Summary
The major part of biological nitrogen fixation is catalyzed by the molybdenum nitrogenase that carries at its active site the iron and molybdenum cofactor (FeMo-co). The nitrogen fixation (nif) genes required for the biosynthesis of FeMo-co are derepressed in the absence of a source of fixed nitrogen. The nifB gene product is remarkable because it assembles NifB-co, a complex cluster proposed to have a [6Fe-9S-X] composition, from simpler [Fe-S] clusters common to other metabolic pathways. NifB-co is a common intermediate of the biosyntheses of the cofactors present in the molybdenum, vanadium and iron nitrogenases. In this work, the expression of the Azotobacter vinelandii nifB gene was uncoupled from its natural nif-regulation to show that NifB protein levels are lower in cells growing diazotrophically than in cells growing at the expense of ammonium. A. vinelandii carries a duplicated copy of the ATPase component of the ubiquitous ClpXP protease (ClpX2) which is induced under nitrogen fixing conditions. Inactivation of clpX2 resulted in the accumulation of NifB and NifEN and a defect in diazotrophic growth, especially when iron was in short supply. Mutations in nifE, nifN and nifX and in nifA also affected NifB accumulation, suggesting that NifB susceptibility to degradation might vary during its catalytic cycle.
Introduction
The major part of biological nitrogen fixation is catalyzed by the molybdenum nitrogenase, although some nitrogen-fixing bacteria additionally contain alternative vanadium or iron-only nitrogenases that are expressed when molybdenum is not available (Eady, 1996). The molybdenum nitrogenase is composed of two proteins that can be separately purified: dinitrogenase and dinitrogenase reductase. Dinitrogenase, is a 220-to 240-kDa tetramer of the nifD and nifK gene products that contains two pairs of two complex metalloclusters. Dinitrogenase reductase, is a 60-kDa dimer of the product of the nifH gene, which contains a single [4Fe-4S] cluster and two Mg-ATP binding sites and is the obligate electron donor to the molybdenum dinitrogenase. The molybdenum nitrogenase carries at its active site a complex iron-molybdenum cofactor (FeMo-co) composed of seven Fe, nine S, one Mo, one homocitrate, and one light atom proposed to be C, N or O (Einsle et al., 2002, Lukoyanov et al., 2007).
Genetic and biochemical studies in free-living diazotrophic bacteria, mainly Azotobacter vinelandii and Klebsiella pneumoniae, have shown that a number of nitrogen fixation (nif) genes are involved in FeMo-co biosynthesis and the formation of an active nitrogenase enzyme. Among them, nifB has been shown to be crucial for global nitrogen fixation because its product, the SAM-radical enzyme NifB, catalyzes the assembly of NifB-co, which is a common precursor for the biosyntheses of FeMo-co and the analogous iron-vanadium (FeV) and iron-only (FeFe) cofactors, carried by the vanadium and the iron-only nitrogenases, respectively (Bishop and Joerger, 1990; Curatti et al., 2006 and 2007).
NifB-co has been proposed to comprise the [6Fe-9S-X] core of FeMo-co (George et al., 2008). Maturation of NifB-co into FeMo-co requires its transfer to the NifEN scaffold protein, onto which additional Fe, the Mo atom, and homocitrate are incorporated into the precursor to generate FeMo-co in a series of reactions, some of which require NifH, Mg-ATP and reductant (see Rubio and Ludden, 2008 for a review).
Because NifB catalyzes the first committed step in dinitrogenase cofactor biosynthesis, cellular NifB levels and activity are expected to be tightly regulated. The expression of nifB has been shown to be under the regulation of the transcriptional activators from the three nitrogenase systems, NifA, VnfA and AnfA (Drummond et al., 1996). However, the existence of additional mechanisms regulating the levels of NifB, is not known.
In A. vinelandii and other diazotrophs, duplicated copies of housekeeping genes have acquired nif regulation to boost or gain specific functions during nitrogen fixation. Two well-studied examples are the nifUS genes, which are homologous to the house keeping iscUS genes, and the rnf1 gene cluster, which is homologous to the rnf2 and to the NADH-ubiquinone oxidoreductase gene clusters. NifU and NifS appear to boost the biosynthesis of [Fe-S] clusters for nitrogenase proteins (Dos Santos et al., 2007, Zhao et al., 2007), whereas rnf1 has acquired roles in modulation of nif genes expression and in NifH maturation (Curatti et al., 2005).
The A. vinelandii genome carries two ClpX-like encoding sequences in the open reading frames Avin23580 and Avin01700. Avin23580 might represent the house keeping clpX copy since it is located in a similar genetic context as clpX genes from non-diazotrophic bacteria and its product exhibits higher similarity to the ClpX subunit of ClpXP proteases. ClpXP is an ATP-dependent protease that is widespread in nature and shares a basic mechanism with the proteasome of eukaryotic organisms (Dougan et al., 2002; Zolkiewski, 2006). The ClpX component is a hexameric AAA+ATPase responsible for substrate recognition, unfolding, and translocation into ClpP, a serine peptidase. ClpX can also act independently to dismantle multimers and remodel proteins (Kim et al., 2000).
On the other hand, Avin01700, hereafter denominated as clpX2, is located between the nifM and nifF genes in the major nif cluster in A. vinelandii (Setubal et al., 2009). Homologs to clpX2 have also been found within nif gene clusters in other diazotrophs. ClpX2 is not essential for nitrogen fixation since disruption of the clpx2 gene in A. vinelandii resulted in a Nif+ phenotype (Jacobson et al., 1989). On the other hand, transposon mutagenesis of a house-keeping type clpX in Azospirillum brasilense resulted in a 3-fold increase in nitrogenase activity among other pleiotropic effects (Rodriguez et al., 2006).
Here we demonstrate that the A. vinelandii ClpX2 protein is part of a nitrogen source-dependent regulatory mechanism that controls the cellular levels of NifB and NifEN proteins by means of protein degradation. Thus, a regulatory layer superimposed on transcriptional control exists in the model diazotrophic organism A. vinelandii to fine tune nitrogenase cofactor biosynthesis.
Results
NifB stability is influenced by the nitrogen source
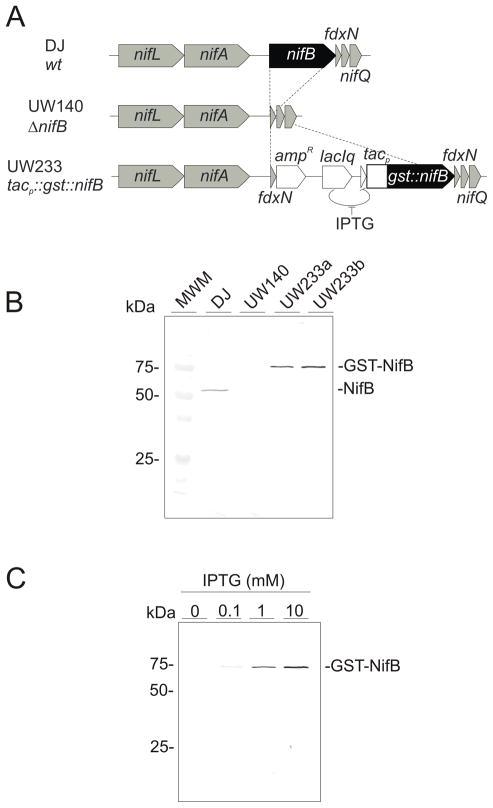
As part of our studies to understand the function and regulation of NifB in A. vinelandii, we constructed an A. vinelandii conditional Nif-defective strain (UW233) in which the original nifB gene had been replaced by a gst::nifB chimeric gene under the control of an IPTG-inducible promoter (Fig. 1A). UW233 was unable to grow diazotrophically unless the medium was supplemented with IPTG, as expected (Fig. S1). The accumulation of GST-NifB in UW233, upon IPTG induction, was verified by immunoblots developed with antibodies to NifB (Fig. 1B). This strategy allowed controlling GST-NifB accumulation in the cells in response to the concentration of IPTG added to the medium (Fig. 1C).
Fig. 1.
Artificially controlled expression of NifB in A. vinelandii. A, comparison of physical maps of A. vinelandii chromosomal regions around the nifB locus in the wild-type strain (DJ), a ΔnifB strain (UW140), and an IPTG-controlled gst::nifB-expressing strain (UW233). B, Immunoblot developed with antibodies to NifB showing accumulation of NifB or GST-NifB in A. vinelandii strains, DJ, UW140 and two clones of strain UW233. All strains were derepressed for nitrogen fixation during 4 h; in addition, UW233 cultures were treated with 1 mM IPTG. C, GST-NifB accumulation in strain UW233 under nitrogenase derepressing conditions in medium supplemented with increasing concentrations of IPTG.
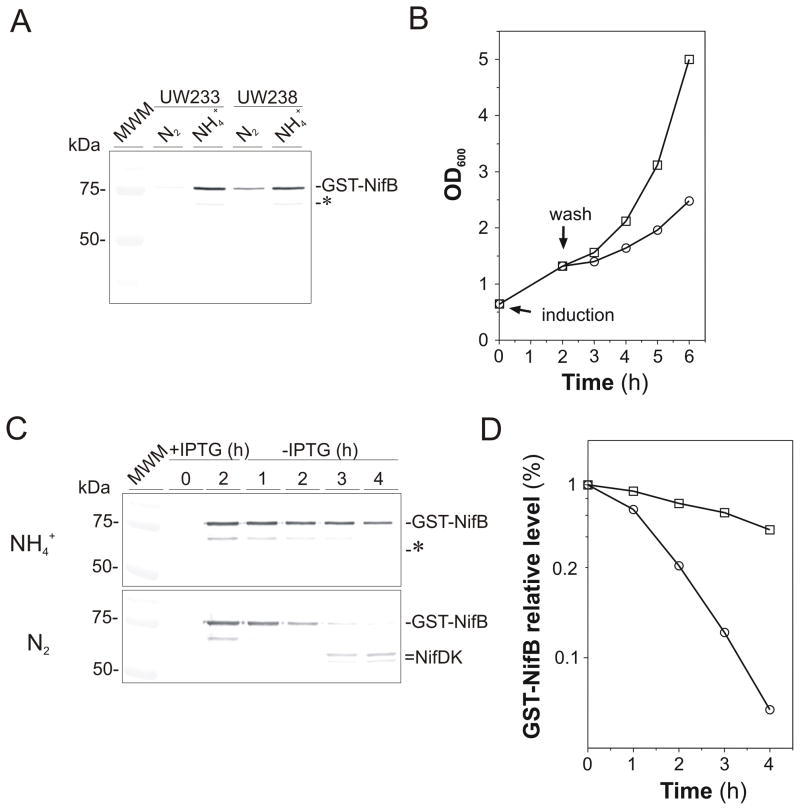
Induction of gst::nifB expression by IPTG addition showed that GST-NifB accumulated to higher levels in A. vinelandii cells growing with ammonium as nitrogen source than in cells growing diazotrophically (Fig. 2A). To determine whether this difference in GST-NifB accumulation was due to changes in NifB stability, we investigated the changes in GST-NifB degradation rates according to the nitrogen source used by the cells. UW233 cells growing with ammonium as nitrogen source were loaded with GST-NifB by supplementing the medium with 10 mM IPTG for 2 h. UW233 cells were then collected, washed with medium lacking ammonium and IPTG, and then incubated in fresh medium lacking or containing ammonium. As shown in Fig. 2B, UW233 cells were able to grow diazotrophically at the expense of the accumulated GST-NifB protein. Immunoblot analysis showed that the degradation of GST-NifB was much slower in cells growing with ammonium as nitrogen source than in those growing diazotrophically (Fig. 2C). Depletion of GST-NifB was more pronounced by the time dinitrogenase polypeptides (NifDK) started to accumulate (Fig. 2C) and was almost undetectable 4 h after the onset of nitrogenase derepressing conditions (Fig. 2D).
Fig. 2.
Differential degradation of GST-NifB under diazotrophic and non-diazotrophic growing conditions. A, Immunoblot analysis showing the IPTG-induced accumulation of GST-NifB in cells of A. vinelandii UW233 and UW238 strains growing under ammonium- or N2-dependent conditions. UW238 is a ΔnifENX::kn derivative of UW233. B, Growth curves of UW233 cells loaded with GST-NifB by IPTG induction in medium containing ammonium and then transferred to diazotrophic (circles) or ammonium-containing (squares) medium lacking IPTG. C, Degradation profile of GST-NifB protein accumulated in UW233 cells treated as described in B. For this analysis, the immunoblot was developed with a mixture of anti-NifB and anti-NifDK antibodies. The asterisk points to a GST-NifB-degradation product. D, Densitometric quantitation of GST-NifB disappearance under ammonium-dependent (squares) or diazotrophic (circles) growing conditions. This representative plot was generated by using the GST-NifB immunoblot shown in C.
Similarly, extracts obtained from A. vinelandii UW140 (ΔnifB) cells grown under diazotrophic conditions were shown to promote the in vitro degradation of GST-NifB present in extracts of IPTG-induced ammonium-grown UW233 cells. In vitro GST-NifB degradation rates were stimulated by the addition of ATP to the reaction mixtures (Fig. S2). Altogether, these results indicate that a proteolytic system involved in NifB degradation is induced under diazotrophic growth conditions.
NifB stability is influenced by mutations in nifENX and nifA
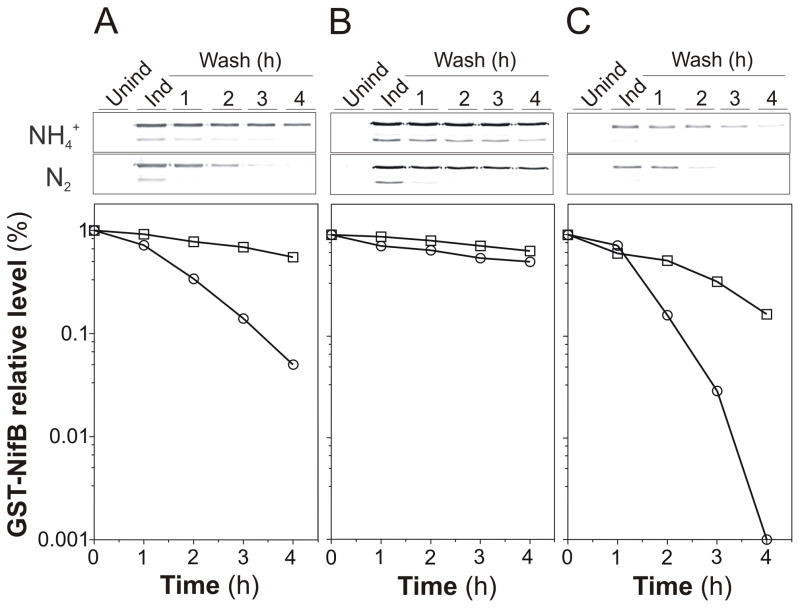
We had previously observed that NifB accumulated to a larger extent in a mutant strain lacking nifENX (UW235) than in the wild-type strain (Fig. S3). Uncoupling nifB expression from its natural nif regulation did not restore the wild-type phenotype (Fig. 2A), suggesting that the accumulation of NifB observed in the cells from ΔnifENX strains was due to increased NifB stability rather than higher nifB transcriptional rates. Moreover, the ΔnifENX strain exhibited low GST-NifB degradation rates regardless of the presence or absence of ammonium in the medium (Fig. 3B).
Fig. 3.
Effect of ΔnifENX and ΔnifA mutations on the degradation rates of GST-NifB protein. Immunoblot analyses of GST-NifB accumulated under diazotrophic or ammonium-amended medium in A. vinelandii strains UW233 (A), UW238 (B), and UW295 (C). UW238 is a ΔnifENX::kn derivative of UW233. UW295 is a ΔnifA::sp derivative of UW233. A. vinelandii cells cultured in medium containing ammonium (Unind) were induced for GST-NifB expression during 2 h by adding IPTG to the medium (Ind). Cells were then treated as described in Fig. 2B and analyzed by immunoblot developed with antibodies to NifB. The asterisk points to a GST-NifB-degradation product. Densitometric quantitations of GST-NifB disappearance under ammonium-dependent (squares) or diazotrophic (circles) growing conditions are shown for each strain.
The stability of GST-NifB was also investigated in the ΔnifA genetic background, in which expression of most (or all) major nif genes is abolished. Surprisingly, GST-NifB became more unstable in the absence of NifA, especially under diazotrophic growth conditions (Fig. 3C), which suggested that the nitrogen source-dependent proteolytic factor involved in NifB degradation was not the product of a NifA-dependent nif gene.
The A. vinelandii genome contains two copies of clpX-like genes
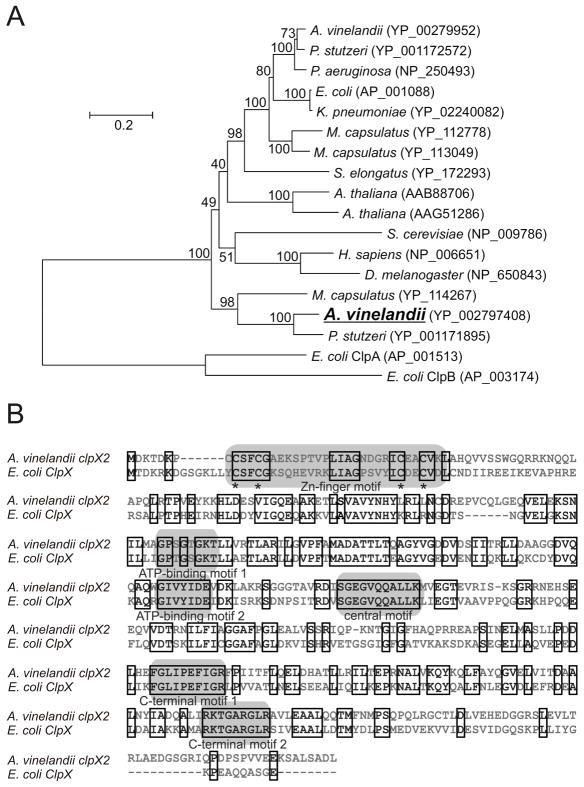
The phylogenetic analysis in Fig. 4A shows that, while the product of the A. vinelandii Avin23580 gene (clpX) is most similar to ClpX proteins found in diverse non-diazotrophic microorganisms, the product of Avin01700 (clpX2) is more similar to ClpX proteins present in other diazotrophs such as Pseudomonas stutzeri. In addition, both in A. vinelandii and in P. stutzeri, the clpX2 genes are located among characterized nif genes and, in the case of A. vinelandii, it was previously denominated as orf9.
Fig. 4.
A. vinelandii ClpX2 sequence analysis. A, Neighbour joining phylogenetic tree of the A. vinelandii ClpX2 polypeptide (shown in bold). Sequence data used to generate the tree were obtained from the NCBI nr database. Numbers adjacent to each node show the bootstrap percentage. B, Sequence alignment of the A. vinelandii ClpX2 and Escherichia coli ClpX polypeptides showing conservation of domains known to be relevant to E. coli ClpX function.
The predicted ClpX2 protein from A. vinelandii exhibited 50% sequence similarity to the Escherichia coli ClpX ortholog and contained the conserved the sequence motifs known to be essential to protease activity in E. coli ClpX (Fig. 4B). However, the C-terminal region of A. vinelandii ClpX2 was especially distinct from the described ClpX proteins.
A. vinelandii clpX2 expression is regulated by the nitrogen source
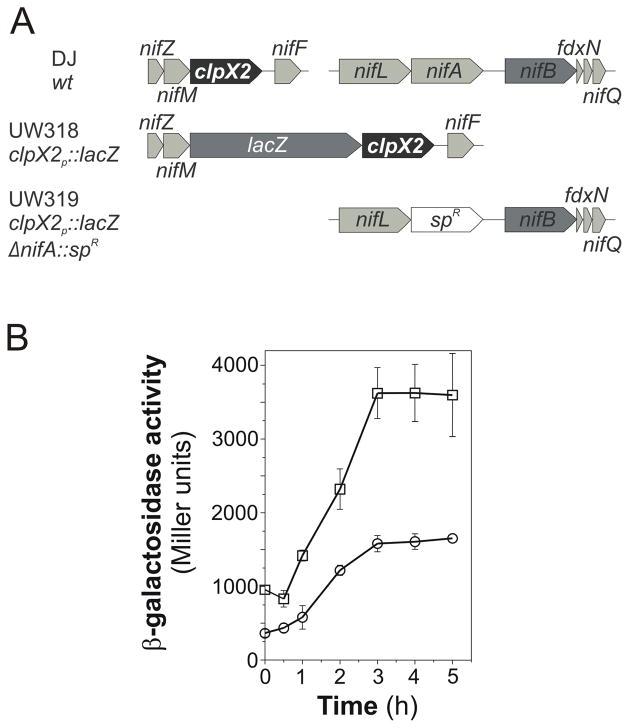
To study the expression of clpX2, A. vinelandii strains containing chromosomal transcriptional fusions between the sequence upstream of clpX2 and the lacZ reporter gene were generated (Fig. 5A). β-galactosidase activity assays revealed that ammonium removal from the medium increased clpX2 gene expression by 3.5-fold in a period of 3 h (Fig. 5B). To ascertain whether such increase was dependent on nifA, β-galactosidase activity was determined in a clpX2::lacZ reporter strain carrying a ΔnifA mutation (Fig. 5A). Interestingly, induction of clpX2 expression in the absence of ammonium appeared not to require NifA (Fig. 5B). Moreover, clpX2 expression is apparently higher in the absence of NifA. The molecular basis of this effect is not currently understood. Expression of clpX2 according to the nitrogen source and the existence of an mRNA species comprising at least part of nifM and clpX2 have been confirmed by semiquantitative RT-PCR (data not shown).
Fig. 5.
A. vinelandii clpX2 expression. A, physical map of the clpX2 locus in A. vinelandii strains DJ, UW318 and UW319. B, β-galactosidase activity following ammonium step-down in strains UW318 (open circles) and UW319 (open squares). Ammonium was removed from the medium at time zero.
ClpX2 is involved in the regulation of NifB and NifEN levels
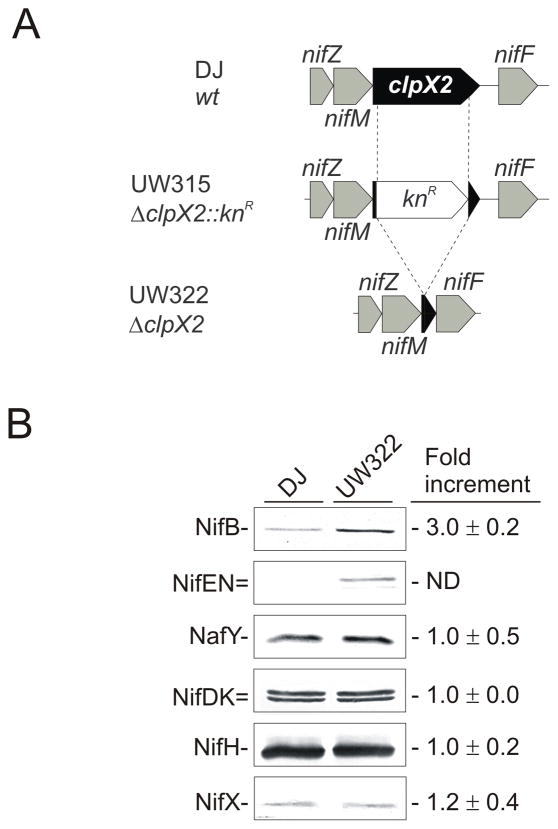
To investigate the possible participation of clpX2 in the nitrogen source-dependent proteolytic system responsible for NifB degradation, a mutant strain (UW322) bearing an in-frame deletion of most of the clpX2 gene was generated (Fig. 6A). Immunoblot analyses showed that, 3 h after ammonium removal from the medium, the NifB and NifEN polypeptide levels were significantly higher in the ΔclpX2 mutant strain than in the wild-type strain, while NifDK, NifH, NifX and NafY polypeptide levels remained almost unchanged (Fig. 6B). Relative NifB levels were 3-fold higher, whereas the difference in NifEN accumulation between the ΔclpX2 and the wild-type strains was even more remarkable, suggesting a participation of ClpX2 in NifB and NifEN polypeptide turnover.
Fig. 6.
Accumulation of Nif proteins in wild-type and clpX2 strains. A, Genetic map of A. vinelandii clpX2 region in the wild-type and the clpX2 strains. B, Immunoblot analyses of NifB, NifEN, NafY, NifDK, NifH and NifX proteins in cell-free extracts of wild-type and clpX2 mutant strains under nitrogenase-derepressing conditions. Representative immunoblots are shown. Changes in protein accumulation were estimated by densitometric quantification of immunoblots using the ImageJ software. Values are the mean and standard deviations of at least two independent experiments. N.D. means not determined due to the very low levels of NifEN in extracts of DJ strain.
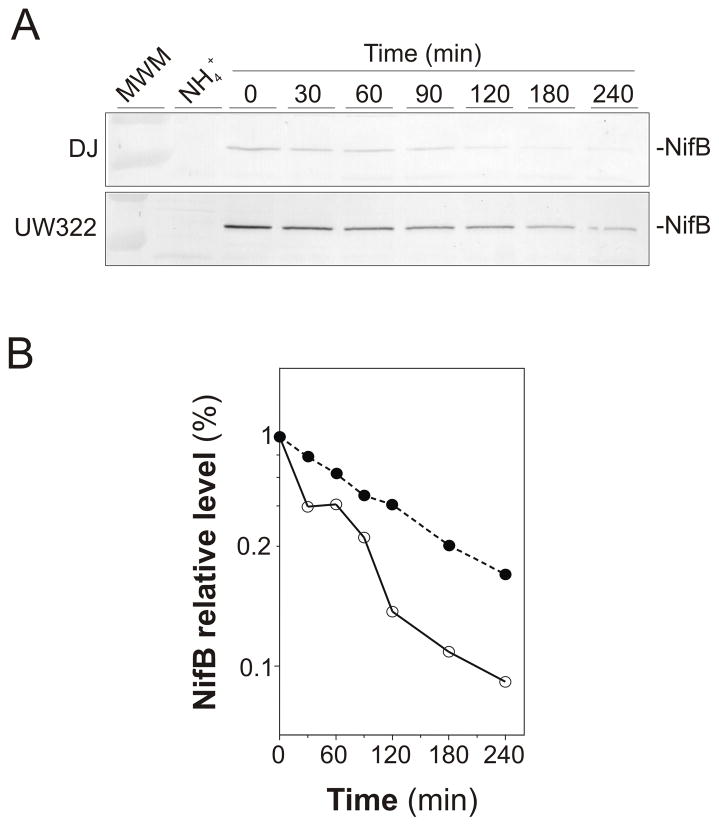
The in vivo decay of NifB polypeptides was analyzed by using A. vinelandii cells treated with spectinomycin 3 h after derepression of nif gene expression in order to stop protein synthesis. Fig. 7 shows that the degradation rate of NifB polypeptides in vivo was slower in the ΔclpX2 mutant than in the wild-type strain. In contrast, the degradation rates of NifD and NifK polypeptides were not affected by the ΔclpX2 mutation (Fig. S4).
Fig. 7.
Degradation of NifB polypeptide over time in the wild-type and the clpX2 strains. A, Time-course analysis of NifB protein accumulation in cell-free extracts from wild-type (DJ) and clpX2 strains derepressed for nitrogenase and treated with spectinomycin (10 μg.ml−1) to stop protein synthesis. Cell-free extracts obtained from cells grown with ammonium were used as controls. Immunoblots were developed with antibodies to NifB. A representative immunoblot is shown. B, Relative NifB levels in the wild-type (open circles) and the clpX2 (closed circles) strains estimated by densitometric quantification of immunoblots shown in A.
The A. vinelandii ΔclpX2 mutant strain exhibits a diazotrophic growth defect that is exacerbated under iron-limiting conditions
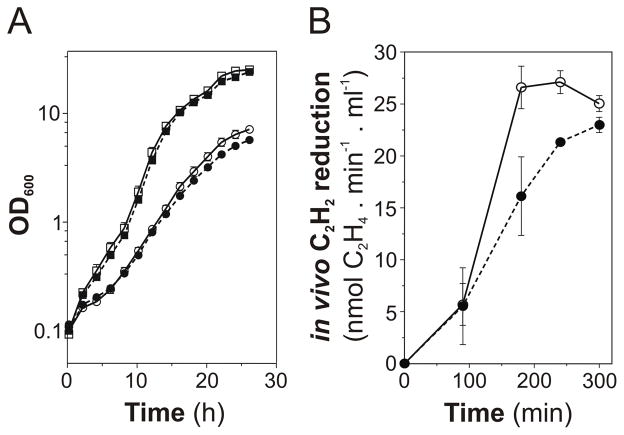
The growth of the ΔclpX2 mutant strain was identical to that of the wild type when using ammonium as nitrogen source. However, the ΔclpX2 mutant grew slightly slower than the wild type under diazotrophic conditions (Fig. 8A). Consistently, the onset of nitrogenase activity in vivo was slower in the ΔclpX2 mutant than in the wild-type strain (Fig. 8B).
Fig. 8.
Effect of deleting A. vinelandii clpX2 on diazotrophic growth and in vivo nitrogen fixation. A, Growth curves of A. vinelandii strains DJ (open symbols) and UW322 (closed symbols) using ammonium (squares) or N2 (circles) as nitrogen source. B, Time-course development of in vivo nitrogen fixation activity in A. vinelandii strains DJ (open symbols) and UW322 (closed symbols) estimated by the acetylene reduction assay. Data represent the mean and standard deviation of three independent experiments.
When determined in vitro at three hours from derepression, the levels of nitrogenase activity present in cell-free extracts of the wild-type andΔclpX2 strains were similar (Table 1). However, activity measurements of the individual components of nitrogenase showed that ΔclpX2 cells accumulated 40% more active NifDK component than the wild type while having similar levels of active NifH. No significant difference in apo-NifDK accumulation was evidenced by supplementing the cell free-extracts with purified FeMo-co.
Table 1.
Comparison of in vitro nitrogenase-component activities between the wild-type and the ΔclpX2 strains1
| DJ (wt) | UW322 ( clpX2) | |
|---|---|---|
| Nitrogenase | 40,62 ± 9,47 | 46,09 ± 12,13 |
| Dinitrogenase2 | 108,92 ± 17,67 | 151,89 ± 21,50 |
| Dinitrogenase reductase3 | 50,88 ± 1,26 | 58,04 ± 7,32 |
| FeMo-co4 | 37,05 ± 14,52 | 46,09 ± 7,31 |
nmol C2H4.min−1.mg protein−1
assayed in the presence of an excess of purified dinitrogenase reductase (NifH)
assayed in the presence of an excess of purified dinitrogenase (NifDK)
assayed in the presence of an excess of purified FeMo-co
We hypothesized that ClpX2 could confer A. vinelandii some advantage during diazotrophic growth under iron-limiting conditions because of the following reasons: (1) the ΔclpX2 mutant strain exhibited a subtle growth defect during late exponential phase of diazotrophic growth; (2) the levels of NifB and NifEN polypeptides are regulated by ClpX2, both of which are critical proteins for the mobilization of cellular iron towards FeMo-co biosynthesis; and (3) ClpX2 homologs are nutritional-stress associated proteins.
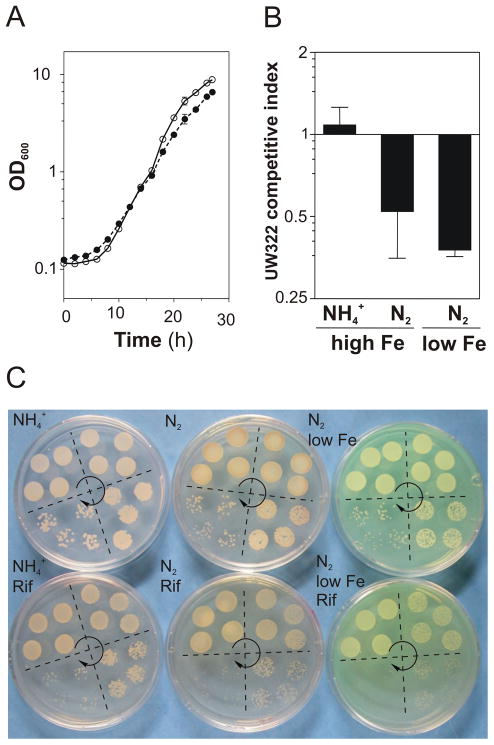
As shown in Fig. 9A, the ΔclpX2 mutant exhibited slower diazotrophic growth rates than the wild-type strain in medium containing low levels of iron (3 μM). The competitive performance of UW322 (ΔclpX2) versus the wild-type strain in different growing conditions is shown in Fig. 9B–C. The ΔclpX2 strain exhibited a competitive index of 1 when growing using ammonium as nitrogen source and 0.5 when growing diazotrophically. The UW322 competitive index was found to be even lower (0.3) when growing diazotrophically on iron-limited medium. This result supports the idea that ClpX2 confers A. vinelandii some advantage during diazotrophic growth, especially under iron limiting conditions.
Fig. 9.
Effect of deleting A. vinelandii clpX2 on diazotrophic growth under iron limiting conditions. A, Growth curves of A. vinelandii DJ (open symbols) and UW322 (closed symbols) under low-iron nitrogenase-derepressing conditions. B, Effect of diazotrophy and iron supply on the competitive index of UW322. Competitive indexes were obtained from mixed cultures (1:1 ratio) of DJ and UW322 strains. Data represent the mean and standard deviation of three independent experiments. C, Representative plates showing the development on solid medium of mixed cultures that were pre-grown in liquid medium for 22 h before plating. Four equivalent drops at different dilutions (10−3, 10−4, 10−5 or 10−6) of DJ and UW322 mixed cultures were plated on each quarter of the plates. Arrows show the direction of the dilutions. Cells were inoculated on media lacking rifampicin (to allow DJ and UW322 growth) and media supplemented with rifampicin (to allow only UW322 growth). Competitive growth was tested in ammonium-containing medium, ammonium-lacking medium, and low-iron (3 μM) ammonium-lacking medium.
Discussion
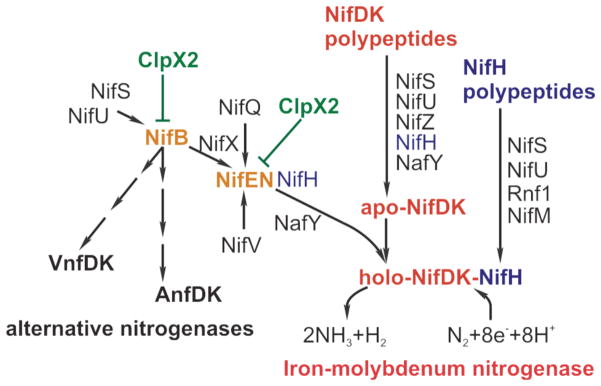
The assembly of active nitrogenase requires a multiplicity of gene products (Fig. 10). While some of them (NifU, NifS, NifB, NifX, NifE, NifN, NifQ, NifH, NifV, NafY and Rnf1) are involved in the biogenesis of either simple or complex [Fe-S] clusters for nitrogenase, others (NifZ, NifW, NafY, NifH and NifM) have roles in the maturation of the nitrogenase polypeptides. The transcription of these genes is coordinately regulated by the NifAL system in response to the presence of ammonium and oxygen (Martinez-Argudo et al., 2004).
Fig. 10.
Diagram of Nif-proteins participation in the formation of active nitrogenase in A. vinelandii. The function of Nif proteins has been reviewed recently (Rubio and Ludden, 2008). Color code: red, NifDK polypeptides; blue, NifH polypeptides; green, ClpX2; yellow, ClpX2 targets; black, other proteins involved in nitrogenase assembly.
The evidence presented in this work demonstrates the existence of another layer of regulation for the FeMo-co biosynthetic pathway in A. vinelandii. This regulatory mechanism comprises the proteolytic control of NifB and NifEN levels by a duplicated copy of ClpX that has gained functional specificity (Fig. 10). A proposed mechanism as a working hypothesis for future studies is that a ClpX2-dependent proteolytic pathway might help control the rate of FeMo-co synthesis, and hence nitrogenase activation, according to nutritional and/or environmental stimuli, as proposed here for iron limitation. This might operate to help allocate limiting nutrients to competing branches of metabolism to optimize balanced growth. Several lines of evidence are consistent with this proposal: i) compromised diazotrophic growth under Fe limiting conditions of the clpX2-defficient mutant strain, ii) nitrogen source dependent expression of clpX2, iii) changes in susceptibility to degradation of NifB ingenetic backgrounds that might change the flux of Fe through NifB (nifA and nifENX), iv) enhanced “in vitro” NifDK activity in clpX2 mutants. In addition, ClpX2 participation in the biosyntheses of the co-factors of the alternative nitrogenases is apparent through regulation of NifB.
ClpX and ClpP orthologs are found in most bacteria, mitochondria, and chloroplasts. In E. coli, ClpP-defective cells show delayed recovery both from stationary phase and following a shift to nutrient poor media. Proteomic analysis of cellular substrates of the ClpXP protease in E. coli has revealed five classes of ClpX-recognition signals (Flynn et al., 2003). No obvious matches were found in the A. vinelandii NifB, NifE or NifN polypeptides.
Unlike the widespread copy of clpXP, the clpX2 gene is not physically linked to a clpP homolog in the A. vinelandii chromosome (Setubal et al., 2009). An additional distantly-related clpP homolog (Avin02290) was identified in the genome, raising the question of which one is the protease-component partner of ClpX2 in this bacterium. Tentatively, ClpX2 could be a nitrogen fixation-specific component of a proteolytic complex with ClpP. On the other hand, the A. vinelandii genome contains homologs to the E. coli adaptor protein SspB, which fine-tunes bacterial stress responses by altering the degradation rates of protein targets by ClpXP, and homologs to the ATP-binding components ClpA and ClpB, as well as the ClpS adaptor protein, which recognizes subsets of specific targets.
Confirming previous mutational analysis (Jacobson et al., 1989), this study shows that clpX2 is not essential for diazotrophic growth of A. vinelandii cultured in Burk’s modified medium, indicating that ClpX2 does not play a critical role in nitrogenase assembly or function, as opposed to NifB, NifEN, or NifH. This is in line with the fact that clpX2 homologs are only present in some diazotrophic bacteria, such as P. stutzeri, which is closely related to A. vinelandii and carries a clpX2 gene in an identical genomic environment (nifM-clpX2-nifF) (Setubal et al., 2009; Yan et al., 2008).
Microarray analysis of genes expressed under nitrogen-fixing conditions in P. stutzeri showed nitrogen source-dependent expression of clpX2. The expression of clpX2 (PST1358) increased 8-fold during nitrogen fixation while decreased to half its value after subjecting diazotrophic cells to an ammonium shock (Yan et al., 2010). Sequence inspection suggested rather distal RpoN and NifA binding motifs in a putative promoter controlling the expression of nine orfs (PST1349 to PST1358) in P. stutzeri. On the other hand, we show here that in A. vinelandii NifA was not required for derepression of clpX2 expression after ammonium depletion. Future work should address the details of the ammonium-dependent regulation of A. vinelandii clpX2 expression.
The differential stability of NifB polypeptides in wild type, nifA and nifENX mutants could indicate the existence of different NifB conformations during the loading and unloading cycle of FeMo-co-precursors, as these conformations might become “frozen” in the different genetic backgrounds. For instance, the instability of GST-NifB in the nifA mutant could be due to its inability to induce transcription of other nif genes, such as nifU and nifS, which act as major providers of [Fe-S] cluster substrates for NifB activity (Zhao et al., 2007). On the other hand, inactivation of nifENX rendered a GST-NifB protein that was much more resistant to degradation. NifX and NifEN are proteins that bind to the metabolic product of NifB (NifB-co), possibly by interacting with NifB, and are involved in further processing of NifB-co into FeMo-co. The differential susceptibility of NifB to proteolysis in those genetic backgrounds that might alter the flux of FeMo-co precursors through NifB could help understand regulatory aspects of the first committed step in FeMo-co biosynthesis.
Disruption of a house keeping-like copy of clpX in Azospirillum brasilense resulted in an increased in vivo nitrogen-fixation activity among other pleiotropic mutant phenotypes (Rodriguez et al., 2006). The modest rise observed for NifDK nitrogenase-component activity in vitro of the A. vinelandii ΔclpX2 mutant strain could be due to enhanced NifB and NifEN activities when they were degraded at a lower rate. However, this increase did not result in higher nitrogen-fixation rates in vivo. Moreover, the A. vinelandii ΔclpX2 strain exhibited slightly lower in vivo nitrogen-fixation activity and diazotrophic growth rate, which was more noticeable when iron was limiting in the medium.
In K. pneumoniae, excess iron has been shown to relieve NifA inhibition by NifL whereas iron depletion abolishes NifA-dependent activation of nif genes (Schmitz, 1996). In A. vinelandii, iron-dependent regulation of nif-gene expression has not been reported yet. In any case, the regulatory mechanism comprising ClpX2-dependent degradation of NifB and NifEN might operate as a second layer of regulation for a more robust control of the commitment to nitrogen fixation according to the availability of iron.
Identification and characterization of the function of other components involved in the regulatory pathway uncovered in this work will lead to a more detailed understanding of the regulation of nitrogen fixation in A. vinelandii and related diazotrophs, which appears to be another important trait regulated by ClpXP proteases.
Experimental procedures
Generation of A. vinelandii mutant strains
A. vinelandii strains UW140 (ΔnifB) (Curatti et al., 2006), UW235 (ΔnifENX) and UW236 (Hernandez et al., 2007) have been previously described.
To generate strains UW233 and UW238, a DNA fragment comprising the open reading frame of A. vinelandii nifB was PCR amplified with the oligonucleotides 5′-GGA TCC ATG GAA CTG AGC GTA CTT GG-3′ and 5′-AAG CTT TCG CTG CCC GCT CAG GCC TTG-3′ and ligated into the BamHI-HindIII sites of pGEX4T-2 to obtain a gst::nifB chimeric gene under the control of the IPTG-inducible tacP promoter (pRHB148). A BglII-XhoI DNA fragment, comprising the 3′ half of the A. vinelandii nifB, the fdxN and the nifO genes, was obtained from plasmid pRHB13 and used to replace the BglII-XhoI DNA fragment of pRHB148 (comprising the 3′ half of nifB) generating pRHB150. Plasmid pRHB150 contains the lac repressor gene, lacI, for tighter repression of gst::nifB expression under non-inducing conditions. This vector was incorporated into the genome of A. vinelandii strains UW140 and UW235 by single homologous recombination, generating strains UW233 and UW238, respectively.
To generate strain UW295 (gst::nifB, ΔnifA), UW233 was transformed with the vector pRHB138, which bears a ΔnifA::sp allele (Curatti et al., 2005).
To generate strain UW318, the lacZ reporter gene was inserted at a position corresponding to the clpX2 start codon. Plasmid pRHB295 contains a 1151-bp DNA fragment upstream of clpX2, which was amplified from the A. vinelandii genome by PCR using oligonucleotides 5′-TTT CGA ATT CCG CGA CGA TGT GAT CGC CAC CCG-3′ and 5′-GTC GGG ATC CTC TCC GCG CCG CAG AAC GAG CAG C-3′, and cloned into the EcoRI-BamHI sites of pUC18 (Promega, Madison, WI). Restriction enzymes sites (EcoRI and BamHI) incorporated into the oligonucleotides to facilitate vector construction are shown underlined in the oligonucleotide sequences? Plasmid pRHB296 contains a 793-bp DNA fragment downstream of clpX2 start codon, which was amplified from the A. vinelandii genome by PCR with oligonucleotides 5′-ATT GTC TAG ATC CAG CGC CAG GCC GAC GAA CTT-3′ and 5′-GCC GAA GAT GGC AGC GGC CGC ATT CAG CCC GAT C-3′ and cloned into the pGEM-T vector (Promega, Madison, WI). Plasmid pRHB298 contains a 3.1-kb DNA fragment with the E. coli lacZ gene amplified by PCR with oligonucleotides 5′-GGA AAC AGC CAT GGC TAA GAT TAC GGA TTC ACT GG-3′ and 5′-AAT ACG GGC AGA CAG CGG CCG CCC GGT TAT TAT TA-3′ and cloned into the pGEM-T vector (Promega, Madison, WI). Plasmid pRHB300 was generated by ligating an NcoI-XbaI fragment from pRHB295 to an NcoI-NotI fragment from pRHB298, and a NotI-XbaI fragment from pRHB296, in order to construct a clpX2P::lacZ transcriptional fusion. Positive clones were selected by blue color on X-gal (5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside) supplemented plates. The fidelity of the construction was confirmed by PCR amplification of the target region using genomic DNA as template. A. vinelandii strain UW318 was generated by transformation of wild-type cells with pRHB300, followed by screening of a blue phenotype on X-gal supplemented plates. Isolated colonies were screened by PCR to confirm the presence and segregation of the clpX2P::lacZ construction.
A. vinelandii strain UW319 (clpX2P::lacZ, ΔnifA::sp) was generated by transforming UW318 with pRHB138 (Curatti et al., 2005). The replacement of nifA by the Spr cassette in UW139 was confirmed by PCR amplification of the nifAL region.
To generate a clpX2 mutant, an in-frame clpX2 deletion was introduced into the A. vinelandii chromosome. Flanking regions of clpX2 gene were generated by PCR using the oligonucleotides 5′-TTT CGA ATT CCG CGA CGA TGT GAT CGA CCA CCC G-3′ and 5′-GTC GGG ATC CTC TCC GCG CCG CAG AAC GAG CAG C-3v for the nifM region upstream of clpX2 and 5′-AGC CGG ATC CAT CTC CGG TAG TGG AGG AAA AA-3′ and 5′-ATT GTC TAG ATC CAG CGC CAG GCC GAC GAA CTT-3′ for the nifF region downstream of clpX2. Restriction enzymes sites (EcoRI, BamHI and XbaI) incorporated into the oligonucleotides to facilitate vector construction are shown underlined in the oligonucleotide sequences. The resulting products were digested with EcoRI-BamHI or BamHI-XbaI, respectively, and used in triple-ligation reactions to pUC18, generating plasmid pRHB305, or in quadruple-ligations together with a BamHI-digested kanamycin-resistance cassette to generate pRHB306. Plasmid pRHB306 was introduced into the chromosome of A. vinelandii DJ strain by homologous recombination to generate strain UW315. Strain UW322, bearing an in-frame ΔclpX2 mutation was isolated by cotransforming (congression) strain UW315 with plasmids pRHB305 and pDB303, which contains a rifampicin-resistance marker (kindly provided by D. Dean). Preliminary identification of ΔclpX2 mutants was verified on rifampicin-containing solid medium by phenotypic loss of kanamycin resistance, which was further confirmed by PCR analysis and sequencing of the locus using genomic DNA of the mutant strains.
All DNA constructions were confirmed by restriction analysis and by DNA sequencing. Generated A. vinelandii strains were confirmed by PCR analysis and, when possible, by immunoblot analysis with appropriate antibodies.
Bacterial growth and nitrogenase derepression
E. coli strains were cultivated in Luria-Bertani medium at 37°C with shaking (250 rpm). A. vinelandii strains were cultivated in Burk’s modified medium at 30°C with modification of the nitrogen or iron sources, when indicated. Antibiotics were added at standard concentrations (Curatti et al. 2005). Growth rates were estimated from the light scattering of culture samples at 600 nm using an Ultrospec 3300 pro UV/Visible spectrophotometer (Amersham Biosciences). For the determination of growth curves, ammonium-grown cultures were diluted in fresh ammonium-containing medium to an OD600 of 0.5–0.8 and further incubated for 2–3 h. Cells were then collected, washed with and resuspended in ammonium-amended medium, ammonium-free medium, or ammonium-free medium with low iron (3 μM FeCl3) at a final OD600 of 0.1. Cells were then incubated at 30°C with shaking (200 rpm).
For nitrogenase derepression, ammonium-grown cells were diluted in fresh ammonium-containing medium to an OD600 of 0.5–0.8 and further incubated for 2–3 h. Cells were then collected, washed with and resuspended in ammonium-free medium at a final OD600 of 0.75 and incubated at 30°C for 3 h with shaking (200 rpm).
For NifB and NifDK polypeptide turnover experiments shown in Fig. 7 and Fig. S4, cultures were derepressed for nitrogenase during 3 h and then supplemented with 10 μg.ml−1 spectinomycin to stop protein synthesis, after which samples were collected at different time points.
Note: typically, pulse-chase radiolabeling followed by immuno pull-down is used for studies of in vivo degradation of proteins (e.g. Mettert and Kiley, 2005). Because it has been stated that the genus Azotobacter has limited capacity for amino acid transport (Kennedy and Bishop, 2004) these kind of complementary analyses were not attempted in this study.
For in vivo loading of A. vinelandii cells with GST-NifB, UW233 and UW233-derivative strains were cultivated in ammonium-containing medium and treated with 10 mM IPTG for 2 h. Cells were then collected, washed with medium free of IPTG and further incubated in the media indicated in each in vivo NifB-depletion studies.
In vivo and in vitro nitrogenase activities
In vivo nitrogenase activity was determined by the acetylene reduction assay at 30°C for 30 min using 1-ml culture samples as described (Stewart et al., 1967). In vitro nitrogenase activity was determined in samples of anaerobic A. vinelandii cell-free extracts prepared by osmotic-shock (Shah et al., 1972). Dinitrogenase and dinitrogenase reductase activities were obtained after titration with an excess of the complementary component as described (Shah and Brill, 1973). The specific activity of each protein is defined as nanomoles of ethylene formed per minute per milligram of protein in the extract.
Protein methods
Protein concentration was determined by the bicinchoninic acid method with BSA as the standard (Smith et al., 1985). Procedures for SDS-PAGE (Laemmli, 1970) and immunoblot analysis (Brandner et al., 1989) have been described. Protein samples for immunoblot analyses were prepared by mixing pelleted cells with Laemmli sample buffer 2X. ImageJ software was used to quantify protein levels in immunoblot membranes (Abramoff et al., 2004). β-galactosidase activity was assayed in permeabilized A. vinelandii cells following the procedure described by Miller (1972).
Competitive index assays
Competitive index (CI) was defined as the mutant-to-wild-type ratio within the output sample, divided by the corresponding ratio in the inoculum (Macho et al., 2007). For CI assays, a mixed inoculum containing equal CFU of wild-type and mutant strains was inoculated in the indicated Burk’s media and incubated at 30°C. Serial dilutions of the mixed cultures were sampled at incubation times 0 (t0) and 22 h (t22) and plated onto Burk’s solid medium (to allow growth of mutant and wild-type strains) and Burk’s solid medium supplemented with rifampicin (to inhibit growth of the wild type strain). The t0 determination was carried out as a control to confirm dose and to obtain the input mutant-to-wild-type ratio. The t22 determination was carried out to obtain the output CI values. CI shown are the mean of three independent experiments and the error bars represent standard error.
Supplementary Material
Acknowledgments
This work is dedicated to the memory of Bob Burris. Authors would like to thank Paul Ludden for his support and valuable discussions and Dennis Dean for calling our attention to orf9/clpX2 and helpful comments on this research. G. Martínez-Noël and L. Curatti are career researchers at the CONICET, Argentina. This work was supported by National Institute of Health Grant 35332 (to P. Ludden), by ERC Starting Grant 205442 (to L. Rubio), by MICINN BIO2009-12661 Grant (to L. Rubio), by Agencia Nacional de Promoción Científica y Tecnológica Grant PICT 01717 (to L. Curatti), and by Midwestern University Intramural Funds (to J. Hernandez).
Footnotes
Additional supporting information may be found in the online version of this article.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Bishop PE, Joerger RD. Genetics and molecular biology of alternative nitrogen fixation systems. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:109–125. [Google Scholar]
- Brandner JP, McEwan AG, Kaplan S, Donohue TJ. Expression of the Rhodobacter sphaeroides cytochrome c2 structural gene. J Bacteriol. 1989;171:360–368. doi: 10.1128/jb.171.1.360-368.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MK, Kim J, Rees DC. The nitrogenase FeMo-cofactor and P-cluster pair: 2.2 A resolution structures. Science. 1993;260:792–794. doi: 10.1126/science.8484118. [DOI] [PubMed] [Google Scholar]
- Curatti L, Brown CS, Ludden PW, Rubio LM. Genes required for rapid expression of nitrogenase activity in Azotobacter vinelandii. Proc Natl Acad Sci USA. 2005;102:6291–6296. doi: 10.1073/pnas.0501216102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curatti L, Ludden PW, Rubio LM. NifB-dependent in vitro synthesis of the iron-molybdenum cofactor of nitrogenase. Proc Natl Acad Sci USA. 2006;103:5297–301. doi: 10.1073/pnas.0601115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curatti L, Hernandez JA, Igarashi RY, Soboh B, Zhao D, Rubio LM. In vitro synthesis of the iron-molybdenum cofactor of nitrogenase from iron, sulfur, molybdenum, and homocitrate using purified proteins. Proc Natl Acad Sci USA. 2007;104:17626–17631. doi: 10.1073/pnas.0703050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos PC, Johnson DC, Ragle BE, Unciuleac MC, Dean DR. Controlled Expression of nif and isc Iron-Sulfur Protein Maturation Components Reveals Target Specificity and Limited Functional Replacement between the Two Systems. J Bacteriol. 2007;189:2854–2862. doi: 10.1128/JB.01734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan DA, Mogk A, Zeth K, Turgay K, Bukau B. AAA+ proteins and substrate recognition, it all depends on their partner in crime. FEBS Letters. 2002;529:6–10. doi: 10.1016/s0014-5793(02)03179-4. [DOI] [PubMed] [Google Scholar]
- Drummond M, Walmsley J, Kennedy C. Expression from the nifB promoter of Azotobacter vinelandii can be activated by NifA, VnfA, or AnfA transcriptional activators. J Bacteriol. 1996;178:788–792. doi: 10.1128/jb.178.3.788-792.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady RR. Structure-function relationships of alternative nitrogenases. Chem Rev. 1996;96:3013–3030. doi: 10.1021/cr950057h. [DOI] [PubMed] [Google Scholar]
- Einsle O, Tezcan FA, Andrade SL, Schmid B, Yoshida M, Howard JB, Rees DC. Nitrogenase MoFe-protein at 1.16 A resolution: a central ligand in the FeMo-cofactor. Science. 2002;297:1696–1700. doi: 10.1126/science.1073877. [DOI] [PubMed] [Google Scholar]
- Flynn JM, Neher SB, Kim YI, Sauer RT, Baker TA. Proteomic discovery of cellular substrates of the ClpXP protease reveal five classes of ClpX-recognition signals. Mol Cell. 2003;11:671–683. doi: 10.1016/s1097-2765(03)00060-1. [DOI] [PubMed] [Google Scholar]
- George SJ, Igarashi RY, Xiao Y, Hernandez JA, Demuez M, Zhao D, Yoda Y, Ludden PW, Rubio LM, Cramer SP. Extended X-ray absorption fine structure and nuclear resonance vibrational Spectroscopy reveal that NifB-co, a FeMo-co precursor, comprises a 6Fe core with an interstitial light atom. J Am Chem Soc. 2008;130:5673–5680. doi: 10.1021/ja0755358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JA, Igarashi RY, Soboh B, Curatti L, Dean DR, Ludden PW, Rubio LM. NifX and NifEN exchange NifB cofactor and the VK-cluster, a newly isolated intermediate of the iron-molybdenum cofactor biosynthetic pathway. Mol Microbiol. 2007;63:177–192. doi: 10.1111/j.1365-2958.2006.05514.x. [DOI] [PubMed] [Google Scholar]
- Jacobson MR, Cash VL, Weiss MC, Laird NF, Newton WE, Dean DR. Biochemical and genetic analysis of the nifUSVWZM cluster from Azotobacter vinelandii. Mol Gen Genet. 1989;219:49–57. doi: 10.1007/BF00261156. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Bishop P. Genetics and regulation of nitrogen fixation in free-living bacteria nitrogen fixation: origins, applications, and research progress. Volume. 2004;2:27–52. [Google Scholar]
- Kim YI, Burton RE, Burton BM, Sauer RT, Baker TA. Dynamics of substrate denaturation and translocation by the ClpXP degradation machine. Mol Cell. 2000;5:639–648. doi: 10.1016/s1097-2765(00)80243-9. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukoyanov D, Pelmenschikov V, Maeser N, Laryukhin M, Yang TC, Noodleman L, Dean DR, Case DA, Seefeldt LC, Hoffman BM. Testing if the interstitial atom, X, of the nitrogenase molybdenum-iron cofactor is N or C: ENDOR, ESEEM, and DFT studies of the S = 3/2 resting state in multiple environments. Inorg Chem. 1989;46:11437–11449. doi: 10.1021/ic7018814. [DOI] [PubMed] [Google Scholar]
- Macho AP, Zumaquero A, Ortiz-Martín I, Beuzón CR. Competitive index in mixed infections: a sensitive and accurate assay for the genetic analysis of Pseudomonas syringae-plant interactions. Mol Plant Path. 2007;8:437–450. doi: 10.1111/j.1364-3703.2007.00404.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Argudo I, Little R, Shearer N, Johnson P, Dixon R. The NifL-NifA System: a Multidomain Transcriptional Regulatory Complex That Integrates Environmental Signals. J Bacteriol. 2004;186:601–610. doi: 10.1128/JB.186.3.601-610.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettert EL, Kiley PJ. ClpXP-dependent proteolysis of FNR upon loss of its O2-sensing [4Fe–4S] cluster. J Mol Biol. 2005;354:220–232. doi: 10.1016/j.jmb.2005.09.066. [DOI] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Lab. Press; Plainview, NY: 1972. [Google Scholar]
- Rodriguez H, Mendoza A, Cruz MA, Holguin G, Glick BR, Bashan Y. Pleiotropic physiological effects in the plant growth-promoting bacterium Azospirillum brasilense following chromosomal labeling in the clpX gene. FEMS Microbiol Ecol. 2006;57:217–225. doi: 10.1111/j.1574-6941.2006.00111.x. [DOI] [PubMed] [Google Scholar]
- Rubio LM, Ludden PW. Biosynthesis of the iron-molybdenum cofactor of nitrogenase. Annu Rev Microbiol. 2008;62:93–111. doi: 10.1146/annurev.micro.62.081307.162737. [DOI] [PubMed] [Google Scholar]
- Setubal JC, Dos Santos P, Goldman BS, Ertesvåg H, Espin G, Rubio LM, et al. Genome sequence of Azotobacter vinelandii, an obligate aerobe specialized to support diverse anaerobic metabolic processes. J Bacteriol. 2009;191:4534–4545. doi: 10.1128/JB.00504-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz RA, He L, Kustu S. Iron is required to relieve inhibitory effects on NifL on transcriptional activation by NifA in Klebsiella pneumoniae. J Bacteriol. 1996;178:4679–4687. doi: 10.1128/jb.178.15.4679-4687.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah VK, Allen JR, Spangler NJ, Ludden PW. In vitro synthesis of the iron-molybdenum cofactor of nitrogenase. Purification and characterization of NifB cofactor, the product of NIFB protein. J Biol Chem. 1994;269:1154–1158. [PubMed] [Google Scholar]
- Shah VK, Davis LC, Brill WJ. Nitrogenase. I. Repression and derepression of the iron-molybdenum and iron proteins of nitrogenase in Azotobacter vinelandii. Biochim Biophys Acta. 1972;256:498–511. doi: 10.1016/0005-2728(72)90078-3. [DOI] [PubMed] [Google Scholar]
- Shah VK, Brill WJ. Nitrogenase. IV. Simple method of purification to homogeneity of nitrogenase components from Azotobacter vinelandii. Biochim Biophys Acta. 1973;305:445–454. doi: 10.1016/0005-2728(73)90190-4. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stewart WDP, Fitzgerald GP, Burris RH. In situ studies on N2 fixation using the acetylene reduction technique. Proc Natl Acad Sci USA. 1967;58:2071–2078. doi: 10.1073/pnas.58.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Yang J, Dou Y, Chen M, Ping S, Peng J, et al. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc Natl Acad Sci USA. 2008;105:7564–7569. doi: 10.1073/pnas.0801093105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Ping S, Peng J, Han Y, Li L, Yang J, et al. Global transcriptional analysis of nitrogen fixation and ammonium repression in root-associated Pseudomonas stutzeri A1501. BMC Genomics. 2010;11:11–24. doi: 10.1186/1471-2164-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Curatti L, Rubio LM. Evidence for nifU and nifS participation in the biosynthesis of the iron-molybdenum cofactor of nitrogenase. J Biol Chem. 2007;282:37016–37025. doi: 10.1074/jbc.M708097200. [DOI] [PubMed] [Google Scholar]
- Zolkiewski M. A camel passes through the eye of a needle: protein unfolding activity of Clp ATPases. Mol Microbiol. 2006;61:1094–1100. doi: 10.1111/j.1365-2958.2006.05309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.