Abstract
Background
Attempts to eradicate alien arthropods often require pesticide applications. An effort to remove an alien beetle from Central Park in New York City, USA, resulted in widespread treatments of trees with the neonicotinoid insecticide imidacloprid. Imidacloprid's systemic activity and mode of entry via roots or trunk injections reduce risk of environmental contamination and limit exposure of non-target organisms to pesticide residues. However, unexpected outbreaks of a formerly innocuous herbivore, Tetranychus schoenei (Acari: Tetranychidae), followed imidacloprid applications to elms in Central Park. This undesirable outcome necessitated an assessment of imidacloprid's impact on communities of arthropods, its effects on predators, and enhancement of the performance of T. schoenei.
Methodology/Principal Findings
By sampling arthropods in elm canopies over three years in two locations, we document changes in the structure of communities following applications of imidacloprid. Differences in community structure were mostly attributable to increases in the abundance of T. schoenei on elms treated with imidacloprid. In laboratory experiments, predators of T. schoenei were poisoned through ingestion of prey exposed to imidacloprid. Imidacloprid's proclivity to elevate fecundity of T. schoenei also contributed to their elevated densities on treated elms.
Conclusions/Significance
This is the first study to report the effects of pesticide applications on the arthropod communities in urban landscapes and demonstrate that imidacloprid increases spider mite fecundity through a plant-mediated mechanism. Laboratory experiments provide evidence that imidacloprid debilitates insect predators of spider mites suggesting that relaxation of top-down regulation combined with enhanced reproduction promoted a non-target herbivore to pest status. With global commerce accelerating the incidence of arthropod invasions, prophylactic applications of pesticides play a major role in eradication attempts. Widespread use of neonicotinoid insecticides, however, can disrupt ecosystems tipping the ecological balance in favor of herbivores and creating pest outbreaks.
Introduction
One of the most ecologically significant outcomes of global change is the rapid redistribution of biota [1]. The number of alien insect species introduced to the United States has increased dramatically during the last two centuries [2] and alien species account for more than $120 billion in damage annually in the United States [3]. Recently, two alien wood-boring beetles, the Asian longhorned beetle, Anoplophora glabripennis, and the emerald ash borer, Agrillus planipennis have killed tens of millions of trees and dramatically altered the composition of forests in cities and natural lands in North America [4], [5].
To halt these rapidly spreading beetles in the United States, federal quarantine and eradication programs destroyed thousands of infested trees and applied insecticides to protect tens of thousands of others [4], [6], [7]. One insecticide widely used in eradication efforts is imidacloprid. Imidacloprid belongs to a relatively new class of insecticides, the neonicotinoids. These nitroguanidine compounds have impressive toxicity against a wide range of economically important pests [8] and long residual activity [9]–[12]. Unlike nicotine, neonicotinoids exhibit a selective affinity for nerve cell receptors of insects [13]. Their broad spectrum of activity kills many insect pests, but one economically important family of herbivorous arachnids, spider mites (Tetranychidae), are insensitive to neonicotinoids [8] and their abundance may increase following imidacloprid applications [9], [14], [15].
Following the discovery of A. glabripennis in Central Park, New York, in 1996, an effort to eradicate the pest and save historically important trees including a stand of American elms, Ulmus americana, resulted in more than 14,000 applications of imidacloprid between 2005 and 2007 [16]. This intensively managed ecosystem provided a unique opportunity to investigate the effects of imidacloprid on community structure of arboreal arthropods, in particular on population dynamics of spider mites on elm trees and physiological responses of non-target arthropods to imidacloprid exposure. Changes in the structure and function of arthropod communities following pesticides applications have been examined in several agricultural [17]-[20] and aquatic [21]–[24] systems, but rarely in urban ecosystems [25], [26]. Results of terrestrial studies in agriculture revealed significant shifts in arthropod communities characterized by reductions in richness, diversity, density, and biomass of many arthropod species following pesticide applications [17]–[20]. Similar changes have been documented in aquatic communities following exposure to insecticides [21]–[23]. Moreover, pesticides restructure heterospecific interactions including competition and predation in invertebrate communities [24].
While pesticides reduce abundance and mitigate the impact of invasive pests, they also disrupt ecological processes resulting in outbreaks of pests and reductions in yields or quality of crops [27]. Early mechanistic explanations for resurgences of primary pests (targets of pesticide applications) and outbreaks of secondary pest (pests not targeted by applications) focused on pesticide-driven elimination of predatory arthropods, which play a major top-down role in suppressing herbivores in managed ecosystems [25]–[28]. Recently, complementary mechanisms have been used to explain increases in pest populations following pesticide applications [27], [29]. These include hormoligosis or hormesis [27], [29]–[31], defined as elevated fecundity of herbivores following sub-lethal exposure to pesticides. Additionally, some insecticide classes such as neonicotinoids may alter physiological pathways within plants [14], [27], [29], [32], [33] and improve their nutritional value for herbivores [14], [29], [32].
Here we report changes in the structure of arboreal arthropod communities and in the populations of arthropods following applications of imidacloprid in Central Park, New York and College Park, Maryland. The study site in New York received imidacloprid applications as part of a federally mandated quarantine and eradication effort and randomization of treatments was restricted by law. A common garden study site was established in Maryland to permit randomization of treatments and examine more thoroughly changes in the arthropod community and populations of arthropods in response to applications of imidacloprid. Moreover, to elucidate mechanisms underlying changes in spider mite abundance documented by earlier published reports [9], [14], [15], in laboratory bioassays, we examined direct and indirect effects of imidacloprid on Tetranychus schoenei (Acari: Tetranychidae), the most abundant spider mite on elm trees in New York and Maryland, and two model insect predators, Stethorus punctillum and Chrysoperla rufilabris. Other studies examined independently effects of imidacloprid on populations of other species within Tetranychidae [9], [14], [15], plant quality [14], [32], [33], and natural enemies [15], [34]–[42]. Our research, however, provides the first report of imidacloprid's impact on arthropod communities in urban landscapes and explores multiple mechanisms underlying changes in structure of arthropod fauna and outbreaks of spider mites. This is also the first study to separate the direct impact of imidacloprid on spider mite fecundity from the plant-mediated effect on spider mite reproduction. We show that imidacloprid enhances quality of plants for spider mites that lay more eggs when feeding on imidacloprid-treated plants. Furthermore, the importance of this research is underscored by the rate at which extensive trade exchange, increasing globalization, and climate change exacerbate pest problems [1], [2], [43] and the fact that insecticides like imidacloprid will be used to mitigate problems caused by alien pests [4]–[6].
Results
Preliminary surveys of arthropods in a quarantine zone in New York and effects of imidacloprid on arthropod communities in a common garden study in Maryland
Owing to the fact that a federal agency mandated exactly which trees were treated with imidacloprid in Central Park, the random assignment of treatments was restricted. Therefore, inferences from preliminary surveys conducted in New York are interpreted conservatively and restricted only to trees in New York. Over the three years of this study, more than 254,990 arthropods were collected from the canopies of elms in New York and Maryland. Arachnids dominated communities of arboreal arthropods at both locations (Figure 1A). The less abundant taxa, grouped into ‘Other’ category, included Aphididae (aphids), Saproglyphidae (scavenger mites), Chrysopidae (green lacewings), Cecidomyiidae (predatory midges), Thripidae (thrips) and Coccinellidae (lady beetles in the genus Stethorus). Each taxon in this category was comprised of a small number of species, usually one or two, and did not differ significantly between trees treated with imidacloprid and untreated elms at either site. Eggs of Phytoseiidae and Chrysopidae were enumerated in addition to active stages of the predators, and eggs comprised 51% and 47% of all Phytoseiidae and Chrysopidae, respectively. We also captured other arthropods that did not account for more than 0.05% of all arthropods such as Anthocoridae, Araneae, Miridae, Reduvidae, and lepidopteran larvae among others.
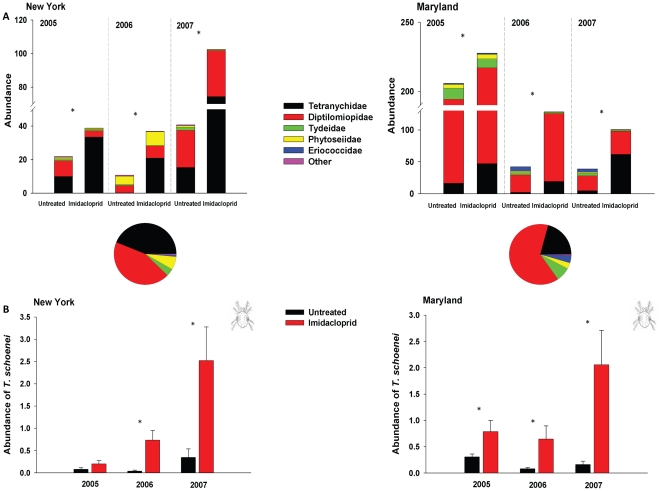
Figure 1. Effect of imidacloprid on arthropod communities and spider mite abundance in New York and Maryland.
(A) Abundance of arthropods (per cm2 of leaf area) on imidacloprid-treated elms (N = 10) and on untreated trees (N = 10). Asterisks mark differences in overall abundance of arthropods that were significant within each year (P<0.05; Monte Carlo permutation test). At both locations, arthropod communities increased on elms that received imidacloprid. Abundance of spider mites, Tetranychidae, explained most of the variation due to imidacloprid treatments. Pie charts represent percent contribution of the most abundant taxa to the sampled arthropod community over three-year period at each location. (B) Abundance (√(number)/cm2) of the spider mite, T. schoenei, on elms treated with imidacloprid (N = 10) and on untreated trees (N = 10) in New York and Maryland. Asterisks mark means±s.e.m. that differed significantly (P<0.05). There was a significant interactive effect of treatment and time for both locations and in most years, and means were compared within each date (Table S1). Elevated densities of mites were found only on elms treated with imidacloprid. Rarely encountered taxa included arthropods in families Chrysopidae, Coccinellidae, Cecidomyiidae, Aphididae, Saproglyphidae and Thripidae. These arthropods were pooled and categorized as ‘Other’.
The first ordination accounted for 10 to 20% of the observed variation in New York and 6 to 18% of the observed variation in Maryland over the course of the study. Imidacloprid significantly altered the structure of arthropod communities at both locations in each year of the study (New York: 2005, F = 11.27, P<0.001; 2006, F = 16.10, P<0.001; 2007, F = 18.89, P<0.001; Maryland: 2005, F = 11.95, P<0.001; 2006, F = 16.58, P<0.001; 2007, F = 16.50, P<0.001) (Figure 1A). With the exception of the first season in New York, T. schoenei, were far more abundant on imidacloprid-treated elms at both locations (New York: 2005, χ2 = 0.13, df = 1, P = 0.722; 2006, χ2 = 8.72, df = 1, P = 0.003; 2007, χ2 = 3.61, df = 1, P = 0.051; Maryland: 2005, χ2 = 5.09, df = 1, P = 0.024; 2006, χ2 = 9.15, df = 1, P = 0.003; 2007, χ2 = 10.86, df = 1, P = 0.001) (Figure 1B). Within most years, significant interactive effects of time and treatment on T. schoenei abundance were evident at both locations (New York: 2005, F 4,89 = 7.27, P<0.001; 2006, F 2,54 = 3.61, P = 0.011; 2007, F 3,72 = 11.57, P<0.001; Maryland: 2005, F 4,85 = 1.93, P = 0.049; 2006, F 2,53 = 0.5, P = 0.737; 2007, F 2,54 = 4.81, P = 0.002). At the beginning of each growing season, T. schoenei were equally abundant on treated and untreated trees, but as the season progressed, T. schoenei became far more abundant on trees treated with imidacloprid (Figure S1, Table S1). In New York, differences in numbers of T. schoenei between treatments waned every fall (Figure S1). Other mites such as the omnivorous tydeids, Homeopronematus anconai and Lorryia sp., were more abundant on untreated trees in most years (Figure 1A, Table S2). H. anconai represented approximately 90% of the tydeid mites collected on all elm trees in New York and Maryland. Predatory phytoseiid mites (all in the genus Galendromus), were more abundant on untreated elms in 2007 in New York and in 2006 and 2007 in Maryland (Figure 1A, Table S2). Herbivorous mites in the family Diptilomiopidae, on the other hand, were more abundant on imidacloprid-treated elms in two out of three years in Maryland (Figure 1A, Table S2). Imidacloprid applications suppressed populations of the scale insect, Gossyparia spuria (Hemiptera: Eriococcidae) on elm trees in Maryland (Table S3). All other arthropods did not contribute significantly to differences in community structure on imidacloprid-treated and untreated elms (Table S4).
Mechanisms underlying outbreaks of T. schoenei: Deleterious effects of imidacloprid on two model insect predators
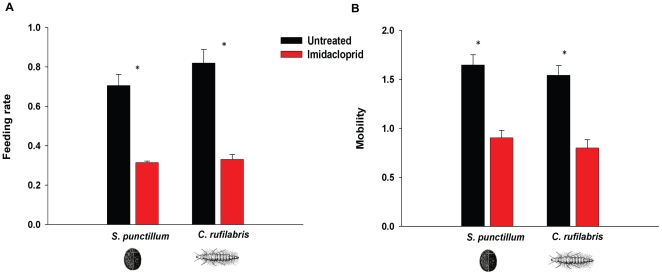
Two important taxa of spider mite predators, Coccinellidae in the genus Stethorus and Chrysopidae were collected at both locations in most years of the study. Several previous studies indicate that lady beetles in the genus Stethorus are highly specialized predators of spider mites and are known to exhibit positive density dependence with increasing prey populations [44]–[46]. Green lacewing larvae, Chrysopidae, are also important generalist predators of spider mites [44], [45]. It was not surprising that Stethorus and Chrysopidae were rare on untreated trees in New York and Maryland, where populations of spider mites remained relatively low in all years of the study (Figures 1 and S1). However, the lack of numerical response by either predator to eruptive mite populations at both sites in all years was perplexing and unexpected. This observation and the fact that others have documented debilitating effects of imidacloprid on predatory insects [35], [36], [41], [42] led us to examine the effects of imidacloprid on the behaviour and longevity of two model predators of spider mites, Stethorus punctillum and Chrysoperla rufilabris. We found that feeding rates of adult S. punctillum and larval C. rufilabris were significantly reduced when T. schoenei from elms treated with imidacloprid were offered as prey for 3.5 h (S. punctillum, F 1,12 = 56.62, P<0.001; C. rufilabris, F1,12 = 44.09, P<0.001; Figure 2A). While there were significant time by treatment interactions for both predators (S. punctillum, F 3,36 = 34.63, P<0.01; C. rufilabris, F 3,36 = 17.79, P<0.01), feeding rates differed significantly between treatments after only 1.5 h and differences grew larger as the experiment progressed (Table S5).
Figure 2. Feeding rate and mobility of S. punctillum and C. rufilabris exposed to imidacloprid through prey.
A) Feeding rates (√(mites eaten)/h) of S. punctillum and C. rufilabris were reduced after 3.5 h when mites were reared on foliage from imidacloprid-treated (N = 7) compared to untreated elms (N = 7). (B) Mobility (√mm/s) of S. punctillum and C. rufilabris was significantly reduced after 3.5 h of exposure to spider mites reared on foliage from imidacloprid-treated elms compared to untreated elms. Means±s.e.m. marked with asterisks are significantly different at P<0.05.
Consumption of prey tainted with imidacloprid directly affected mobility of predators thereby contributing to reduced feeding rates. S. punctillum and C. rufilabris were rapidly intoxicated when exposed to T. schoenei that consumed foliage from trees treated with imidacloprid. Their mobility was reduced after 3.5 h of exposure to T. schoenei from imidacloprid-treated elms (S. punctillum, F 1,12 = 30.95, P<0.001; C. rufilabris, F 1,12 = 32.08, P<0.001; Figure 2B), and the effect was evident after 0.5 h of exposure (Table S6). To determine if the route of exposure was through prey or through contact with imidacloprid-treated foliage, S. punctillum and C. rufilabris were maintained on leaves without T. schoenei. When predators were exposed to leaves from treated or untreated plants, no differences in mobility were observed (S. punctillum, F 1, 84.9 = 0.70, P = 0.4; C. rufilabris, F 1,86.4 = 0.18, P = 0.67; Table S6). Time did not interact with treatment with respect to mobility of the predators (S. punctillum, F 9,61 = 0.5, P<0.87; C. rufilabris, F 9,61 = 1.12, P<0.36). Exposure to imidacloprid or its metabolites appears to occur by ingesting contaminated prey rather than via cuticular absorption from contaminated leaf surfaces. In addition to impaired mobility, predators exhibited clear signs of intoxication including partial or complete lack of response to touch, tremors, regurgitation, excessive grooming, and inability to right themselves when placed on their back.
Consuming prey from leaves of imidacloprid-treated plants dramatically reduced longevity of both predators (S. punctillum, t = 23.04, df = 10.6, P<0.01; C. rufilabris, t = 4.66, df = 6.1, P<0.01). S. punctillum that consumed mites from untreated trees lived 9.34±0.30 (s.e.m.) out of a 10-day observation period, while those that consumed prey from treated plants lived less than one day on average, 0.89±0.21 (s.e.m.). C. rufilabris lived 12.64±2.18 (s.e.m.) days out of a possible 20 days after consuming mites from untreated plants compared to 2.13±0.18 (s.e.m.) days when offered T. schoenei from treated plants.
Mechanisms underlying outbreaks of T. schoenei: Stimulatory effects of imidacloprid on spider mite reproduction
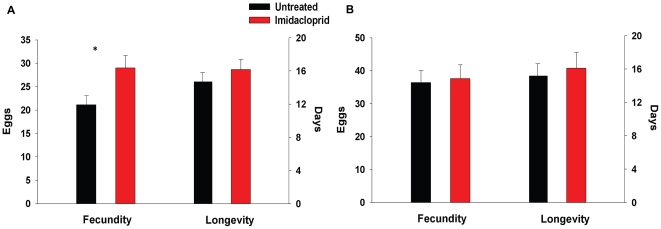
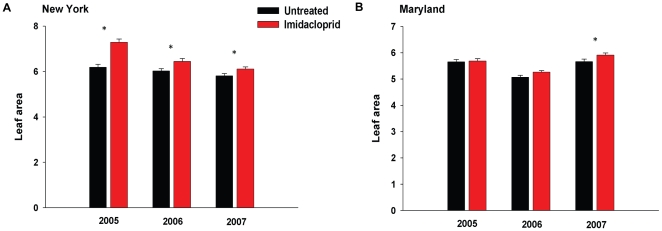
Imidacloprid clearly affected reproduction of T. schoenei thereby revealing another deleterious consequence of its use. In microcosms containing leaves, imidacloprid directly enhanced fecundity of T. schoenei. Adult T. schoenei fed foliage from elms treated with imidacloprid laid more eggs than T. schoenei that consumed leaves from untreated elms (F 1,15 = 4.93, P = 0.042; Figure 3A). While fecundity was enhanced by almost 40%, longevity was not affected (F 1,15 = 1.54, P = 0.23; Figure 3A). Conversely, a direct stimulatory effect of imidacloprid on T. schoenei fecundity was absent when mites were directly sprayed with the pesticide and then offered foliage from elms free of imidacloprid (F 1,58 = 0.52, P = 0.49; Figure 3B). The longevity of T. schoenei was similarly unaffected by dermal exposure to imidacloprid (F 1,58 = 1.45, P = 0.23; Figure 3B). Females sprayed directly with imidacloprid may have been exposed to lower doses of the pesticide than T. schoenei feeding on imidacloprid-treated foliage for extended period of time, thus explaining lack of effect of dermal sprays on T. schoenei fecundity and longevity. Stimulation of T. schoenei's reproductive performance, however, could also be mediated through a physiological response of elms to imidacloprid. Evidence for altered plant physiology was evident in comparisons of leaf areas of treated and untreated elms. Despite housing greater numbers of spider mites, imidacloprid-treated trees had significantly larger leaves in New York each year (2005, F 1,89 = 17.89, P<0.001; 2006, F 1,54 = 6.83, P = 0.009; 2007, F 1,72 = 4.57, P = 0.033), and in 2007 in Maryland (F 1,54 = 5.54, P = 0.02; Figure 4). The interaction between time and treatment did not have significant effects on leaf sizes (New York: 2005, F 4,89 = 0.15, P = 0.963; 2006, F 2,54 = 0.09, P = 0.915; 2007, F 3,72 = 0.13, P = 0.944; Maryland: 2007, F 2,54 = 1.2, P = 0.303). Contrary to an earlier report [14], we found that increased leaf size was not accompanied by increased nitrogen content (Table S7).
Figure 3. Effect of exposure to imidacloprid on lifetime fecundity and longevity of T. schoenei.
(A) Effect of imidacloprid delivered through plant tissue. T. schoenei consuming foliage from elms treated with imidacloprid (N = 8) laid significantly more eggs than spider mites feeding on leaves from untreated trees (N = 8), while their longevity was not affected. (B) Topical application of imidacloprid to T. schoenei. Females (N = 30) sprayed with imidacloprid did not exhibit higher fecundity or longevity than T. schoenei sprayed with water (N = 30). Means±s.e.m. marked with asterisks are significantly different at P<0.05.
Figure 4. Effect of imidacloprid on elm leaf area in New York (A) and Maryland (B).
Figure depicts area of leaves (√cm2) used in surveys of arthropod community presented in Figure 1. Elms (treated: N = 10; untreated: N = 10) in New York had a significantly greater leaf area following exposure to imidacloprid in all sampling years, while trees in Maryland (treated: N = 10; untreated: N = 10) were larger in the last year of the study. Means±s.e.m. marked with asterisks are significantly different at P<0.05.
Discussion
Applications of imidacloprid significantly altered the community of arboreal arthropods associated with elms in Central Park, New York and College Park, Maryland. Spider mites responded strongly to applications of imidacloprid and were far more abundant on treated elms (Figure 1, Table S2). These findings accord with others were pesticides other than imidacloprid reduced the richness, biomass, diversity or abundance of arthropods in apple orchards [17], tomato farms [19], oat fields [20] and corn fields [18]. One striking similarity between this investigation and studies in agricultural systems was the increased abundance of mites following application of pesticides [17], [18]. Several of these studies noted significant reductions in the abundance of many predatory taxa following these applications [18]–[20].
Although previous studies did not examine changes in community structure, elevated populations of spider mites following applications of imidacloprid have been documented previously in systems involving Gleditsia triacanthos (honeylocust) and Platytetranychus multidigituli (honeylocust spider mite) [15], Tsuga canadensis (Canadian hemlock) and Oligonychus ununguis (spruce spider mite) [9] and Rosa sp. (rose) and Tetranychus urticae (twospotted spider mite) [14]. It is noteworthy that elevated populations of mites occurred in several distinct plant families and the possibility of common mechanisms is intriguing. Debilitation of key natural enemies has been suggested as a likely cause of mite outbreaks [15], and our field studies suggest that a loss of top-down suppression of T. schoenei played a role in spider mite outbreaks observed in New York and Maryland. We found evidence that imidacloprid removed the most abundant natural enemy of spider mites in our study, Galendromus spp.. The abundance of Galendromus spp. was lower on imidacloprid-treated trees in 2007 in New York and in 2006 and 2007 in Maryland, while the predatory mites did not differ in their abundance between imidacloprid-treated and untreated elms in the remaining years of the study (Table S2). The fact that imidacloprid did not consistently remove Galendromus spp. from imidacloprid-treated elms in each year suggests that other factors played a role in population dynamics of this predator. While the response of Galendromus spp. to imidacloprid exposure in the field has not been examined before, several laboratory [38], [39] and greenhouse [37] studies report toxicity of imidacloprid to Galendromus spp.. Moreover, in a laboratory experiment, imidacloprid exposure had a negative impact on functional responses of phytoseiid mites from other genera (Neoseiulus and Phytoseiulus) [40], and it is possible that foraging of Galendromus spp. was affected by imidacloprid in the present study as well. Further research is necessary to establish if imidacloprid exposure dampens foraging efficiency and lowers survival of Galendromus spp. in the field.
Whereas the phytoseiid predators were likely able to persist on untreated elms owing to abundant alternative prey, the eriophyid mites, untreated trees had few arthropods that could serve as prey to Stethorus and Chrysopidae. Thus, lack of the key insect predators on these trees is not surprising. Their absence on imidacloprid-treated trees, however, suggests that imidacloprid had a negative effect on the insect predators of spider mites. Laboratory assays clearly demonstrated debilitating consequences of imidacloprid exposure to two model insect predators of spider mites, S. punctillum and C. rufilabris. Consumption of T. schoenei fed leaves from imidacloprid-treated elms reduced feeding and mobility and shortened longevity of both predators. Our results concur with others that show lethal and sublethal effects of imidacloprid exposure to lady beetles, lacewings, and parasitic wasps [36], [41], [42], [47]. It is clear from our laboratory experiments that S. punctillum and C. rufilabris gained exposure to imidacloprid through consumption of tainted prey and were adversely affected. Prey-mediated toxicity of imidacloprid to predators such as Coccinellidae and Chrysopidae, may have precluded successful colonization and positive density dependence of important insect predators on elms treated with imidacloprid.
Enhanced fecundity of T. schoenei on elms treated with imidacloprid likely contributed in a significant way to eruptive populations of spider mites in New York and Maryland. Elevated fecundity of T. schoenei in this report is consistent with studies of imidacloprid's effects on a related spider mite, T. urticae, in some [31], [32], but not other studies [48]. Enhanced fecundity can play an important contributory role in outbreaks especially for multivoltine pests like spider mites [29], [49]. Dramatic increases in T. schoenei abundance late in the growing season are consistent with previously documented outbreaks of spider mites in urban settings related to elevated temperatures, pollution, or loss of top-down suppression by natural enemies [49], [50].
Notably, this is the first study to show that imidacloprid enhances spider mite fecundity via a plant-mediated mechanism. We demonstrated that imidacloprid exposure enhances reproduction of spider mites only when spider mites feed on foliage from imidacloprid-treated elms. This bottom-up effect of the insecticide implies altered quality of the plant for spider mites. Other studies tie imidacloprid's influence on plant physiology to pest outbreaks as well. Increased fecundity and resurgence of a lepidopteran herbivore, pyralid moth Tryporyza incertulas, was linked to decreased activity of a detoxification enzyme, glutathione S-transferase in rice treated with imidacloprid [51], [52]. Moreover, Rosa sp. treated with imidacloprid housed greater numbers of spider mites and had significantly greater chlorophyll indices, leaf area, and nitrogen concentration than untreated plants [14]. We suggest that imidacloprid alters plants in a way that enhances the quality of plants as hosts for T. schoenei and consistently larger elm leaves lend support to this hypothesis. Further, imidacloprid was recently shown to induce expression of genes involved in plant defenses against pathogens [53] and reduce expression of protease inhibitor genes employed in plant defense against herbivores [54]. It is noteworthy that separate pathways regulate plant defenses against pathogens and herbivores, and in several model plants, the two pathways exhibit antagonistic interactions [55]. If imidacloprid applications result in deployment of pathogen-related responses, pivotal plant defenses against herbivores could be adversely affected. Decreased expression of genes for protease inhibitors, for example, which reduce digestibility of plant proteins, would allow spider mites to assimilate nutrients more efficiently and may explain their increased fecundity on imidacloprid-treated plants. Certainly, the mechanisms by which imidacloprid alters plant physiology and leads to enhanced herbivore performance and ultimately changes the entire arthropod community deserve further examination.
Previous accounts attributed secondary outbreaks of pests coincident with area-wide eradication programs such as the one is Central Park to disruption of top-down regulation by predatory arthropods [26], [29], [56]. Our study indicates that plant-mediated enhancement of mite reproduction may conspire with relaxation of suppression by predators to elevate mite populations on trees treated with imidacloprid. A holistic approach that considers both top-down and bottom-up forces will lead to a clearer understanding of the mechanisms underlying pest outbreaks following the application of pesticides [27], [29]. This is especially important in light of increasing pressure to mitigate deleterious ecological and economic impacts linked to the ongoing global deluge of invasive species.
Materials and Methods
Applications of imidacloprid
In Central Park, applications of imidacloprid were administered in the spring of each year, and doses of the pesticide were calibrated based on trunk diameter of each U. americana measured at a standardized height of approximately 150 cm from the ground. This is a standard procedure for determining appropriate doses of pesticides applied to trees. Certified pesticide applicators used two formulations and three methods of application for imidacloprid. All methods and doses of pesticides were in compliance with manufacturers' requirements and recommendations mandated by APHIS, the federal agency imposing the treatments. In 2005, 1323 trees received trunk injections of liquid imidacloprid formulated as 10% active ingredient (Imicide® Hp, J.J. Mauget Co., Arcadia, CA, USA) applied at the rates of 2 ml for every 2.54 cm of trunk diameter for trees ranging between 5.1 to 27.9 cm of trunk diameter, 4 ml per 2.54 cm for trees with trunk diameters of 30.5 to 58.42 cm, 8 ml per 2.54 cm for trees 61.0 to 88.9 cm of trunk diameter, and 12 ml per 2.54 cm for trees greater than 88.9 cm trunk diameter. Twenty eight trees received a soil drench of imidacloprid formulated as a 75% active ingredient wettable soluble powder and 3483 trees received a soil injection of Merit® 75 WSP or Bandit® 75 WSP (Bayer Environmental Science, Research Triangle Park, NC, USA) at the rate of 227 g per 2.54 cm of trunk diameter. In 2006, 1448 trees received trunk injections of imidacloprid (Imicide® Hp), 12 trees received soil drenches of imidacloprid (Merit® 75 WSP) at the rate of 113 g per 2.54 cm of trunk diameter, and 3401 received imidacloprid as soil injections (Merit® 75 WSP or Bandit® 75 WSP) at the rate of 113 g per 2.54 cm of trunk diameter. In 2007, 301 trees received trunk injections of imidacloprid (Imicide® Hp) and 4716 received imidacloprid as soil injections (Merit® 75 WSP or Bandit® 75 WSP) at the rate of 113 g per 2.54 cm of trunk diameter. Table S8 details the number of trees in different sizes classes treated by different methods of application for all years of the study. All trees outdoors and in the greenhouse at College Park received imidacloprid formulated as a 75% active ingredient wettable powder (Merit® 75 WP) at the rate of 1.96 g per 2.54 cm of trunk diameter applied by A.S., S.F.C., and M.J.R. All methods of imidacloprid applications used in New York and Maryland are commonly employed to suppress susceptible insect herbivores on trees in urban and residential landscapes. Imidacloprid at both locations was applied at label dose specified by the manufacturer of each pesticide formulation.
Community structure in New York and Maryland
Central Park was in the federal quarantine zone established by USDA-APHIS to eradicate A. glabripennis [57]. The agency imposed mandatory applications of pesticides to trees within an 800-m radius of every confirmed A. glabripennis infestation [57]. Thus, while each tree was treated independently, there was a spatial limitation to the random assignment of trees to treatments. We recognize that this limitation restricts inferences only to trees sampled in Central Park, hence, the need for a second study site in Maryland. In Central Park, 86th Street marked the boundary for the eradication zone, and all elms located south of 86th Street received individual treatments of imidacloprid. Each year, ten different treated and untreated elms were sampled. All trees were mature, and measured approximately 10–30 m in height. In 2005, trees on two east-west transects across the park north and south of 86th Street were sampled in untreated and treated zones, respectively. In 2006 trees were sampled on the western boundary of the park parallel to 8th Avenue north and south of the treatment demarcation line at 86th Street and in 2007, trees along the eastern boundary along 5th Avenue north and south of the demarcation boundary were included in the study. Elms were sampled every three to six weeks and leaf samples were collected five times in 2005, three times in 2006 and four times in 2007. The experiment was repeated in Maryland using a stand of U. americana in a maintained margin lining a boulevard. In Maryland, 20 U. americana were randomly assigned to two treatments. Half received annual applications of imidacloprid each spring and half served as untreated controls. U. americana in the Maryland study were younger than those in New York and measured approximately 3–5 m in height. Ten treated and ten untreated elms were sampled every two to six weeks for three consecutive years.
In all years at both locations, four branches approximately 15–30 cm long were removed from each cardinal position on each tree. Due to the height of the canopies of trees in New York, pole pruners were used in combination with hand pruners to collect samples. Small trees in Maryland with lower canopies were sampled with hand pruners. The excised foliage from each tree was collectively bagged, placed in a cooler, and brought back to the laboratory and refrigerated until arthropods were counted using a dissecting microscope. This method of sampling has been used to estimate densities of mites and their predators in a wide variety of trees in landscapes [15], [58]–[60] and agricultural fields [61]–[63]. All arthropods on the adaxial and abaxial surfaces of the two most terminal leaves were counted, and natural enemies and their eggs were noted on three additional leaves occupying position 3–5 on the branch's terminus. Abundance of all arthropods was tallied as a function of measured leaf area (cm2) recorded for each leaf using an area meter (LI-COR Environmental, Lincoln, NE, USA). In 2005, all leaves collected at both sites were dried at 70°C for seven days and ground in a plant mill (Capitol Scientific, Austin, USA). Percent nitrogen content of the samples was analyzed by the Environmental Analysis Research Lab at the University of Maryland using dry combustion method [64].
Mechanisms underlying outbreaks of T. schoenei: Deleterious effects of imidacloprid on two model insect predators
In a greenhouse, 14 U. americana planted in containers were randomly assigned to one of two treatments. Half received imidacloprid applications as described above for the Maryland study and half were assigned as untreated controls. Trees were arranged in a randomized complete block design in two rows within the greenhouse space. Trees were 1.8 m apart and canopies of individual trees were not in contact with adjacent elms. After imidacloprid applications, all trees received branches infested with T. schoenei from an untreated elm to establish colonies of mites. T. schoenei were allowed to multiply for two months prior to bioassays. To assess insecticide-related changes in foraging and mobility of the predators we followed the protocol of James and Price [31] previously used to measure spider mite fecundity. Leaves were removed from treated and untreated trees and leaf disks 22 mm in diameter were punched from each leaf with an apple corer (Progressive International, Kent, WA, USA). Leaf disks were cleaned of mites and placed lower side down in a 40 mm Petri dish filled with saturated cotton gauze. Ten adult female mites were transferred from each tree and placed on the respective leaf disk. Commercially purchased (IPM Labs, Locke, NY, USA) predators, a single adult S. punctillum or a larva of C. rufilabris was then introduced to the leaf disk. The number of mites consumed was recorded after 0.5, 1.5, 2.5, and 3.5 h of exposure. At each time-interval, predators were removed from arenas to measure their mobility. Mobility was assessed by placing a predator at the center of a 40 mm diameter circle, and recording the time required to escape from the circle.
In a separate experiment, predators were also exposed to leaves from treated and untreated trees without prey to determine if dermal exposure to contaminated leaf surfaces affected mobility thereby separating the effect of intoxication through contact with treated leaves from the effect of intoxication by ingestion of prey. An arena was constructed of a 118 mL Solo Cup (Solo Cup Company, Urbana, IL, USA). A water source was included which consisted of a trimmed micropipette tip stuffed with water-saturated cotton gauze. Each arena was supplied with 2–3 excised leaves from treated or untreated elms from which all life stages of mites were removed. Mobility was assessed as in the previous assay at the same intervals of time. The bioassays for each predator species included at least three subsamples in all replicates and treatment combinations (n = 95 for S. punctillum and n = 84 for C. rufilabris).
The effect of consuming tainted prey on predator longevity was assessed by exposing S. punctillum adults and C. rufilabris larvae to the following treatments. S. punctillum adults or the C. rufilabris larvae were individually placed in a 118 mL Solo Cup and supplied with a trimmed micropipette tip filled with cotton saturated with a sugar-water solution (10 mg sugar: 100 mg water). Leaves infested with T. schoenei from treated or untreated elms described in the previous study were provided at the beginning of the experiment and replaced every other day thereafter to ensure that prey were not limiting. The arenas were held under lighted ambient laboratory conditions (23±2°C) for the duration of the assays. Every 24 hours, predators were observed and considered dead if they were completely unresponsive to the touch of a probe and not making any movements. Assays with C. rufilabris were conducted for 20 days and assays for S. punctillum were conducted for 10 days. Trees were replicates and predators were subsamples (2 or 3 predators per tree × 7 trees).
Mechanisms underlying outbreaks of T. schoenei: Stimulatory effects of imidacloprid on spider mite reproduction
In a common garden experiment, an additional 16 U. americana were planted at the University of Maryland Turf Research Farm at College Park, Maryland in May 2005. The trees were uniform in size and measured 1.5 m at the time of planting. The elms were randomly assigned to one of two treatments. Half received imidacloprid and half of the trees were untreated controls. Imidacloprid was applied in June 2006 and May 2007. Foliage from these elms was used in all experiments assessing T. schoenei fecundity, which were carried out in August and September 2007. One month before bioassays with T. schoenei were initiated, T. schoenei colonies were established in the laboratory from naturally occurring populations and maintained on leaves from untreated elms in growth chambers (Percival Scientific, Perry, IA, USA) at 23±2°C and 16∶8 light∶ dark. T. schoenei were then removed from colonies of mites reared on insecticide-free trees for at least two generations and used in the assays. One hundred and forty four T. schoenei females of the same age were randomly assigned to treated or untreated leaves and enclosed in 60 mm plastic clip cages. Mites were transferred to new leaves every other day. Leaves with mites in clip cages were placed in growth chambers maintained at 23±2°C and 16∶8 light∶ dark. Lifetime fecundity and longevity were recorded for all females maintained on foliage from eight treated and eight untreated elms. Trees were replicates and individual T. schoenei were subsamples (nine per replicate).
To test effects of direct exposure to imidacloprid, 60 even-aged females reared on foliage from insecticide-free trees were randomly assigned to one of two treatments. Half of the females received sprays of imidacloprid and half received sprays of distilled water delivered by a Potter Spray Tower® (Burkard, Rickmansworth, UK). Two mL of flowable formulation of Admire® (2 g of imidacloprid/L, Bayer Environmental Science) were delivered at 50 kPa, resulting in an average application of 1.6–1.8 mg of liquid per cm2. Imidacloprid applied at this rate to bean leaves was previously shown to enhance spider mite fecundity [31]. Females were enclosed in clip cages and maintained on insecticide-free leaves for the duration of the experiment in growth chambers under conditions described previously. Lifetime fecundity and longevity were measured. In this experiment, individual females were replicates.
Statistical analyses
To test and visualize how the community of arthropods responded to imidacloprid treatment through time, we utilized a constrained form of principal components analysis called principal response curve (PRC), a multivariate approach based on redundancy analysis [18], [65], [66]. It performs weighted least-squares regression of values of inert and latent variables, referred to as axes, extracted from the species abundance data on treatment and time. The weights are based on abundance of each taxon relative to its accumulation in the control treatment; therefore, response of the sampled arthropod fauna is expressed as deviation from the community in control treatment. The analysis provides an exact significance test. Monte-Carlo permutations are used to test for significance of the response curve. An F test statistic is calculated and the permutations produce 1,000 new data sets that are equally likely under a null hypothesis of canonical coefficients equalled zero. Significance is then computed based on the proportion of F values greater or equal to the F value of the original data set [66]. In addition to examining community structure with canonical coefficients, PRC scores were used to examine responses of individual taxa to insecticide applications. Following the convention of Dively [18], taxa with coefficients near zero (0.5 to −0.5) were considered to have no response or one unrelated to the overall pattern expressed by the PRCs.
Effects of imidacloprid treatments on spider mite densities, prey consumption, predator mobility, predator longevity, and mite fecundity and longevity were evaluated by analysis of variance with repeated measures, randomized complete block analysis of variance, or two sample t-tests. Transformations corrected heteroschedastic data prior to analyses. Non-parametric Kruskal-Wallis tests (χ2 statistic) were used when assumptions of parametric analysis could not be satisfied [67].
Supporting Information
Abundance (√number/cm2) of the spider mite, T. schoenei, on elms treated with imidacloprid (N = 10) and on untreated trees (N = 10) in New York (A) and Maryland (B). Asterisks mark means±s.e.m. that differed significantly within each sampling date (P<0.05) (Tukey's test).
(TIF)
Comparisons of abundance of T. schoenei on elms treated with imidacloprid and untreated elms in New York (NY), and Maryland (MD).
(DOC)
Comparison of abundance of Tydeidae, Diptilomiopidae and Phytoseiidae on elms treated with imidacloprid and untreated trees in New York (NY) and Maryland (MD).
(DOC)
Comparisons of abundance (number/cm2) of Eriococcidae on elms treated with imidacloprid and untreated elms in Maryland.
(DOC)
Species scores were generated by PRC analysis to examine responses of individual taxa to imidacloprid applications.
(DOC)
Comparison of feeding rates of S. punctillum and C. rufilabris exposed to spider mites that consumed foliage from imidacloprid-treated elms and untreated elms.
(DOC)
Comparison of mobility of S. punctillum and C. rufilabris exposed to imidacloprid in prey and foliage.
(DOC)
Comparison of nitrogen levels in elm trees treated with imidacloprid in New York (NY) and Maryland (MD) in 2005
(DOC)
The number of trees in different size classes treated by different methods of application for 2005, 2006, and 2007.
(DOC)
Acknowledgments
We thank S. Bealmear, S. Grimard, K. Hand, B. Raupp, E. Raupp, J. Rogers, C. Tierno, S. Wagner, M. Vogel, and Z. Vogel for assistance in the field and laboratory; W. Berliner and N. Calvanese for assistance at Central Park; Department of Physical Plant, University of Maryland, for permitting pesticide applications to elms; G. Dively for help with statistical analyses; and R. Ochoa, R. Gordon, and C. Welbourn for identifying the arthropods. Authors are grateful to M.D. Eubanks and D.S. Gruner for friendly review of the manuscript and three anonymous reviewers, who provided several comments that greatly improved the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by the National Research Initiative Competitive Grants Program of USDA 2005-35303-16269 and International Society of Arboriculture Tree Fund grants to M.J.R. and Gahan and Bamford Fellowships to A.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Walther GR, Roques A, Hulme PE, Sykes MT, Pysek P, et al. Alien species in a warmer world: risks and opportunities. Trends in Ecology & Evolution. 2009;24:686–693. doi: 10.1016/j.tree.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Corn ML, Buck EH, Rawson J, Segarra A, Fischer E. Invasive non-native species: Background and issues for Congress. RL30123. Congressional Research Service. 2002:1–91. [Google Scholar]
- 3.Pimentel D, Morrison D, Zuniga R. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics. 2005;52:273–288. [Google Scholar]
- 4.Kovacs KF, Haight RG, McCullough DG, Mercader RJ, Seeger NW, et al. Cost of potential emerald ash borer damage in U.S. communities, 2009–2019. Ecological Economics. 2009;69:569–578. [Google Scholar]
- 5.Raupp MJ, Buckelew Cumming A, Raupp EC. Street tree diversity in eastern North America and its potential for tree loss to exotic pests. Journal of Arboriculture. 2006;32:297–304. [Google Scholar]
- 6.Raupp MJ, Szczepaniec A, Buckelew Cumming A. Prophylactic pesticide applications and low species diversity: Do they create pest outbreaks in the urban forest? Interagency Research on Invasive Species [USDA Proc forum] 2007:59–61. [Google Scholar]
- 7.Cappaert D, McCullough DC, Poland TM, Siegert NW. Emerald ash borer in North America: A research and regulatory challenge. American Entomologist. 2005;51:152–165. [Google Scholar]
- 8.Mullins JW. Imidacloprid: a new nitroguanidine insecticide. American Chemical Society. 1993;524:183–198. [Google Scholar]
- 9.Raupp MJ, Webb R, Szczepaniec A, Booth D, Ahern R. Incidence, abundance, and severity of mites on hemlocks following applications of imidacloprid. Journal of Arboriculture. 2004;30:108–13. [Google Scholar]
- 10.Frank S, Ahern R, Raupp MJ. Does imidacloprid reduce defoliation by Japanese beetles on linden for more than one growing season? Journal of Arboriculture. 2007;33:392–396. [Google Scholar]
- 11.Szczepaniec A, Raupp MJ. Residual toxicity of imidacloprid to hawthorn lace bug, Corythuca cydoniae, feeding on cotoneasters in landscapes and containers. Journal of Environmental Horticulture. 2007;25:43–46. [Google Scholar]
- 12.Cowles RS, Montgomery ME, Cheah CSJ. Activity and residues of imidacloprid applied to soil and tree trunks to control hemlock woolly adelgid (Hemiptera: Adelgidae) in forests. Journal of Economic Entomology. 2006;99:1258–1267. doi: 10.1603/0022-0493-99.4.1258. [DOI] [PubMed] [Google Scholar]
- 13.Tomizawa M, Casida JE. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annual Review of Entomology. 2003;48:339–64. doi: 10.1146/annurev.ento.48.091801.112731. [DOI] [PubMed] [Google Scholar]
- 14.Gupta G, Krischik VA. Professional and consumer insecticides for the management of adult Japanese beetle on hybrid tea rose. Journal of Economic Entomology. 2007;100:830–837. doi: 10.1603/0022-0493(2007)100[830:pacifm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Sclar DC, Gerace D, Cranshaw WS. Observations of population increase and injury by spider mites (Acari: Tetranychidae) on ornamental plants treated with imidacloprid. Journal of Economic Entomology. 1998;91:250–255. [Google Scholar]
- 16.Berliner W. Associate Vice-President of Horticulture, Central Park Conservancy, Personal communication. 2008.
- 17.Brown MW, Adler CRL. Community structure of phytophagous arthropods on apple. Environmental Entomology. 1989;18:600–607. [Google Scholar]
- 18.Dively GP. Impact of transgenic VIP3A x Cry1Ab lepidopteran-resistant field corn on the nontarget arthropod community. Environmental Entomology. 2005;34:1267–1291. [Google Scholar]
- 19.Letourneau DK, Goldstein B. Pest damage and arthropod community structure in organic vs. conventional tomato production in California. Journal of Applied Ecology. 2001;38:557–570. [Google Scholar]
- 20.Suttman CE, Barrett GW. Effects of sevin on arthropods in an agricultural and old-field plant community. Ecology. 1979;60:628–641. [Google Scholar]
- 21.Berenzen N, Kumke T, Schulz HK, Schulz R. Macroinvertebrate community structure in agricultural streams: impact of runoff-related pesticide contamination. Ecotoxicology and Environmental Safety. 2005;60:37–46. doi: 10.1016/j.ecoenv.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Schafer RB, Caquet T, Siimes K, Mueller R, Lagadic L, et al. Effects of pesticides on community structure and ecosystem functions in agricultural streams of three biogeographical regions in Europe. Science of the Total Environment. 2007;382:272–285. doi: 10.1016/j.scitotenv.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 23.Fairchild WL, Eidt DC. Perturbation of the aquatic invertebrate community of acidic bog ponds by the insecticide fenitrothion. Archives of Environmental Contamination and Toxicology. 1993;25:170–183. [Google Scholar]
- 24.Rohr JR, Crumrine PW. Effects of an herbicide and an insecticide on pond community structure and process. Ecological Applications. 2005;15:1135–1147. [Google Scholar]
- 25.Luck RF, Dahlsten DL. Natural decline of a pine needle scale (Chionaspis pinifoliae (Fitch)) outbreak at South Lake Tahoe, California, following cessation of adult mosquito control with malathion. Ecology. 1975;56:893–904. [Google Scholar]
- 26.Dreistadt SH, Dahlsten DL. Medfly eradication in California: lessons from the field. Environment. 1986;28(6):18–20, 40–44. [Google Scholar]
- 27.Dutcher JD. Amsterdam: Springer Dordrecht; 2007. A review of resurgence and replacement causing pest outbreaks in IPM. In: Ciancio A, Mukerji KG, editors. General Concepts in Integrated Pest and Disease Management. pp. 27–43. [Google Scholar]
- 28.Costamagna AS, Landis DA, Difonzo DC. Suppression of soybean aphid by generalist predators results in a trophic cascade in soybeans. Ecological Applications. 2007;17:441–451. doi: 10.1890/06-0284. [DOI] [PubMed] [Google Scholar]
- 29.Raupp MJ, Shrewsbury PM, Herms DH. Ecology of herbivorous arthropods in urban landscapes. Annual Review of Entomology. 2010;55:19–38. doi: 10.1146/annurev-ento-112408-085351. [DOI] [PubMed] [Google Scholar]
- 30.Luckey TD. Insect hormoligosis. Journal of Economic Entomology. 1968;61:7–12. doi: 10.1093/jee/61.1.7. [DOI] [PubMed] [Google Scholar]
- 31.James DG, Price TS. Fecundity of twospotted spider mite (Acari: Tetranychidae) is increased by direct and systemic exposure to imidacloprid. Journal of Economic Entomology. 2002;27:151–156. doi: 10.1603/0022-0493-95.4.729. [DOI] [PubMed] [Google Scholar]
- 32.Chiriboga A. MSc thesis. The Ohio State Univ; 2009. Physiological responses of woody plants to imidacloprid formulations.130 [Google Scholar]
- 33.Tenczar EG, Krischik VA. Management of cottonwood leaf beetle (Coleoptera: Chrysomelidae) with a novel transplant soak and biorational insecticides to conserve coccinellid beetles. Journal of Economic Entomology. 2006;99:102–108. doi: 10.1093/jee/99.1.102. [DOI] [PubMed] [Google Scholar]
- 34.Rebek EJ, Sadof CS. Effects of pesticide applications on the euonymus scale (Homoptera: Diaspididae) and its parasitoid, Encarsia citrina (Hymenoptera: Aphelinidae). Journal of Economic Entomology. 2003;96:446–452. doi: 10.1093/jee/96.2.446. [DOI] [PubMed] [Google Scholar]
- 35.Cole PG, Horne PA. The impact of aphicide drenches on Micromus tasmaniae (Walker) (Neuroptera: Hemerobiidae) and the implications for pest management in lettuce crops. Australian Journal of Entomology. 2006;45:244–248. [Google Scholar]
- 36.Rogers MA, Krischik VA, Martin LA. Effect of soil application of imidacloprid on survival of adult green lacewing, Chrysoperla carnea, (Neuroptera: Chrysopidae), used for biological control in greenhouse. Biological Control. 2007;42:171–177. [Google Scholar]
- 37.Stavrinides MC, Mills NJ. Demographic effects of pesticides on biological control of Pacific spider mite (Tetranychus pacificus) by the western predatory mite (Galendromus occidentalis). Biological Control. 2009;48:267–273. [Google Scholar]
- 38.Bostanian NJ, Thistlewood HA, Hardman JM, Laurin MC, Racette G. Effect of seven new orchard pesticides on Galendromus occidentalis in laboratory studies. Pest Management Science. 2009;65:635–639. doi: 10.1002/ps.1721. [DOI] [PubMed] [Google Scholar]
- 39.James DG. Toxicity of imidacloprid to Galendromus occidentalis, Neoseiulus fallacies, Amblyseius andersoni (Acari: Phytoseiidae) from hops in Washington State, USA. Experimental and Applied Acarology. 2003;31:275–281. doi: 10.1023/b:appa.0000010383.33351.2f. [DOI] [PubMed] [Google Scholar]
- 40.Poletti M, Maia AHN, Omoto C. Toxicity of neonicotinoid insecticides to Neoseiulus californicus and Phytoseiulus macropilis (Acari: Phytoseiidae) and their impact on functional response to Tetranychus urticae (Acari: Tetranychidae). Biological Control. 2007;40:30–36. [Google Scholar]
- 41.James DG. Pesticide susceptibility of two coccinellids (Stethorus punctum picipes and Harmonia axyridis) important in biological control of mites and aphids in Washington hops. Biocontrol Science and Technology. 2003;13:253–259. [Google Scholar]
- 42.Papachristos DP, Milonas PG. Adverse effects of soil applied insecticides on the predatory coccinellid Hippodamia undecimnotata (Coleoptera: Coccinellidae). Biological Control. 2008;47:77–81. [Google Scholar]
- 43.Mooney HA, Cleland EE. The evolutionary impact of invasive species. Proceedings of National Academy of Sciences. 2001;98:5446–5451. doi: 10.1073/pnas.091093398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chazeau J. in Spider Mites. In: Helle W, Sabelis MW, editors. Their Biology, Natural Enemies and Control. Amsterdam: Elsevier, Vol B; 1985. pp. 211–246. [Google Scholar]
- 45.McMurtry MA, Huffaker CB, Van de Vrie M. Ecology of tetranychid mites and their natural enemies: A review. In: Tetranychid mites: their biological characteristics and the impact of spray practices. Hilgardia. 1970;40:331–390. [Google Scholar]
- 46.Hull LA, Asquith D, Mowery PD. Distribution of Stethorus punctum in relation to densities of the European red mite. Environmental Entomology. 1976;5:337–342. [Google Scholar]
- 47.Rogers ME, Potter DA. Effects of spring imidacloprid application for white grub control on parasitism of Japanese beetle (Coleoptera: Scarabaeidae) by Tiphia vernalis (Hymenoptera: Tiphiidae). Journal of Economic Entomology. 2003;96:1412–1419. doi: 10.1093/jee/96.5.1412. [DOI] [PubMed] [Google Scholar]
- 48.Ako M, Poehling HM, Borgemeister C, Nauen R. Effect of imidacloprid on the reproduction of acaricide-resistant and susceptible strains of Tetranychus urticae Koch (Acari: Tetranychidae). Pest Management Science. 2006;62:419–424. doi: 10.1002/ps.1182. [DOI] [PubMed] [Google Scholar]
- 49.Kropczynska D, van de Vrie M, Tomczyk A. Bionomics of Eotetranychus tiliarium and its phytoseiid predators. Experimental and Applied Acarology. 1988;5(1):65–81. [Google Scholar]
- 50.Schneider K, Balder H, Jackel B, Pradel B. Bionomics of Eotatranychus tiliarum as influenced by key factors. In: Backhaus GF, Balder H, Idczak E, editors. International Symposium on Plant Health in Urban Horticulture. Berlin: Parey Buchverlag; 2000. pp. 102–108. [Google Scholar]
- 51.Wu JC, et al. Impacts of pesticides on physiology and biochemistry of rice. Scientia Agriculatura Sinica. 2003;36:536–541. [Google Scholar]
- 52.Wang AH, Wu JC, Yu YS, Liu JL, Yue JF, et al. Selective insecticide-induced stimulation on fecundity and biochemical changes in Tryporyza incertulas (Lepidoptera: Pyralidae). Journal of Economical Entomology. 2005;98:1144–1149. doi: 10.1603/0022-0493-98.4.1144. [DOI] [PubMed] [Google Scholar]
- 53.Ford KA, Casida JE, Chandran D, Gulevich AG, Okrent RA, et al. Neonicotinoid insecticides induce salicylate-associated plant defense responses. Proceedings of National Academy of Science. 2010;107:17527–17532. doi: 10.1073/pnas.1013020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szczepaniec A. The Univ. of Maryland; 2009. Mechanisms underlying outbreaks of spider mites following applications of imidacloprid. PhD thesis.163 [Google Scholar]
- 55.Doares SH, Narvaez-Vasquez J, Conconi A, Ryan CA. Salicylic acid inhibits synthesis of proteinase inhibitor in tomato leaves induced by systemin and jasmonic acid. Plant physiology. 1995;108:1741–1746. doi: 10.1104/pp.108.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeBach P, Rose M. Environmental upsets caused by chemical eradication. California Agriculture. 1977;31:8–10. [Google Scholar]
- 57.USDA APHIS. Riverdale, MD: Environmental Assessment; 2007. Asian Longhorned Beetle Cooperative Eradication Program in the New York Metropolitan Area. [Google Scholar]
- 58.Kropczynska D, Czajkowska B, Tomczyk A, Kielkiewicz M. Mite communities on linden trees (Tilia sp.) in an urban environment. In: Bernini F, Nannelli R, Nuzaci G, DeLillio E, editors. In Acarid phylogeny and evolution: Adaptation in mites and ticks. Dordrecht: Springer; 2002. pp. 303–313. [Google Scholar]
- 59.Balder H, Jackel B, Pradel B. Investigations on the existence of beneficial organisms on urban trees in Berlin. Acta Horticulturae. 1999;496:189–194. [Google Scholar]
- 60.Shrewsbury P, Hardin M. Evaluation of predatory mite (Acari: Phytoseiidae) releases to suppress spruce spider mites, Oligonychus ununguis (Acari: Tetranychidae), on juniper. Journal of Economic Entomology. 2003;96:1675–1684. doi: 10.1093/jee/96.6.1675. [DOI] [PubMed] [Google Scholar]
- 61.Le Roy M, Brodeur J, Cloutier C. Seasonal abundance of spider mites and their predators on red raspberry in Quebec, Canada. Environmental Entomology. 1999;28:735–747. [Google Scholar]
- 62.Pratt P, Croft B. Screening of predatory mites as potential control agent of pest mites in landscape plant nurseries of the Pacific Northwest. Journal of Environmental Horticulture. 2000;18:218–223. [Google Scholar]
- 63.Croft B, Slone D. Equilibrium densities of European red mite (Acari: Tetranychidae) after exposure to three levels of predacious mite diversity on apple. Environmental Entomology. 1997;26:391–399. [Google Scholar]
- 64.Yeomans JC, Bremner JM. Carbon and nitrogen analysis of soils by automated combustion techniques. Communications in Soil and Plant Analysis. 1991;22:843–850. [Google Scholar]
- 65.van den Brink PJ, ter Braak CJF. Principal response curves: analysis of time-dependent multivariate responses of a biological community to stress. Environmental Toxicology and Chemistry. 1999;18:138–148. [Google Scholar]
- 66.Prasifka JR, Hellmich RL, Dively GP, Lewis LC. Assessing the effects of pest management on nontarget arthropods: the influence of plot size and isolation. Environmental Entomology. 2005;34:1181–1192. [Google Scholar]
- 67.Zar J. Upper Saddle River: Prentice Hall; 1999. Biostatistical Analysis. 4th ed.929 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Abundance (√number/cm2) of the spider mite, T. schoenei, on elms treated with imidacloprid (N = 10) and on untreated trees (N = 10) in New York (A) and Maryland (B). Asterisks mark means±s.e.m. that differed significantly within each sampling date (P<0.05) (Tukey's test).
(TIF)
Comparisons of abundance of T. schoenei on elms treated with imidacloprid and untreated elms in New York (NY), and Maryland (MD).
(DOC)
Comparison of abundance of Tydeidae, Diptilomiopidae and Phytoseiidae on elms treated with imidacloprid and untreated trees in New York (NY) and Maryland (MD).
(DOC)
Comparisons of abundance (number/cm2) of Eriococcidae on elms treated with imidacloprid and untreated elms in Maryland.
(DOC)
Species scores were generated by PRC analysis to examine responses of individual taxa to imidacloprid applications.
(DOC)
Comparison of feeding rates of S. punctillum and C. rufilabris exposed to spider mites that consumed foliage from imidacloprid-treated elms and untreated elms.
(DOC)
Comparison of mobility of S. punctillum and C. rufilabris exposed to imidacloprid in prey and foliage.
(DOC)
Comparison of nitrogen levels in elm trees treated with imidacloprid in New York (NY) and Maryland (MD) in 2005
(DOC)
The number of trees in different size classes treated by different methods of application for 2005, 2006, and 2007.
(DOC)