean childhood BP levels. Hypertensives had significantly higher values of s.d. of both SBP and DBP than normotensives. Furthermore, the mean childhood BP levels were significantly correlated with s.d. for SBP (r = 0.151, P < 0.001), but not for DBP (r = −0.020, P = 0.399). In addition, the trends of the differences in childhood BP variability between hypertensives and normotensives within sex or race subgroups were similar to those in the total sample although the differences became nonsignificant in some subgroups due to reduced sample size (data not shown).
Figure 1.
Covariates-adjusted standard deviation of BP during childhood by hypertension status in adulthood: The Bogalusa Heart Study. Covariates included race, sex, adulthood BMI, adulthood age, mean childhood age, standard deviation of childhood BMI and mean childhood BP levels. BMI, body mass index; BP, blood pressure.
Table 2 shows the association of BP variability in childhood with hypertension and BP levels in adulthood measured by ORs and standardized regression coefficients (β) of childhood BP s.d. (Z-scores) derived in logistic and linear regression models, respectively, adjusting for covariates. Adults were 1.28 or 1.36 times likely to have hypertension when the variability of SBP or DBP, respectively, increased 1 standardized unit (1 s.d.) during childhood based on the ORs derived from logistic regression models. In linear regression analyses, s.d.s of SBP and DBP were both significantly associated with adulthood SBP and DBP levels, respectively. Of note, despite the significant associations of childhood BP variability with adult hypertension status and BP levels, the association parameters (ORs and βs) of BP variability were considerably and consistently lower than those of mean childhood BP levels.
Table 2.
Association of s.d. of blood pressure during childhood with hypertension and blood pressure levels in adulthood, adjusting for covariates in logistic and linear regression models
| Logistic regression | Linear regression | |
|---|---|---|
| Dependent variable: adulthood hypertension | Dependent variable: adulthood SBP levels | |
| OR (95%CI) | β; P value | |
| Female sex | 0.68* (0.49, 0.93) | −0.201; <0.001 |
| Black race | 2.63** (1.90, 3.65) | 0.150; <0.001 |
| Mean childhood agea | 0.65** (0.53, 0.81) | −0.111; <0.001 |
| Adulthood agea | 2.40** (1.96, 2.95) | 0.194; <0.001 |
| Adulthood BMIa | 1.93** (1.64, 2.28) | 0.249; <0.001 |
| s.d. of childhood BMIb | 0.82* (0.69, 0.98) | 0.001; 0.962 |
| Mean childhood SBPa | 2.09** (1.74, 2.51) | 0.372; <0.001 |
| s.d. of childhood SBPa | 1.28** (1.09, 1.51) | 0.086; <0.001 |
| Dependent variable: adulthood hypertension | Dependent variable: adulthood DBP levels | |
| Female sex | 0.44** (0.32, 0.60) | −0.245; <0.001 |
| Black race | 2.71** (1.96, 3.74) | 0.090; 0.018 |
| Mean childhood agea | 0.68 (0.55, 0.84) | −0.187; <0.001 |
| Adulthood agea | 2.22** (1.81, 2.71) | 0.331; <0.001 |
| Adulthood BMIa | 2.07** (1.77, 2.42) | 0.260; <0.001 |
| s.d. of childhood BMIb | 0.80* (0.67, 0.95) | −0.018; 0.438 |
| Mean childhood DBPa | 1.91** (1.59, 2.29) | 0.355; <0.001 |
| s.d. of childhood DBPa | 1.36** (1.16, 1.58) | 0.105; <0.001 |
β, standardized regression coefficient; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; OR, odds ratio; SBP, systolic blood pressure.
Standardized into Z-scores with mean = 0 and variance = 1.
Mean BMI-adjusted and standardized into Z-scores (mean = 0 and variance = 1).
P < 0.05;
P < 0.01.
Table 3 presents the ORs and βs of childhood BP variability for adulthood hypertension and BP levels by race or sex groups, adjusting for the same covariates included in Table 2. The association parameters were all significant except the ORs of s.d. of SBP in whites and males. Further, the association of childhood BP variability with adulthood hypertension and BP levels did not differ significantly between sex or race subgroups as tested in the interaction models.
Table 3.
Association of s.d. of childhood blood pressure with hypertension and blood pressure levels in adulthood, adjusting for covariates in logistic and linear regression models by race or sex
| Whites (OR, β) | Blacks (OR, β) | Males (OR, β) | Females (OR, β) | |
|---|---|---|---|---|
| s.d. of childhood SBPa | 1.23, 0.11** | 1.34**, 0.10** | 1.16, 0.11** | 1.44**, 0.10** |
| s.d. of childhood DBPa | 1.38**, 0.12** | 1.31*, 0.12** | 1.28*, 0.12** | 1.36**, 0.12** |
The same covariates (except race and sex) as listed in Table 2.
β, standardized regression coefficient; DBP, diastolic blood pressure; OR, odds ratio; SBP, systolic blood pressure.
Standardized into Z-scores with mean = 0 and variance = 1.
P < 0.05;
P < 0.01.
Discussion
The significance of transient hypertension has long been a topic of discussion. As early as in 1921, excessive variability of BP with temporary rises above the usual normal was found in the early stages of hypertension in two independent studies, and then data emerging in 1940s were in support of the view that transient elevation of BP, induced by emotion or other stimuli, should be regarded as evidence of a possible prehypertensive state.15–17 Of particular interest, the importance of the transient BP elevations as a predictor of later fixed hypertension was suggested in a large study of 22,741 army officers conducted during 1944–1945.15,16 Muscatine study examined the level, trend, and variability of BP during childhood over 5–6 years and found that 7.4 and 7.2% of children had high levels and variability of SBP and DBP, respectively; however, the association between childhood BP variability and future hypertension as adults was not examined.28 In this study, we demonstrated that adult hypertension was associated with BP variability in terms of s.d. of serial BP measurements during childhood, even after adjusting for the mean childhood BP levels. Previous studies have shown that greater variability either over 24 h29,30 or over a prolonged period15,16 is associated with hypertension during adulthood. However, data on the relationship between BP variability in childhood and adult hypertension are not available for comparison in spite of the overwhelming evidence for the prediction of elevated childhood BP levels for adult hypertension.23–25
In previous studies, BP levels have been reported to be highly correlated with the magnitude of BP variability with respect to both long-term3 and short-term31 measurements. We also observed a significantly positive correlation between the level and variability for SBP (r = 0.151, P < 0.001) in this study. The observed significant correlation, although low, indicated the need of adjustment for the mean childhood BP levels in the BP variability analysis. After adjustment for the mean childhood BP levels, the increased childhood BP variability was still associated with adult hypertension status. However, childhood BP variability appeared to be less predictive for adult hypertension than elevated childhood BP levels. Further studies are needed in this important and interesting research area.
The relationships of long-term BP variability to cardiovascular2– 4 and cerebrovascular5–9 disease have been demonstrated in previous studies, using various indices of long-term BP variability. A coefficient of variation of serial office BP measurements over a period of 12 months, calculated as s.d./mean, was used in a Japanese population to examine the predictive value for stroke and myocardial infarction.2,5 This measure is identical to the index of long-term BMI variability employed in the Framingham Heart Study.32 In fact, the coefficient of variation, by its definition, is the s.d. corrected for the mean before further analyses. In this study, the mean childhood BP values were included in the model for adjustment. By using the model adjustment, not only the effect of BP variability independent of the mean BP levels can be examined, but also the contributions of BP levels vs. variability to the development of hypertension can be compared using standardized scales. In the Framingham Heart Study cohort, various indices of BP variability were evaluated using eight repeated measurements of BP over a 14-year period. Variance and s.d. of individual’s multiple BP values proved to be better predictors for the future coronary heart disease than other indices.4 Another reason for choosing s.d. as a measure of BP variability over time is that this measure has been widely used in this regard.2,4,5,7–9,18,20
It is well known that blacks vs. whites have higher BP levels and prevalence of hypertension.33 The racial difference in BP was observed even in children, with blacks showing higher mean levels and a faster rate of change.34,35 In this study blacks also had significantly higher long-term SBP variability in terms of s.d. during childhood; however, the strength of the association between childhood BP variability and adult hypertension did not differ significantly between blacks and whites, indicating that the BP variations in early life contribute to the development of hypertension equally in both races. To the best of our knowledge, information on the relationship between BP variability in childhood and hypertension in adulthood is very limited. Therefore, the findings on racial contrasts from this study need to be confirmed and replicated in other population studies. In particular, black–white differences in the impact of neural and hormonal regulations and urine electrolyte excretions on the within-individual variability of BP need to be elucidated. Studies have shown that BP and heart rate variabilities were positively correlated to each other, suggesting a primary role of central nervous mechanisms in regulating these hemodynamic parameters.1,30 In addition, the genetic background may also play an important role in fluctuations of BP over time. Berg36 proposed the concept of “variability gene” to distinguish the variability gene effect from the level gene effect. Based on this concept, the genes, whose expression depends on environmental exposures, are referred to as “variability genes”. The notion of “variability gene” for BP is supported by observations from longitudinal family studies.37,38 The genetic background of BP variability and black–white contrasts regarding the gene–environment interaction is a promising research area of interest although our observational study cannot address this issue.
This community-based study has certain limitations. First, the present longitudinal study cohort has been identified by linking multiple cross-sectional surveys of children and adults. Different number of BP measurements during childhood (4–8 times) among study subjects might have a confounding effect on BP variability analysis. For this reason, we also analyzed s.d. of BP using the first four BP measurements in childhood. The analyses yielded similar results. Second, usual techniques of the BP measurement in clinical settings reflect some degree of environmental stress and therefore would be expected to exhibit greater variability than corresponding home BP values. Third, the BP measurement error was not taken into account in this study under the assumption that the deviation of errors from the “true value” is normally distributed.
In summary, blacks showed greater long-term BP variability during childhood than whites, and the BP variability is a significant predictor of adult hypertension, independently of mean childhood BP levels. Investigation of the BP variability as a new phenotype may yield important information about the etiology and pathogenesis of hypertension and related disorders, and thus its prevention.
Acknowledgments
This study was supported by grants HD-061437 and HD-062783 from the National Institute of Child Health and Human Development, 0855082E from American Heart Association, 546145G1 from Tulane University, and AG-16592 from the National Institute on Aging.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension. 1995;25:1276–1286. doi: 10.1161/01.hyp.25.6.1276. [DOI] [PubMed] [Google Scholar]
- 2.Hata Y, Muratani H, Kimura Y, Fukiyama K, Kawano Y, Ashida T, Yokouchi M, Imai Y, Ozawa T, Fujii J, Omae T. Office blood pressure variability as a predictor of acute myocardial infarction in elderly patients receiving antihypertensive therapy. J Hum Hypertens. 2002;16:141–146. doi: 10.1038/sj.jhh.1001301. [DOI] [PubMed] [Google Scholar]
- 3.Grove JS, Reed DM, Yano K, Hwang LJ. Variability in systolic blood pressure–a risk factor for coronary heart disease? Am J Epidemiol. 1997;145:771–776. doi: 10.1093/oxfordjournals.aje.a009169. [DOI] [PubMed] [Google Scholar]
- 4.Hathaway DK, D’Agostino RB. A technique for summarizing longitudinal data. Stat Med. 1993;12:2169–2178. doi: 10.1002/sim.4780122303. [DOI] [PubMed] [Google Scholar]
- 5.Hata Y, Kimura Y, Muratani H, Fukiyama K, Kawano Y, Ashida T, Yokouchi M, Imai Y, Ozawa T, Fujii J, Omae T. Office blood pressure variability as a predictor of brain infarction in elderly hypertensive patients. Hypertens Res. 2000;23:553–560. doi: 10.1291/hypres.23.553. [DOI] [PubMed] [Google Scholar]
- 6.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging study. Stroke. 2002;33:26–30. doi: 10.1161/hs0102.101890. [DOI] [PubMed] [Google Scholar]
- 7.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 8.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 9.Howard SC, Rothwell PM. Reproducibility of measures of visit-to-visit variability in blood pressure after transient ischaemic attack or minor stroke. Cerebrovasc Dis. 2009;28:331–340. doi: 10.1159/000229551. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Li S, Srinivasan SR, Boerwinkle E, Berenson GS. Autosomal genome scan for loci linked to blood pressure levels and trends since childhood: the Bogalusa Heart Study. Hypertension. 2005;45:954–959. doi: 10.1161/01.HYP.0000161881.02361.11. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Srinivasan SR, Li S, Boerwinkle E, Berenson GS. Gender-specific influence of NO synthase gene on blood pressure since childhood: the Bogalusa Heart Study. Hypertension. 2004;44:668–673. doi: 10.1161/01.HYP.0000145474.23750.2b. [DOI] [PubMed] [Google Scholar]
- 12.Pierdomenico SD, Di Nicola M, Esposito AL, Di Mascio R, Ballone E, Lapenna D, Cuccurullo F. Prognostic value of different indices of blood pressure variability in hypertensive patients. Am J Hypertens. 2009;22:842–847. doi: 10.1038/ajh.2009.103. [DOI] [PubMed] [Google Scholar]
- 13.Eguchi K, Ishikawa J, Hoshide S, Pickering TG, Schwartz JE, Shimada K, Kario K. Night time blood pressure variability is a strong predictor for cardiovascular events in patients with type 2 diabetes. Am J Hypertens. 2009;22:46–51. doi: 10.1038/ajh.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, de Leeuw PW, Jaaskivi M, Nachev C, Parati G, O’Brien ET, Tuomilehto J, Webster J, Bulpitt CJ, Fagard RH Syst-Eur investigators. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21:2251–2257. doi: 10.1097/00004872-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Levy RI, Stroud WD, White PD. Transient hypertension: Its significance in terms of later development of sustained hypertension and cardiovascular-renal diseases. JAMA. 1944;126:829–833. [Google Scholar]
- 16.Levy RI, White PD, Stroud WD. Transient hypertension: The relative prognostic importance of various systolic and diastolic levels. JAMA. 1945;128:1059–1061. [Google Scholar]
- 17.Hines EA., Jr Range of normal blood pressure and development of hypertension: a follow-up study of 1,522 patients. JAMA. 1940;115:271. [Google Scholar]
- 18.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 19.White WB. Importance of blood pressure control over a 24-hour period. J Manag Care Pharm. 2007;13(suppl S-b):S34–S39. doi: 10.18553/jmcp.2007.13.s8-b.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Poulter NR, Sever PS ASCOT-BPLA and MRC Trial Investigators. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480. doi: 10.1016/S1474-4422(10)70066-1. [DOI] [PubMed] [Google Scholar]
- 21.Voors AW, Berenson GS. Search for the determinants of the early onset of essential hypertension. In: Onesti G, Kim KE, editors. Phasic Pressor Mechanisms: Hypertension in the Young and the Old. Grune and Stratton; New York: 1981. pp. 43–55. [Google Scholar]
- 22.Lever AF, Harrap SB. Essential hypertension: a disorder of growth with origins in childhood? J Hypertens. 1992;10:101–120. doi: 10.1097/00004872-199202000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Bao W, Threefoot SA, Srinivasan SR, Berenson GS. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa Heart Study. Am J Hypertens. 1995;8:657–665. doi: 10.1016/0895-7061(95)00116-7. [DOI] [PubMed] [Google Scholar]
- 24.Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633–641. [PubMed] [Google Scholar]
- 25.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berenson GS, McMahan CA, Voors AW, Webber LS, Srinivasan SR, Frank GC. The Early Natural History of Atherosclerosis and Essential Hypertension. Oxford University Press; Oxford: 1980. Cardiovascular Risk Factors in Children; pp. 47–123. [Google Scholar]
- 27.Elkasabany AM, Urbina EM, Daniels SR, Berenson GS. Prediction of adult hypertension by K4 and K5 diastolic blood pressure in children: the Bogalusa Heart Study. J Pediatr. 1998;132:687–692. doi: 10.1016/s0022-3476(98)70361-0. [DOI] [PubMed] [Google Scholar]
- 28.Lauer RM, Clarke WR, Beaglehole R. Level, trend, and variability of blood pressure during childhood: the Muscatine study. Circulation. 1984;69:242–249. doi: 10.1161/01.cir.69.2.242. [DOI] [PubMed] [Google Scholar]
- 29.Parati G, Mancia G. Blood pressure variability as a risk factor. Blood Press Monit. 2001;6:341–347. doi: 10.1097/00126097-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, Grassi G, di Rienzo M, Pedotti A, Zanchetti A. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res. 1983;53:96–104. doi: 10.1161/01.res.53.1.96. [DOI] [PubMed] [Google Scholar]
- 31.Watson RD, Stallard TJ, Flinn RM, Littler WA. Factors determining direct arterial pressure and its variability in hypertensive man. Hypertension. 1980;2:333–341. doi: 10.1161/01.hyp.2.3.333. [DOI] [PubMed] [Google Scholar]
- 32.Lissner L, Odell PM, D’Agostino RB, Stokes J, 3rd, Kreger BE, Belanger AJ, Brownell KD. Variability of body weight and health outcomes in the Framingham population. N Engl J Med. 1991;324:1839–1844. doi: 10.1056/NEJM199106273242602. [DOI] [PubMed] [Google Scholar]
- 33.Cornoni-Huntley J, LaCroix AZ, Havlik RJ. Race and sex differentials in the impact of hypertension in the United States. The National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Intern Med. 1989;149:780–788. [PubMed] [Google Scholar]
- 34.Voors AW, Berenson GS, Dalferes ER, Webber LS, Shuler SE. Racial differences in blood pressure control. Science. 1979;204:1091–1094. doi: 10.1126/science.451554. [DOI] [PubMed] [Google Scholar]
- 35.Manatunga AK, Jones JJ, Pratt JH. Longitudinal assessment of blood pressures in black and white children. Hypertension. 1993;22:84–89. doi: 10.1161/01.hyp.22.1.84. [DOI] [PubMed] [Google Scholar]
- 36.Berg K. Molecular genetics and genetic epidemiology of cardiovascular diseases and diabetes. Introductory remarks: risk factor levels and variability. Ann Med. 1992;24:343–347. doi: 10.3109/07853899209147835. [DOI] [PubMed] [Google Scholar]
- 37.Friedlander Y, Austin MA, Newman B, Edwards K, Mayer-Davis EI, King MC. Heritability of longitudinal changes in coronary-heart-disease risk factors in women twins. Am J Hum Genet. 1997;60:1502–1512. doi: 10.1086/515462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke WR, Schrott HG, Burns TL, Sing CF, Lauer RM. Aggregation of blood pressure in the families of children with labile high systolic blood pressure. The Muscatine Study. Am J Epidemiol. 1986;123:67–80. doi: 10.1093/oxfordjournals.aje.a114225. [DOI] [PubMed] [Google Scholar]