Abstract
Background
Leishmania transmission occurs in the presence of insect saliva. Immunity to Phlebotomus papatasi or Lutzomyia longipalpis saliva or salivary components confers protection against an infection by Leishmania in the presence of the homologous saliva. However, immunization with Lutzomyia intermedia saliva did not protect mice against Leishmania braziliensis plus Lu. intermedia saliva. In the present study, we have studied whether the immunization with Lu. longipalpis saliva or a DNA plasmid coding for LJM19 salivary protein would be protective against L. braziliensis infection in the presence of Lu. intermedia saliva, the natural vector for L. braziliensis.
Methodology/Principal Findings
Immunization with Lu. longipalpis saliva or with LJM19 DNA plasmid induced a Delayed-Type Hypersensitivity (DTH) response against Lu. longipalpis as well as against a Lu. intermedia saliva challenge. Immunized and unimmunized control hamsters were then intradermally infected in the ears with L. braziliensis in the presence of Lu. longipalpis or Lu. intermedia saliva. Animals immunized with Lu. longipalpis saliva exhibited smaller lesion sizes as well as reduced disease burdens both at lesion site and in the draining lymph nodes. These alterations were associated with a significant decrease in the expression levels of IL-10 and TGF-β. Animals immunized with LJM19 DNA plasmid presented similar findings in protection and immune response and additionally increased IFN-γ expression.
Conclusions/Significance
Immunization with Lu. longipalpis saliva or with a DNA plasmid coding LJM19 salivary protein induced protection in hamsters challenged with L. braziliensis plus Lu. intermedia saliva. These findings point out an important role of immune response against saliva components, suggesting the possibility to develop a vaccine using a single component of Lu. longipalpis saliva to generate protection against different species of Leishmania, even those transmitted by a different vector.
Author Summary
Leishmaniasis, caused by parasitic protozoa Leishmania, is transmitted by bites of female sand flies that, during blood-feeding, inject humans with parasites and saliva. Sand fly saliva has been investigated as a potential vaccine candidate. It was previously shown that immunization with Lutzomyia longipalpis saliva or salivary proteins protects against cutaneous and visceral leishmaniasis. In the present study, we evaluated if immunization with Lu. longipalpis saliva or DNA plasmid coding for a specific sand fly salivary protein (LJM19) can protect hamsters against L. braziliensis plus another sand fly saliva. Immunization with saliva or LJM19 DNA plasmid induced a mononuclear cell infiltrate which can be a marker of protection. The immune response induced by immunization with these insect molecules was able to protect animals against L. braziliensis infection as shown by the significant reduction in lesion size, parasite load in the ear and draining lymph node. These data show the important role of immune response against sand fly saliva components, suggesting the possibility to develop vaccines using a single component of saliva against Leishmania transmitted by different vectors.
Introduction
Transmitted by sand flies, the parasitic protozoa of the genus Leishmania are the etiological agents of tegumentar or visceral diseases in humans. Differences in Leishmania species and the genetic background or immunological status of the host underlie the diverse clinical manifestations in leishmaniasis. Leishmania braziliensis is the etiologic agent of American Cutaneous Leishmaniasis (ACL), which is characterized by its chronicity and the possibility to metastasize leading to the muco-cutaneous clinical form [1].
Saliva from sand flies and other blood feeders contains potent pharmacologic components that facilitate blood meal acquisition and evasion of host inflammatory and immune response [2], [3]. Arthropod vector saliva also plays an important role in pathogen transmission. A small amount of vector saliva can intensify parasite or virus infectivity [4]–[6]. On the other hand, immune response to arthropod saliva or to insect bites precludes establishment of the pathogen in the vertebrate host. Recent reports have shown the importance of salivary proteins from sand fly vectors as potential targets for vaccine development against Leishmania infection [4], [7]–[9].
Valenzuela et al showed that immunization with DNA plasmid coding for Phlebotomus papatasi SP15 protein was effective in providing protection against co-inoculation of L. major plus Salivary Gland Sonicate (SGS) in mice [8]. Our group also demonstrated that hamsters immunized with DNA plasmid coding LJM19 Lu. longipalpis protein were protected against a challenge composed by L. infantum chagasi plus Lu. longipalpis SGS [9]. These two studies, as proposed before [10], suggest that a Delayed-Type Hypersensitivity (DTH) response is the mechanism underlying the protective anti-Leishmania immunity.
Exposure to sand fly saliva does not always result in a protective outcome. Mice immunized with Lu. intermedia SGS developed larger lesions and increase in parasite load when challenged with L. braziliensis and Lu. intermedia SGS [5]. These data suggests that immune responses against components present in saliva from different vectors could alter the outcome of infection, leading to protection or exacerbation of disease. Therefore, we hypothesized that immunization with Lu. longipalpis SGS or DNA plasmid coding for LJM19 protein, one of the most abundant Lu. longipalpis salivary proteins [11], could revert the non protective effect of Lu. intermedia SGS.
Methods
Sand flies and salivary gland lysates
Lutzomyia intermedia, Corte de Pedra strain, and Lutzomyia longipalpis, Cavunge strain, were reared at Centro de Pesquisas Gonçalo Moniz – FIOCRUZ, as described elsewhere [12]. Female sand flies from 5- to 7-day-old were dissected, the salivary glands were removed and transferred to 10 or 20 µl Hepes, 10 mM pH 7.0 NaCl 0.15 M in 1.5 mL polypropilene vials, usually in groups of 20 pairs of glands in 20 µl of Hepes saline, or individually in 10 µl of Hepes saline. Salivary glands were kept at −75 °C until needed. Salivary glands were disrupted by sonication using a Branson Sonifier 450 homogenizer (Branson, Danbury, CT). Salivary homogenates (1 pair of salivary gland per 1 µL) were centrifuged at 10,000 g for 2 min; the supernatants were used in the experiments (SGS).
DNA plasmid and immunization
DNA plasmid coding for Lu. longipalpis salivary protein LJM19 was cloned into the VR2001-TOPO vector and purified as described [13]. Male Golden Syrian Hamsters (Mesocricetus auratus) at 10–12 weeks old were obtained from Centro de Pesquisa Gonçalo Moniz Institute FIOCRUZ facility. Groups of hamsters were immunized or not (20 µL) intradermally (i.d.) in the right ear with Lu. longipalpis SGS (5 experiments repeated with 3 hamsters per group per time point) equivalent to 0.5 pair of salivary glands (∼1 µg of protein measured by Bradford method [14]) or 20 µg of DNA plasmid (3 experiments repeated with 5 hamsters per group per time point) for three times at 14 day intervals.
Analysis of the inflammatory immune response in the ear dermis
Following three i.d. injections with saline (8 hamsters per group total), Lu. longipalpis SGS (9 hamsters per group total) or LJM19 DNA plasmid (5 hamsters per group total) in the right ear, hamsters were challenged in the left ear dermis with 0.5 pair of Lu. longipalpis SGS, that corresponds to approximately 1 µg of protein, or 1 pair of Lu. intermedia SGS, which protein concentration is the same. We used different amount of Lu. longipalpis and Lu. intermedia saliva in order to allow the same concentration of protein from both SGS. Forty-eight hours post challenge, left sample ears were removed and fixed in 10% formaldehyde. Following fixation, tissues were processed, embedded in paraffin and 5 µm sections were stained with hematoxylin and eosin (H&E) and analyzed by light microscopy.
Leishmania parasites
L. braziliensis (strain MHOM/BR/01/BA788) promastigotes were cultivated in Schneider's Insect Medium (Sigma Chemical Co., St Louis, Mo, USA) supplemented with 20% heat-inactivated fetal bovine serum (Gibco, USA), L-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 µg/ml) at 23 °C for 5–7 days when the parasites reached the stationary-phase.
Experimental infection
Two weeks after the last immunization, hamsters were challenged i.d. in the left ear with 20 µL of 105 stationary phase promastigotes in the presence of either (a) L. braziliensis plus the equivalent to 0.5 pair of salivary gland of Lu. longipalpis SGS (∼1 µg of protein) or in the presence of (b) L. braziliensis plus one pair of salivary gland-equivalent of Lu. intermedia SGS (∼1 µg of protein). Control animals received saline and the same challenge that immunized hamsters (105 stationary phase promastigotes in the presence of either Lu. longipalpis or Lu. intermedia SGS). Lesion size was measured weekly using a digital caliper (Thomas Scientific, USA). All procedures involving animal experimentation were conducted according to relevant Brazilian guidelines and approved by the Ethical Committee in Animal Use of Centro de Pesquisa Gonçalo Moniz in 2006/03/03, under the number 083.
Limiting Dilution Assay (LDA)
Parasite load was determined 3, 5 and 8 weeks post-infection using the quantitative Limiting Dilution Assay (LDA) as described by Titus et al [15]. Briefly, infected ears and retromaxillar draining lymph nodes were aseptically removed from individual hamster. Tissues were homogenized and diluted in Schneider's Insect Medium (Sigma, St. Louis, MO) supplemented with 10% heat inactivated fetal bovine serum (Gibco, USA), 100 U of penicillin/ml and 100 µg/ml of streptomycin. Homogenate samples were serially diluted into 96-wells plates containing biphasic blood agar (Novy-Nicolle-McNeal) medium and incubated for one week at 23°C. Wells with positive growth were noted at specific dilutions and applying the ELIDA 1986 software to calculate the parasites burdens in the tissues.
RNA isolation and quantitative Real-Time PCR
Total RNA was extracted from the draining lymph nodes using Trizol reagent (Invitrogen, USA) 3, 5 and 8 weeks after infection. First-strand cDNA synthesis was performed with 1–2 µg of RNA in a total volume of 25 µL by using SuperScript II (Gibco, Carlsbad, CA, USA). DNA was amplified in the thermocycler (Mastercycler gradient – Eppendorf, USA) with an initial pre-incubation at 72°C for 5 minutes, followed by amplification of the target DNA at 42°C for 50 minutes. A standard curve was generated for each set of primers and efficiency of each reaction was determined. The expression levels of genes were normalized to GAPDH levels. The results are expressed in fold change over control. Oligonucleotide primers used were: GAPDH (reverse 5′-CTGACATGCCGCCCTGGAG-3′ and forward 3′-TCAGTGTAGCCCAGGATGCC-5′); IFN-γ (reverse 5′-GAAGCTCACCAAGATTCCGGTAA-3′ and forward 3′-TTTTCGTGACAGGTGAGGCAT-5′); IL-10 (reverse 5′-AGACGCCTTTCTCTTGGAGCTTAT-3′ and forward 3′-GGCAACTGCAGCGCTGTC-5′); and TGF-β (reverse 5′-GCTACCACGCCAACTTCTGTC-3′ and forward 3′-TGTTGGTAGAGGGCAAGG-5′).
Statistical analysis
The experiments with total saliva were repeated five times with three hamsters per groups per time point, whereas experiments using LJM19 DNA plasmid were repeated three times with five hamsters per group per time point. Results were expressed as mean ± standard error of the mean or median with interquartile range. Comparisons among immunized and non-immunized control groups were done by Mann-Whitney non-parametric t test, in the following situations: 1. unimmunized and challenged with Lu. longipalpis SGS + L. braziliensis X immunized with Lu. longipalpis SGS and challenged with Lu. longipalpis SGS + L. braziliensis; 2. unimmunized and challenged with Lu. intermedia SGS + L. braziliensis X immunized with Lu. longipalpis SGS and challenged with Lu. intermedia SGS + L. braziliensis. In the experiments using hamsters immunized with LJM19 DNA plasmid, we employed the same statistical analysis, but the control group consisted of animals injected with empty plasmid. One-way ANOVA (Kruskal-Wallis) analysis with Dunn's post-test was done to compare different groups non-immunized or immunized with SGS and LJM19 DNA plasmid (Figure S2). All the statistical analysis was done using Graph Pad 5.0 software program. The course of disease was plotted individually and the area under each resulting curve (AUC) was calculated using Graph Pad 5.0 software program. The results were considered statistically significant when p<0.05.
Results
Immunization with Lu. longipalpis SGS or LJM19 salivary protein induces cellular response in hamsters
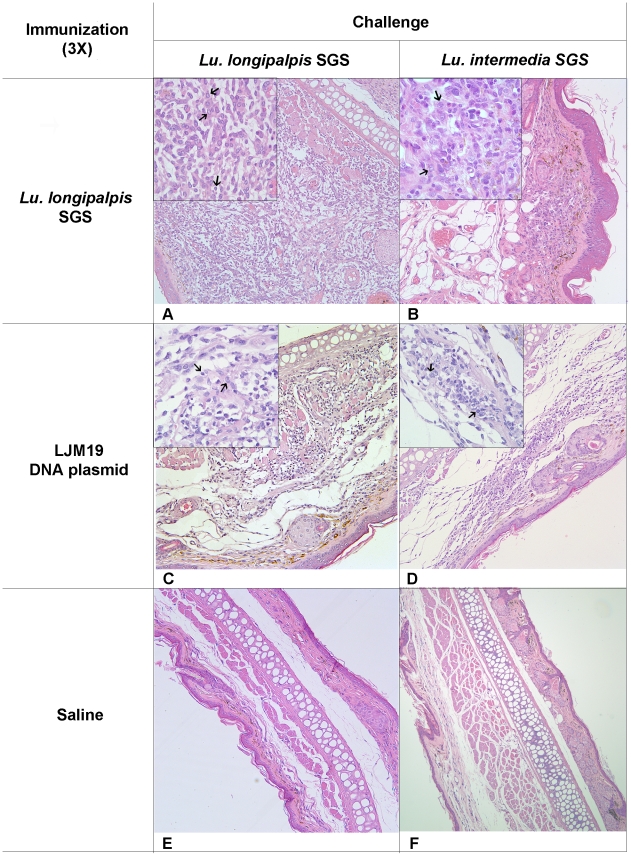
DTH response against salivary proteins can be a marker or correlate of protection against a challenge of parasite plus saliva [8], [9]. To evaluate the induction of DTH reaction by immunization with Lu. longipalpis SGS or by LJM19 DNA plasmid, immunized hamsters were challenged in the contralateral ear using either Lu. longipalpis or Lu. intermedia SGS. Immunization and challenge with Lu. longipalpis SGS induced an intense mononuclear cell infiltrate and edema, whereas the challenge with Lu. intermedia SGS elicited a smaller recruitment of mononuclear cells and edema (Fig. 1A). Hamsters immunized with LJM19 DNA plasmid and challenged with Lu. longipalpis or Lu. intermedia SGS also resulted in a DTH response, but with less recruitment of mononuclear cells and more pronounced edema. Control hamsters injected with saline and challenged with SGS exhibited no edema and scarce inflammatory cells (Fig. 1E,F). The observed inflammatory response in all immunized groups consisted predominantly of mononuclear cells (inserts in Fig. 1A–D), characteristic of a typical DTH response.
Figure 1. Cellular response against sand fly saliva in hamsters.
Hamsters were immunized three times with Lu. longipalpis SGS (A–B; 9 hamsters per group total), LJM19 DNA plasmid (C–D; 5 hamsters per group total) or were inoculated with saline (E–F; 8 hamsters per group total) in the right ear. After the last immunization, hamsters were challenged in the left ear dermis with Lu. longipalpis or with Lu. intermedia SGS. The cellular infiltration was observed by light microscopy stained using H&E at 100x (A–F) and the left corner inserts at 1000x with arrows indicating mononuclear cells (A–D). Experiments were repeated three times and the photographs are representative from each group.
Immunization with Lu. longipalpis SGS induced anti-saliva antibodies as assayed by ELISA. LJM19 immunization did not elicit antibodies to saliva as previously shown [9] (Fig. S1).
The outcome of immunization with Lu. longipalpis SGS followed by infection with L. braziliensis
The onset of lesion development in naive hosts was at two weeks post-infection and lesions progressed until 12 weeks (data not shown), confirming the high susceptibility of hamsters to Leishmania infection [9], [16], [17]. After this period, the animals were euthanized due to the lesions severity. Disease burden was calculated by weekly measure of ear thickness and by comparison of the area under the resulting curves, as explained in statistical analysis section in Material and Methods. Immunization with Lu. longipalpis SGS resulted in significantly (p = 0.011) smaller ear thickness following challenge with parasites plus Lu. intermedia SGS as compared to the control group (Fig. 2A–B). The mean of ear thickness ranged between 0.5 mm and 1.0 mm in immunized hamsters during the infection compared with the range of 1.0 mm to 2.0 mm in the control group (Fig. 2A). Parasite load in the ear (from 1.4×108 in unimmunized group to 1.1×107 in immunized group, p = 0.029) and in the lymph node (from 4.6×108 to 9.0×105, p = 0.001) was also significantly reduced in this group (Fig. 2C–F). No significant differences in ear thickness were observed in the group challenged with parasites plus Lu. longipalpis SGS (Fig 2A–B). However, there was a significant reduction in ear (from 1.7×108 to 2×107, p = 0.036) and lymph node (from 8×107 to 4.6×106, p = 0.009) parasite load in this group (Fig. 2C–F).
Figure 2. Ear thickness and parasite load in Lu. longipalpis SGS immunized hamsters following infection with L. braziliensis.
Hamsters were inoculated three times in the right ear dermis with Lu. longipalpis SGS (closed symbols) or with saline (open symbols) and were infected in the left ear with 105 L. braziliensis stationary promastigotes in the presence of Lu. intermedia SGS (squares; 7 hamsters per group total) or Lu. longipalpis SGS (circles, 8 hamsters per group total). The course of lesion development was monitored weekly (A). The points represent the means and the vertical bars represent standard errors of the means. The areas contained under the curves (AUC) obtained in A for each group was compared (B). The parasite number in the ear (C, D) or draining lymph node (E, F) was evaluated by Limiting Dilution Assay (LDA), estimated by ELIDA in 3, 5 and 8 weeks after the infection. Each bar represents the mean and the vertical bars represent standard errors of the means. The disease burden of the ear (C) or draining lymph node (E) show reduced parasite load in immunized hamsters evaluated by Mann-Whitney non-parametric t test. The areas contained under the curves (AUC) obtained in C and E for each group was compared (D, F) and the experiments were repeated five times. *p<0.05; **p<0.01.
Reduction of IL-10 and TGF-β is involved in the protection against L. braziliensis infection in hamsters immunized with Lu. longipalpis SGS
We studied the cytokine profile of immunized and infected hamsters three, five and eight weeks post challenge by real time PCR. At five weeks post-infection, IFN-γ levels were similar among all groups (Fig. 3A). Similar results were observed at all time points studied (Fig. S2A and D). There was a significant reduction in IL-10 expression (p = 0.026) in hamsters immunized with Lu. longipalpis SGS and challenged with L. braziliensis plus Lu. intermedia SGS (Fig. 3B) compared to control unimmunized group. A significant reduction was also observed at three (p = 0.021) and eight (p = 0.043) weeks post infection (Fig. S2B and E). We also observed a significant reduction in the expression of TGF-β (p = 0.030) in hamsters immunized with Lu. longipalpis SGS and challenged with L. braziliensis + Lu. intermedia SGS as compared to unimmunized control group (Fig. 3C). Similar results were obtained at 3 weeks post-infection (p = 0.040, Fig. S2C). However, the group of hamsters immunized with Lu. longipalpis SGS and challenged with L. braziliensis plus Lu. longipalpis SGS showed significant reduction in TGF-β expression only at three weeks after infection (p = 0.050, Fig. S2C). We also analyzed the ratio IFN-γ/TGF-β and there were not significant differences between the immunized and non-immunized groups (data not shown).
Figure 3. Cytokine expression in lymph node cells from hamsters immunized with Lu. longipalpis followed by L. braziliensis infection.
Hamsters (7 per group total) were inoculated three times in the right ear with Lu. longipalpis SGS (closed symbols) or with saline (open symbols) and were challenged in the left ear with 105 L. braziliensis in the presence of Lu. intermedia (squares) or Lu. longipalpis SGS (circles). The IFN-γ (A), IL-10 (B) and TGF-β (C) relative mRNA expression was evaluated by Real-Time PCR, 5 weeks after the infection. Points represent each animal, experiments were repeated five times and were evaluated by Mann-Whitney non-parametric t test. *p<0.05; **<0.01.
The influence of immunization with DNA plasmid coding LJM19 protein from Lu. longipalpis sand fly in hamsters infected with L. braziliensis
We recently showed that immunization with a DNA plasmid coding for the salivary protein LJM19, one of the most abundant secreted proteins in Lu. longipalpis saliva, protected hamsters against visceral leishmaniasis [9]. Since Lu. longipalpis SGS protect hamsters against an infection with L. braziliensis (Fig. 2), we tested if immunizations with LJM19 DNA plasmid could also protect against L. braziliensis infection. This hypothesis is based on the fact that immunization with LJM19 DNA plasmid also induced a DTH response against Lu. intermedia SGS (Fig. 1D). Hamsters immunized with LJM19 DNA plasmid were challenged with L. braziliensis + Lu. intermedia SGS. As shown in Fig. 4A, the onset of lesion development is at three weeks post infection in both groups: LJM19 DNA plasmid immunized and empty DNA plasmid control. At four weeks, the ear thickness in immunized hamsters peaked around 1.0 mm and is maintained without alterations. In control group, the ear thickness increased up to 1.5 mm until eight weeks post infection (Fig. 4A). Importantly, a significant reduction (p = 0.038) in disease burden was observed in immunized compared to control group (Fig. 4B).
Figure 4. Ear thickness and parasite load in LJM19 immunized hamsters following infection with L. braziliensis.
Hamsters (12 per group total) were inoculated three times in the right ear with DNA plasmid coding LJM19 salivary protein (closed triangle) or empty DNA plasmid (CTR - open triangle) and were challenged intradermally in the left ear with 105 L. braziliensis stationary promastigotes in the presence of Lu. intermedia SGS. The course of lesion development was monitored weekly and points represent the means and standard errors of the means (A). The areas contained underneath the curves (AUC) obtained in A for each group was compared (B). Five weeks after the infection, the parasite load was evaluated in the ear (C) and draining lymph node (D) by LDA, estimated by ELIDA. Experiments were repeated three times and were evaluated by Mann-Whitney non-parametric t test. *p<0.05; **p<0.01.
At the same time point, we also evaluated the control of L. braziliensis infection measuring the parasite load of infected ears (Fig. 4C) and in draining lymph nodes (Fig. 4D). The results showed a significant reduction in parasite load in LJM19 DNA plasmid immunized group (8.9×104 in the ear and 2.3×103 in the lymph nodes) compared with empty DNA plasmid control group (1.3×105 in the ears, p = 0.05 and 2.3×105 in the lymph nodes p = 0.032). Similar results in parasite load of the dLN were obtained at 3 weeks post-infection (Fig. S3). However, at 8 weeks post-infection there is no difference in parasite load between immunized and control groups (data not shown). Interestingly, the protection induced by immunization with Lu. longipapis SGS or LJM19 DNA plasmid was similar and no significant differences were observed between them (Fig. S4).
Increased IFN-γ/TGF-β ratio correlates with the protection of LJM19 immunized hamsters against L. braziliensis infection
In order to investigate the mechanism involved in the protection conferred by LJM19 DNA plasmid immunization, the cytokine expression in draining lymph node cells was evaluated 5 weeks after challenge with L. braziliensis + Lu. intermedia SGS by Real-Time PCR (Fig. 5). There was a significant increase in the IFN-γ expression in LJM19 DNA plasmid immunized group, p = 0.011 (Fig. 5A). However, no differences were found in IL-10 (Fig. 5B) or TGF-β (Fig. 5C) expression in hamsters immunized with LJM19 DNA plasmid compared to control group. Moreover, the ratio of IFN-γ/TGF-β expression was significantly higher (p = 0.011) in LJM19 DNA plasmid immunized hamsters compared with the control group (Fig. 5D). Together, these results suggest that immunization with a defined protein induces a protective immune response, with higher expression of pro-inflammatory cytokine (IFN-γ).
Figure 5. Cytokines production by lymph node cells from hamsters immunized with LJM19 DNA plasmid in L. braziliensis infection.
Hamsters were inoculated three times in the right ear with DNA plasmid LJM19 salivary protein (closed triangle; 9 hamsters per group total) or empty DNA plasmid (CTR – open triangle; 10 hamsters per group total) and were challenged in the left ear with 105 L. braziliensis in the presence of Lu. intermedia SGS. The IFN-γ (A), IL-10 (B) and TGF-β (C) relative mRNA expression was evaluated by Real-Time PCR 5 weeks after the infection. The ratio IFN-γ/TGF-β obtained in A and C for each group was compared (D). Points represent each animal, experiments were repeated three times and were evaluated by Mann-Whitney non-parametric t test. *p<0.05.
Discussion
In this study, we observed that Lu. longipalpis SGS or DNA plasmid coding for the salivary protein LJM19 protected hamsters against a challenge composed of L. braziliensis plus SGS from either Lu. longipalpis or from Lu. intermedia. Hamsters are a good model to evaluate protection against L. braziliensis, since they are highly susceptible to this infection [16], [17]. It has been shown that exposure to sand fly saliva induces a protective immune response against Leishmania [7], [8], [18]. The mechanism of protection conferred by vectors' saliva is not fully understood. However, reports in the literature have shown that the induction of a DTH response by whole saliva or by single salivary protein is a marker of protection [8], [9], [19]. Our results using a DNA plasmid coding LJM19 salivary protein from Lu. longipalpis indicate that a cellular immune response could be the mechanism involved in the protection. The immunization with DNA coding this protein does not induce antibody production [9], but a DTH reaction was observed in hamsters immunized and challenged with either Lu. longipalpis or with Lu. intermedia SGS.
In hamsters immunized with Lu. longipalpis SGS, the presence of Lu. longipalpis SGS in the inoculum did not lead to a reduction in ear thickness. This result suggests dissociation between parasite load and ear thickness, as already shown in other studies [20], [21]. Surprisingly, we did not observe any exacerbation in the infection caused by L. braziliensis plus Lu. longipalpis SGS in unimmunized hamsters, as shown by other studies [4], [22]. Since hamsters are highly susceptible to Leishmania [16], [17], possibly sand fly saliva did not exacerbate the infection. The dissociation between parasite load and lesion development has already been demonstrated [20], [21]. In fact, the ear thickness observed in the hamsters challenged with L. braziliensis plus Lu. longipalpis SGS could be attributed to differences in the inflammatory infiltrate, characterized by an intense cellular recruitment to the site of infection, as observed in the DTH reaction. On the other hand, the challenge with Lu. intermedia SGS in hamsters immunized with Lu. longipalpis induced a less pronounced cell recruitment, but still able to control the infection, as observed by the reduced ear thickness. In this way, the intensity of cell recruitment induced by SGS in the inoculum seems to be a key factor to detect lesion development. Using mice infected with L. amazonensis, another model of high susceptibility infection, immunization with Lu. longipalpis SGS also protected mice against L. amazonensis infection in the absence or presence of saliva [23]. On the other hand, BALB/c mice immunized with Lu. intermedia SGS and challenged with L. braziliensis plus Lu. intermedia SGS showed an exacerbation of L. braziliensis infection [5]. The possible mechanism underlying these different outcomes is the cytokine profile induced in each case. In Lu. longipalpis SGS immunized mice, there was an induction of Th1-type cytokines [23] whereas in Lu. intermedia SGS immunized mice, a Th2 profile was observed [5]. Besides that, Lu. intermedia immunization did not induce cellular immune response, but did generate antibody production and a mixed cellular (Th1–Th2) response, which favored parasite establishment and survival [5]. It is important to consider that the differences observed in these papers are not possibly due only to different sand flies, but also to the amount of Leishmania promastigotes as well as the parasite species used in the inoculum. Another aspect to consider is the type of challenge employed: natural transmission using infected sand flies or only Leishmania injected by needle. Recently, Peters et al [19] demonstrated that the use of natural transmission of Leishmania abrogates the immunity conferred by killed parasite vaccine, which was shown to be protective against a challenge by needle with L. major only. Although we used a challenge by needle, our infection was composed by L. braziliensis with saliva, which is important in the outcome of infection. Our attempt is to induce an immune response against saliva present at the moment of infection which may collaborate to create an inhospitable environment for the establishment of Leishmania infection that may involve direct killing of parasite. Sand fly saliva alters the local microenvironment by modulating the cytokine profiles and the co-stimulatory molecules expression [24]. Therefore, the immune response against salivary components and its presence in the challenge possibly favors the host in the early events after the infection.
Immunization with Lu. longipalpis SGS decreased the expression of IL-10 and TGF-β in draining lymph node cells of hamsters challenged with L. braziliensis + Lu. intermedia SGS. Although no differences were observed in IFN-γ expression, the decrease of inhibitory cytokines can contribute to the protective effect during the infection [25]. Surprisingly, the immunization with Lu. longipalpis SGS did not change the levels of anti-inflammatory cytokines in the hamsters challenged with parasites plus Lu. longipalpis SGS, although the animals showed a reduced parasite load in the ears and lymph nodes. Possibly, in hamsters immunized with whole saliva there were different levels and types of immune response induced by distinct proteins present in the saliva, which could mask some difference in cytokines expression, such as IFN-γ/TGF-β ratio.
Recently, our group demonstrated that immunization with DNA plasmid coding LJM19 protected hamsters against a fatal infection by L. chagasi plus Lu. longipalpis SGS [9]. In the present study, we investigated whether immunization with this DNA plasmid also protects against L. braziliensis plus Lu. intermedia SGS. Interstingly, the protection conferred by immunization with LJM19 DNA plasmid is very similar to that obtained with total Lu. longipalpis SGS. The immunization with LJM19 lead to a pronounced Th1 response with an increase in IFN-γ expression and no differences in IL-10 or TGF-β were noticed. Therefore, the ratio IFN-γ/TGF-β expression was higher than controls. The same cytokine response was observed in protected hamsters immunized with LJM19 and challenged with L. chagasi plus Lu. longipalpis SGS [9].
The results showed in this study suggest cross-reactivity between Lu. longipalpis and Lu. intermedia SGS. De Moura et al (2007) showed that SDS-PAGE profiles from Lu. intermedia and Lu. longipalpis SGS displayed bands migrating at similar molecular weight. However, Lu. intermedia immune sera from BALB/c mice did not recognize any proteins present in Lu. longipalpis SGS, with the exception of the ∼45 kDa one [5]. Although few studies in the literature have shown the direct effect of Lu. longipalpis saliva on innate immune response cells [26], [27], we can also speculate that some of these mechanisms, such as gamma/delta+ T cells in skin or parasite-saliva sharing antigens in their glycan moiety, could also contribute to the protection conferred by the immunization with Lu. longipalpis SGS. This has already been suggested in another model where immunization with Lu. longipalpis SGS was able to protect mice against L. amazonensis without saliva [23]. However, it is intriguing that LJM19 also protected hamsters in a similar way. Further studies are necessary to clarify whether LJM19 and Lu. intermedia proteins share common motifs and whether DTH induced by immunization with LJM19 is a consequence of this cross reactivity.
In conclusion, our results confirm the immunogenic ability of Lu. longipalpis saliva and a defined salivary protein, LJM19, in hamsters and, for the first time, we show the protective potential of this compounds against L. braziliensis infection plus Lu. intermedia SGS. These findings suggest that LJM19 could be applied as a component vaccine candidate in different models or even as adjuvant against other pathogens.
Supporting Information
Production of antibody against saliva in serum samples from hamsters immunized with L. longipalpis SGS or LJM19 DNA plasmid. Hamsters were inoculated three times in the right ear with Lu. longipalpis SGS (closed squares) or LJM19 DNA plasmid (closed triangles) or with saline (open squares). After 48 hours, the serum samples were collected and the total IgG against L. longipalpis SGS was measured by ELISA. Points represent each animal, experiments were repeated three times and were evaluated by Kruskal-Wallis non-parametric test. **p<0.01; ***p<0.001.
(TIF)
Cytokine expression in lymph node cells from hamsters immunized with Lu. longipalpis followed by L. braziliensis infection. Hamsters were inoculated three times in the right ear with Lu. longipalpis SGS (closed symbols) or with saline (open symbols) and were challenged in the left ear with 105 L. braziliensis in the presence of Lu. intermedia (squares) or Lu. longipalpis SGS (circles). IFN- γ, IL-10 and TGF-β relative mRNA expression was evaluated by Real-Time PCR at three (left column) and eight (right column) weeks after the infection. Points represent each animal, experiments were repeated three times and were evaluated by Mann-Whitney non-parametric t test. *p<0.05.
(TIF)
Parasite load in LJM19 immunized hamsters following infection with L. braziliensis . Hamsters (12 per group total) were inoculated three times in the right ear with DNA plasmid coding LJM19 salivary protein or empty DNA plasmid (CTR) and were challenged intradermally in the left ear with 105 L. braziliensis stationary promastigotes in the presence of Lu. intermedia SGS. Five weeks after the infection, the parasite load was evaluated in the draining lymph node by LDA and estimated by ELIDA. Experiments were repeated three times and were evaluated by Mann-Whitney non-parametric t test. *p<0.05.
(TIF)
Parasite load in LJM19 DNA plasmid or Lu. longipalpis SGS immunized hamsters following infection with L. braziliensis . Hamsters (9 per group total) were inoculated three times in the right ear with DNA plasmid coding LJM19 salivary protein or Lu. longipalpis SGS (closed bars) or empty DNA plasmid (CTR) or Saline (open bars) and were challenged intradermally in the left ear with 105 L. braziliensis stationary promastigotes in the presence of L. intermedia SGS. The parasite load was evaluated 5 weeks after infection in the ear (A) and draining lymph node (B) by LDA, estimated by ELIDA. Bars represent the median and standard errors of the means. Experiments were repeated three times and were evaluated by ANOVA (Kruskal-Wallis) analysis with Dunn's post-test. *p<0.05; **p<0.01.
(TIF)
Footnotes
The authors have declared that no competing interests exist.
This work was supported by FAPESB, Renorbio (554753/2006-5) and CNPq (472973/2006-1). AB, CIO, MB-N and CB are senior investigators from CNPq. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bittencourt A, Barral-Netto M. Evaluation of the histopathological classifications of American cutaneous and mucocutaneous leishmaniasis. Mem. Inst. Oswaldo Cruz. 1991;86:51–56. doi: 10.1590/s0074-02761991000100009. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro JM, Francischetti IM. Platelet-activating-factor-hydrolyzing phospholipase C in the salivary glands and saliva of the mosquito Culex quinquefasciatus. The Journal of experimental biology. 2001;204:3887–94. doi: 10.1242/jeb.204.22.3887. [DOI] [PubMed] [Google Scholar]
- 3.Andrade BB, Oliveira CI de, Brodskyn CI, Barral A, Barral-Netto M. Role of sand fly saliva in human and experimental leishmaniasis: current insights. Scandinavian journal of immunology. 2007;66:122–7. doi: 10.1111/j.1365-3083.2007.01964.x. [DOI] [PubMed] [Google Scholar]
- 4.Titus RG, Ribeiro JM. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science (New York, N.Y.) 1988;239:1306–8. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 5.Moura Tde, Oliveira F, Novais FO, Miranda J, Clarêncio J, et al. Enhanced Leishmania braziliensis infection following pre-exposure to sandfly saliva. PLoS Negl Trop Dis. 2007. e84. [DOI] [PMC free article] [PubMed]
- 6.Labuda M, Jones LD, Williams T, Nuttall PA. Enhancement of tick-borne encephalitis virus transmission by tick salivary gland extracts. Medical and veterinary entomology. 1993;7:193–6. doi: 10.1111/j.1365-2915.1993.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 7.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science (New York, N.Y.) 2000;290:1351–4. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- 8.Valenzuela JG, Belkaid Y, Garfield MK, Mendez S, Kamhawi S, et al. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. The Journal of experimental medicine. 2001;194:331–42. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes R, Teixeira C, Teixeira MJ, Oliveira F, Menezes MJ, et al. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7845–50. doi: 10.1073/pnas.0712153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belkaid Y, Valenzuela JG, Kamhawi S, Rowton E, Sacks DL, et al. Delayed-type hypersensitivity to Phlebotomus papatasi sand fly bite: An adaptive response induced by the fly? Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6704–9. doi: 10.1073/pnas.97.12.6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valenzuela JG, Garfield MK, Rowton ED, Pham V. Identification of the most abundant secreted proteins from the salivary glands of the sand fly Lutzomyia longipalpis, vector of Leishmania chagasi. J Exp Biol. 2004;207:3717–29. doi: 10.1242/jeb.01185. [DOI] [PubMed] [Google Scholar]
- 12.Modi G, Tesh R. A simple technique for mass rearing Lutzomyia longipalpis and Phlebotomus papatasi (Diptera: Psychodidae) in the laboratory. J Med Entomol. 1983;20:568–9. doi: 10.1093/jmedent/20.5.568. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira F, Kamhawi S, Seitz A, Pham V, Guigal P, et al. From transcriptome to immunome: identification of DTH inducing proteins from a Phlebotomus ariasi salivary gland cDNA library. Vaccine. 2006;24:374–90. doi: 10.1016/j.vaccine.2005.07.085. [DOI] [PubMed] [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 15.Titus RG, Marchand M, Boon T, Louis J. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 16.Herrer A, Telford S, Christensen H. Leishmania braziliensis: dissemination of Panamanian strains in golden hamsters. Exp Parasitol. 1979;48:359–63. doi: 10.1016/0014-4894(79)90120-6. [DOI] [PubMed] [Google Scholar]
- 17.Wilson R, Dieckmann B, Childs G. Leishmania braziliensis and Leishmania mexicana: experimental cutaneous infections in golden hamsters. Exp Parasitol. 1979;47:270–83. doi: 10.1016/0014-4894(79)90079-1. [DOI] [PubMed] [Google Scholar]
- 18.Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. The Journal of experimental medicine. 1998;188:1941–53. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collin N, Gomes R, Teixeira C, Cheng L, Laughinghouse A, et al. Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania. PLoS pathogens. 2009;5:e1000441. doi: 10.1371/journal.ppat.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira CI, Teixeira MJ, Teixeira CR, Jesus JR, Rosato AB, et al. Leishmania braziliensis isolates differing at the genome level display distinctive features in BALB/c mice. Microbes and infection / Institut Pasteur. 2004;6:977–84. doi: 10.1016/j.micinf.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Teixeira MJ, Fernandes JD, Teixeira CR, Andrade BB, Pompeu ML, et al. Distinct Leishmania braziliensis isolates induce different paces of chemokine expression patterns. Infection and immunity. 2005;73:1191–5. doi: 10.1128/IAI.73.2.1191-1195.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima HC, Titus RG. Effects of sand fly vector saliva on development of cutaneous lesions and the immune response to Leishmania braziliensis in BALB/c mice. Infection and immunity. 1996;64:5442–5. doi: 10.1128/iai.64.12.5442-5445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiakaki M, Rohousova I, Volfova V, Volf P, Chang K-P, et al. Sand fly specificity of saliva-mediated protective immunity in Leishmania amazonensis-BALB/c mouse model. Microbes and infection / Institut Pasteur. 2005;7:760–6. doi: 10.1016/j.micinf.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Costa DJ, Favali C, Clarêncio J, Afonso L, Conceição V, et al. Lutzomyia longipalpis salivary gland homogenate impairs cytokine production and costimulatory molecule expression on human monocytes and dendritic cells. Infection and immunity. 2004;72:1298–305. doi: 10.1128/IAI.72.3.1298-1305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melby PC, Tabares A, Restrepo BI, Cardona AE, McGuff HS, et al. Leishmania donovani: evolution and architecture of the splenic cellular immune response related to control of infection. Experimental parasitology. 2001;99:17–25. doi: 10.1006/expr.2001.4640. [DOI] [PubMed] [Google Scholar]
- 26.Moura TR de, Oliveira F, Rodrigues GC, Carneiro MW, Fukutani KF, et al. Immunity to Lutzomyia intermedia Saliva Modulates the Inflammatory Environment Induced by Leishmania braziliensis. PLoS Neglected Tropical Diseases. 2010;4:e712. doi: 10.1371/journal.pntd.0000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Araújo-Santos T, Prates DB, Andrade BB, Nascimento DO, Clarêncio J, et al. Lutzomyia longipalpis Saliva Triggers Lipid Body Formation and Prostaglandin E(2) Production in Murine Macrophages. PLoS neglected tropical diseases. 2010;4:e873. doi: 10.1371/journal.pntd.0000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Production of antibody against saliva in serum samples from hamsters immunized with L. longipalpis SGS or LJM19 DNA plasmid. Hamsters were inoculated three times in the right ear with Lu. longipalpis SGS (closed squares) or LJM19 DNA plasmid (closed triangles) or with saline (open squares). After 48 hours, the serum samples were collected and the total IgG against L. longipalpis SGS was measured by ELISA. Points represent each animal, experiments were repeated three times and were evaluated by Kruskal-Wallis non-parametric test. **p<0.01; ***p<0.001.
(TIF)
Cytokine expression in lymph node cells from hamsters immunized with Lu. longipalpis followed by L. braziliensis infection. Hamsters were inoculated three times in the right ear with Lu. longipalpis SGS (closed symbols) or with saline (open symbols) and were challenged in the left ear with 105 L. braziliensis in the presence of Lu. intermedia (squares) or Lu. longipalpis SGS (circles). IFN- γ, IL-10 and TGF-β relative mRNA expression was evaluated by Real-Time PCR at three (left column) and eight (right column) weeks after the infection. Points represent each animal, experiments were repeated three times and were evaluated by Mann-Whitney non-parametric t test. *p<0.05.
(TIF)
Parasite load in LJM19 immunized hamsters following infection with L. braziliensis . Hamsters (12 per group total) were inoculated three times in the right ear with DNA plasmid coding LJM19 salivary protein or empty DNA plasmid (CTR) and were challenged intradermally in the left ear with 105 L. braziliensis stationary promastigotes in the presence of Lu. intermedia SGS. Five weeks after the infection, the parasite load was evaluated in the draining lymph node by LDA and estimated by ELIDA. Experiments were repeated three times and were evaluated by Mann-Whitney non-parametric t test. *p<0.05.
(TIF)
Parasite load in LJM19 DNA plasmid or Lu. longipalpis SGS immunized hamsters following infection with L. braziliensis . Hamsters (9 per group total) were inoculated three times in the right ear with DNA plasmid coding LJM19 salivary protein or Lu. longipalpis SGS (closed bars) or empty DNA plasmid (CTR) or Saline (open bars) and were challenged intradermally in the left ear with 105 L. braziliensis stationary promastigotes in the presence of L. intermedia SGS. The parasite load was evaluated 5 weeks after infection in the ear (A) and draining lymph node (B) by LDA, estimated by ELIDA. Bars represent the median and standard errors of the means. Experiments were repeated three times and were evaluated by ANOVA (Kruskal-Wallis) analysis with Dunn's post-test. *p<0.05; **p<0.01.
(TIF)