Abstract
Previous studies comparing interleukin 4 receptor α (IL-4Rα)-/- and interleukin 4 (IL-4)-/- BALB/c mice have indicated that interleukin 13 (IL-13), whose receptor shares the IL-4Rα subunit with IL-4, plays a protective role during visceral leishmaniasis. We demonstrate that IL-13-/- BALB/c mice were less able to control hepatic growth of Leishmania donovani compared with wild-type mice. This correlated with significantly retarded granuloma maturation in IL-13-/- mice, defective interferon γ (IFN-γ) production, and elevated IL-4 and interleukin 10 (IL-10) levels. L. donovani–infected IL-13-/- mice also responded poorly to sodium stibogluconate-mediated chemotherapy compared with wild-type BALB/c mice. Because murine lymphocytes do not have IL-13 receptors, we examined the ability of macrophage/neutrophil-specific IL-4Rα-/- mice to control primary infection with L. donovani and to respond to chemotherapy. Macrophage/neutrophil-specific IL-4Rα-/- mice were as resistant to leishmaniasis as wild-type mice, and chemotherapy retained its efficacy. Consequently, in L. donovani infected BALB/c mice, IL-13 promotes hepatic granuloma formation and controls parasite burdens independently of direct effects on macrophages/neutrophils.
Infection with the intracellular protozoan parasite Leishmania donovani causes a potentially fatal disease wherein macrophages of the viscera including the spleen, liver, and bone marrow become infected, leading to splenomegaly and hepatomegaly. Resistance to infection with L. donovani in the well-characterized BALB/c mouse model is associated with an interleukin 1 (IL-1)–driven type 1 response, leading to the production of interferon-γ (IFN-γ) and activation of macrophages [1]. In contrast, overproduction of interleukin 10 (IL-10) is associated with disease exacerbation [2, 3]. Control of parasite growth in the liver is associated with the ability to produce sterile granulomas [4], a mechanism driven by T-cell–derived IFN-γ [2]. Paradoxically, studies using interleukin 4 (IL-4)-/- mice have also demonstrated an important protective role for this cytokine during primary infection [4]. Enhanced susceptibility of IL-4-/- mice was associated with downregulated type 1 responses [5] and markedly retarded granuloma maturation [4].
A number of chemotherapeutic options are available to treat visceral leishmaniasis. Pentavalent antimony (sodium stibogluconate [SSG]) is composed of stibonic and gluconic acids [6] and has been among the most commonly used antileishmanial drugs. Several studies have highlighted the importance of T lymphocytes and associated cytokines in the efficacy of SSG treatment, indicating that the host cell–mediated immune response is an important factor in SSG chemotherapy [2, 5, 7]. Animal studies have also demonstrated that successful treatment of visceral leishmaniasis with SSG requires the presence of both CD4+ and CD8+ T cells [7] accompanied by the type 1 cytokines interleukin 12 (IL-12) and IFN-γ [2]. Our previous studies of primary L. donovani infection using IL-4-/- BALB/c mice demonstrated that IL-4 plays a protective role and facilitates successful chemotherapy by promoting a type 1 response [4, 5]. Additional studies of the primary disease model demonstrated that interleukin 4 receptor α (IL-4Rα)-/- BALB/c mice were significantly more susceptible to L. donovani infection than were IL-4-/- mice, as measured by liver parasite burdens early in infection [4]. Because the IL-4 and interleukin 13 (IL-13) receptors share the IL-4Rα subunit, this clearly suggests a role for IL-13 in the protective response. Studies using IL-13-/- BALB/c mice have been less conclusive, and although IL-13 deficiency was identified as promoting granuloma maturation, which is correlated with protection, no effect of IL-13 deficiency on parasite burdens was observed [8]. Indeed, a study using mice deficient in the IL-13 decoy receptor IL-13Rα2, and consequently producing excess functional IL-13, indicated that IL-13 inhibited a type 1 response and promoted disease [9]. Surprisingly, IL-13 was found to have little effect on SSG chemotherapy in either study [8, 9], despite the demonstrated involvement of the related cytokine IL-4 in this process [5]. However, previous studies on cutaneous leishmaniasis have demonstrated that although IL-13 can substitute for IL-4 in its absence, IL-13 can also have independent properties [10].
Consequently, to more specifically characterize the role of IL-13 and its functional targets during infection with L. donovani, we have used IL-13-/-, IL-4Rα-/-, and macrophage/neutrophil-specific IL-4Rα-/- BALB/c mice both during primary infection with L. donovani and following SSG chemotherapeutic intervention. We demonstrate that IL-13 plays a significant role in controlling hepatic visceral leishmaniasis both during primary infection and following SSG chemotherapy by promoting a type 1 response and hepatic granuloma maturation. Furthermore, using macrophage/neutrophil-specific IL-4Rα-/- BALB/c mice [11], we demonstrate that the protective influence of IL-13 is independent of these cellular targets. Because murine lymphocytes do not possess IL-13 receptors, these results raise intriguing questions regarding the mode of action and cellular targets of this cytokine. Data reported here suggest that IL-13 functions through dendritic cells to promote a protective response.
MATERIALS AND METHODS
Animals and Parasites
We used age- and sex-matched, in-house, inbred IL-13-/- BALB/c mice (20–25 g, female) and their wild-type counterparts along with macrophage/neutrophil-specific IL-4Rα-/- mice and their negative littermates [11]. We intercrossed IL-4Rαlox/lox BALB/c [11] mice with BALB/c mice expressing Cre recombinase under the control of the LysM promoter [12] and then further crossed with IL-4Rα BALB/c mice [13] to generate hemizygous LysMcreIL-4Rα-/lox mice. Polymerase chain reaction genotyping was used to identify LysMcreIL-4Rα-/lox mice. LysMcre-negative IL-4Rα-/lox BALB/c mice were used as wild-type equivalents. Commercially obtained golden Syrian hamsters (Mesocricetus auratus) were used for maintenance of L. donovani strains (Harlan Olac). L. donovani strains 200016 and LV82 were used in our study [14]. Mice were infected on day 0 by intravenous injection (tail vein, no anaesthetic) with 1–2 × 107 L. donovani amastigotes [15]. Animal experiments were performed after institutional ethical review and in accordance with UK Home Office regulations.
In Vivo Studies
To compare the course of infection in cytokine deficient mice and their wild-type counterparts (n = 4 treatment), we infected mice with L. donovani and killed them on different days postinfection. In drug studies, infected mice were treated intravenously on days 14 and 15 with phosphate-buffered saline (controls) or SSG solution (Glaxo-Wellcome, equivalent to a final dose of 111 mg Sbv/kg), and we determined parasite burdens in control and drug-treated animals at day 40 postinfection.
To determine parasite burdens in the spleen and liver, impression smears were prepared on an individual glass microscope slide for each mouse at experiment termination. We prepared bone marrow smears by exposing the mouse femur, cutting behind the kneecap, and inserting a needle into the bone to retrieve the bone marrow. We smeared each bone marrow sample onto the end of a slide using the needle. We fixed the slides in methanol for 2 minutes, stained them with a 10% Giemsa solution (BDH, VWR International Ltd) for 20 minutes, washed them in tap water, and allowed them to dry. We determined the number of parasites per 1000 host nuclei for each sample at x1000 magnification.
Generation and Stimulation of Bone Marrow–Derived Dendritic Cells
We obtained bone marrow–derived dendritic cells from BALB/c mice (aged 10 wk). We flushed the femurs with 5 mL of Roswell Park Memorial Institute 1640 medium (RPMI 1640), washed the cell suspension, adjusted the bone marrow cell concentration to 5 x105 cells/mL, and cultured the cells in 6-well plates (Corning Costar) at 37°C and 5% CO2 in RPMI 1640, 10% vol/vol heat-inactivated fetal bovine serum (FBS, Sigma-Aldrich), 1% vol/vol 2 mM L-glutamine solution, 1% vol/vol 100 IU/mL penicillin, 100 mg/mL streptomycin (PAA Laboratories, GmbH), and 10% culture supernatant from X63 myeloma cells transfected with mouse GM-CSF cDNA. We added fresh medium to the cell cultures every 3 days, generating many CD11c+ dendritic cells largely free from granulocyte and monocyte contamination. We harvested the cells on day 10, seeded them at 5 ×104 cells per well in 96-well tissue culture plates, and treated them with 100 ng/mL recombinant (r)IL-13 (R&D Systems) or 100 ng/mL lipopolysaccharide (LPS). The cells were either preincubated overnight with rIL-13 before being stimulated with LPS for a further 48 hours or incubated with rIL-13 and LPS together for 72 hours. We froze the supernatants at −20°C before we measured IL-12p40 production by ELISA.
Cytokine Production
We used enzyme-linked immunosorbent assay (ELISA) to determine IL-4, IL-10, IL-12p40, IL-13, and IFN-γ levels in the sera of L. donovani–infected mice, as previously described [5].
Histology
At termination, we removed sections of liver and fixed them in neutral-buffered formalin, then processed and stained them with Haematoxylin and Eosin (Fisher Scientific). We scored granulomas as infected Kupffer cells (parasitized macrophages), immature (developing granuloma consisting of CD4+ and CD8+ T cells and monocytes surrounding infected Kupffer cells), mature (more developed than immature granulomas), or sterile (parasite-free granuloma). Granulomas were scored as previously described in a study by Murray [16].
Statistical Analysis of Data
We analyzed parasite data from in vivo experiments using a 1-way analysis of variance (ANOVA, using log10-transformed parasite burden for the liver data). We then analyzed differences between treatments using a Fisher protected least significant difference (PLSD) test with the Statview version 5.0.1 software package. We analyzed cytokine, granuloma, and flow cytometry data using an unpaired t test.
RESULTS
Liver Parasite Burdens Are Increased and Granuloma Formation Inhibited in IL-13-/- BALB/c Mice Infected With L. donovani
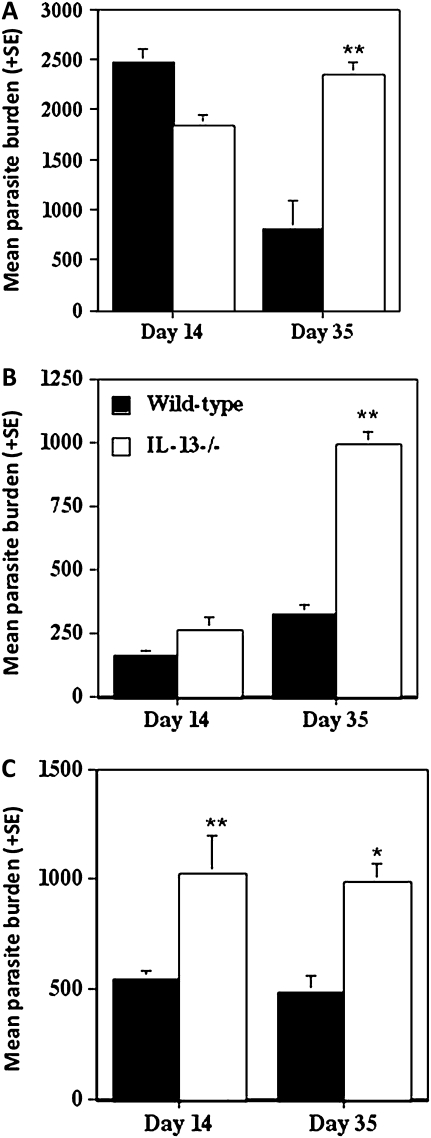
Parasite burdens in the livers of wild-type and IL-13-/- BALB/c mice infected intraveneously with 1–2 × 107 L. donovani amastigotes were enumerated at days 14 and 35 postinfection. At day 14 postinfection, liver parasite burdens were similar in both wild-type and IL-13-/- BALB/c mice (Figure 1A). Whereas parasite burdens in the livers of wild-type mice had declined by day 35 postinfection, they did not decline over this period in IL-13-/- BALB/c mice, and we found them to be significantly higher (P < .001) in IL-13-/- than in wild-type mice at this time (Figure 1A). Parasite numbers were also found to be significantly greater in the spleens and bone marrow of L. donovani–infected IL-13-/- compared with those found in wild-type BALB/c mice (Figures 1B and C).
Figure 1.
The L. donovani liver (A), spleen (B), and bone marrow (C) parasite burdens at days 14 and 35 postinfection following intravenous infection with 1–2 × 107 amastigotes. The data are means ± standard errors for individual mice (n = 4) and are representative of 1 of 4 independent experiments.
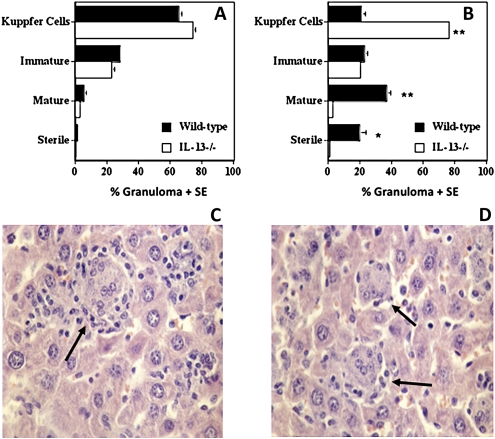
We also assessed granuloma maturation in livers from wild-type and IL-13-/- mice over this period (Figures 2A and 2B). We scored granulomas as described previously [16]: infected Kupffer cells (parasitized macrophages), immature granuloma (consisting of T cells and monocytes surrounding infected Kupffer cells), mature (developed), or sterile (parasite-free) granuloma. IL-13-/- BALB/c mice showed normal early inflammatory responses, with the formation of immature granulomas with similar frequency as wild-type mice on day 14 postinfection (Figure 2A). Whereas approximately 60% of the inflammatory foci had progressed to mature or sterile granulomas in wild-type mice at day 35 postinfection (Figures 2B–C), granulomas failed to mature in IL-13-/- mice (Figures 2B–D) and the histological profile was similar to that of day 14. Because the overall granulomatous response at day 35 is indicative of a good healing response [16], which is reflected by the reduction in parasite burdens in wild-type mice, these data suggest that host-protective granulomatous responses were inhibited in IL-13-/- mice.
Figure 2.
Granuloma maturation in L. donovani–infected wild-type and IL-13-/- mice at days 14 (A) and 35 (B) postinfection. The data are means ± standard errors for 1 of 4 independent experiments. *P < .05 and **P < .01 wild-type versus IL-13-/-. Photomicrographs showing the hepatic granuloma response in two L. donovani–infected mice, wild-type (C) and IL-13-/- (D), at day 35 postinfection. Wild-type mice show well-developed mature granulomas (arrows) surrounding a core of infected Kupffer cells, whereas IL-13-/- mice show abrogated granuloma development characterized by the presence of immature granulomas and amastigotes within the cytoplasm of Kupffer cells at day 35 postinfection (arrows). Magnification x400.
Serum Cytokine Levels Correspond to a Deficient Type-1 Response in L. donovani Infected IL-13-/- Compared With Wild-Type BALB/c Mice
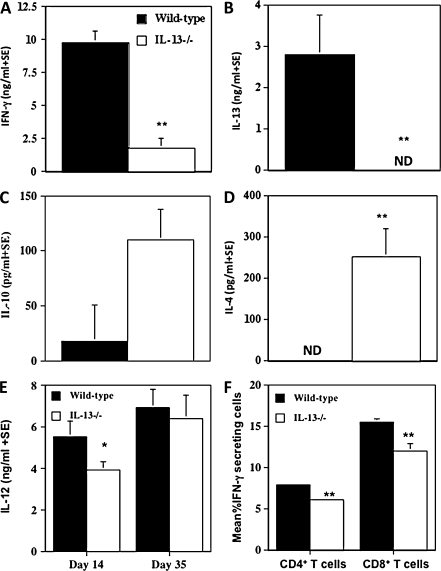
IFN-γ is important in controlling granuloma maturation; Murray et al have previously shown that IFN-γ-deficient mice are unable to control parasite burdens and granuloma formation [2]. We found serum IFN-γ levels at day 35 postinfection to be significantly greater (P < .0001) in L. donovani–infected wild-type mice compared with levels in IL-13-/- BALB/c mice (Figure 3A). We also found L. donovani to induce high serum IL-13 levels in L. donovani–infected wild-type mice (Figure 3B). Conversely, both IL-4 and IL-10 serum levels were greater in IL-13-/- mice compared with levels in wild-type mice (Figure 3C and D respectively). Together these results show a positive association of IFN-γ and IL-13 production with granuloma maturation and a negative association with IL-4 and IL-10 levels. Consistent with these observations, IL-12 serum levels peaked earlier in infection in wild-type mice compared with levels in IL-13-/- mice (Figure 3E). As well as systemic cytokine levels, the percentage of IFN-γ-secreting CD4+ T cells and CD8+ T cells was significantly greater in the spleens of wild-type mice than in IL-13-/- mice (Figure 3F; wild-type mice, 7.9 ± 0.265% CD4+ IFN-γ+ T cells and 15.5 ± 0.354% CD8+ IFN-γ+ T cells; IL-13-/- mice, 6.1 ± 0.24% CD4+ IFN-γ+ T cells and 12.0 ± 0.841% CD8+ IFN-γ+ T cells).
Figure 3.
Mean serum IFN-γ (A), IL-13 (B), IL-10 (C), and IL-4 (D) levels ± standard errors in wild-type and IL-13-/- BALB/c mice (n = 4) at day 35, and IL-12 p40/70 (E) at days 14 and 35 postinfection with L. donovani as measured in individual serum samples by enzyme-linked immunosorbent assay. Representative data from 4 independent experiments are shown. No measurable cytokine levels were observed in sera from uninfected wild-type and IL-13-/-mice. Analysis of intracellular IFN-γ expression (F) was performed on day 35 postinfection on splenocytes stimulated in the presence of 50 ng/mL phorbol myristate acetate (Sigma-Aldrich) and 500 ng/mL of ionomycin (Sigma-Aldrich) for 4 hours, with the addition of Brefeldin A during the last 2 hours as previously described. The antimouse antibody PerCP-labeled anti-CD4+, activated protein C–labeled anti-CD8+, peridinin chlorophyll protein–labeled, and activated protein C–labeled IgG isotype controls were used to stain the cell surfaces, and the protein E–labeled anti-mouse IFN-γ and protein E–labeled IgG isotype control were used for intracellular staining. All antibodies were obtained from BD Biosciences. Cells were analyzed with the FACSCanto. The FACsDiva software was used to analyze results. Representative of 2 separate experiments. *P < .05, **P < .01 wild-type versus IL-13-/-. ND = not detected.
Sodium Stibogluconate Drug Treatment Was Ineffective in IL-13-/- BALB/c Mice and Granulomas Failed to Mature
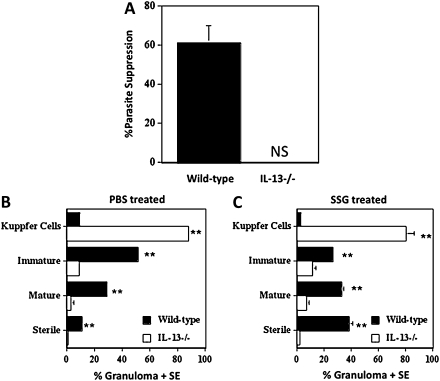
We treated L. donovani–infected wild-type and IL-13-/- BALB/c mice with SSG (111 mg Sbv/Kg) on days 14 and 15 postinfection, and we compared parasite burdens with those of non–drug-treated control mice at day 40 postinfection. We calculated SSG parasite suppression by comparing each experimental value with the mean control value. As expected, SSG drug treatment was successful in wild-type animals, resulting in 61% suppression of parasite numbers in the liver (Figure 4A). Interestingly, SSG drug treatment was ineffective in IL-13-/- mice, as parasite burdens at day 40 postinfection were similar to control values.
Figure 4.
Sodium stibogluconate–mediated suppression of parasite burdens in the liver and granuloma maturation in IL-13-/- and wild-type BALB/c mice infected with L. donovani. The mean suppression (± standard error [SE]) of parasite burdens induced by treatment with 111 mg Sbv/kg sodium stibogluconate (SSG) at days 14 and 15 postinfection in the livers of wild-type and IL-13-/- mice was assessed at day 40 postinfection (A). Parasite suppression was measured by comparing each experimental value with the mean control value (NS = no suppression). Granuloma maturation in non–drug-treated (B) and drug-treated (C) L. donovani–infected wild-type and IL-13-/- mice at day 40 postinfection was also measured. Paraffin-embedded liver sections from wild-type and IL-13-/- mice (n = 4) were stained with haematoxylin and eosin, and granuloma formation per 100 microscopic fields was determined. The data are means (±SE) for 1 of 2 independent experiments. **P < .01 wild-type versus IL-13-/-.
We assessed granuloma maturation in livers from control and drug-treated mice of both wild-type and IL-13-/- BALB/c strains as we described previously. As expected, wild-type mice demonstrated a significant amount of granuloma maturation whereby there were few infected Kupffer cells remaining without associated inflammation, although immature and, to a lesser extent, mature granulomas were predominant; and some sterile granulomas were also evident (Figure 4B). In drug-treated wild-type mice, however, there was a significant increase in mature but particularly sterile granulomas compared with granulomas in non–drug-treated animals (P < .001), whereas levels of infected Kupffer cells and immature granulomas were reduced greatly (Figure 4C). In non–drug-treated IL-13-/- BALB/c mice, granulomas failed to mature as infected Kupffer cells were dominant, and there were few mature and sterile granulomas (Figure 4B). Furthermore, SSG treatment did not promote any significant granuloma maturation in IL-13-/-BALB/c mice (Figure 4C).
The L. donovani Protective Influences of IL-13 Are Independent of Macrophage/Neutrophil Involvement
Murine lymphocytes are not responsive to IL-13, suggesting that the effects of IL-13 are indirect. Previous studies suggest that in Listeria monocytogenes infection, IL-13 drives IL-12 production by monocytes and a protective type 1 response against the infection [17, 18]. To test the possible role of IL-13–responsive macrophages in the protective responses against L. donovani, we used recently established macrophage/neutrophil-specific IL-4Rα-/- BALB/c mice [11] for comparative L donovani infection studies.
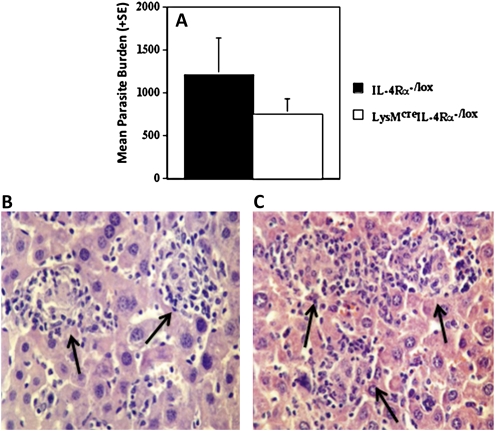
Primary infection of macrophage/neutrophil-specific IL-4Rα-/- mice (LysMcreIL-4Rα-/lox) and their control littermates (IL-4Rα-/lox) with L. donovani resulted in similar liver parasite burdens (Figure 5A) and granuloma maturation (Figures 5B and 5C). As previously published, global IL-4Rα-/- BALB/c mice were more susceptible to infection than wild-type mice and have retarded granuloma development [4] with a histological phenotype similar to IL-13-/- animals (results not shown).
Figure 5.
Liver parasite burdens in wild-type (IL-4Rα-/lox) and macrophage/neutrophil-specific IL-4Rα-/- BALB/c (LysMcreIL-4Rα-/lox) mice at day 40 postinfection with 1–2 × 107 L. donovani amastigotes. Part A shows representative data from 3 independent experiments. Parts B and C are light micrographs from an IL-4Rα-/lox mouse and a LysMcreIL-4Rα-/lox mouse, respectively, at day 40 postinfection; they show mature granulomas (arrows) consisting of well-developed mononuclear cell mantles (magnification 400x).
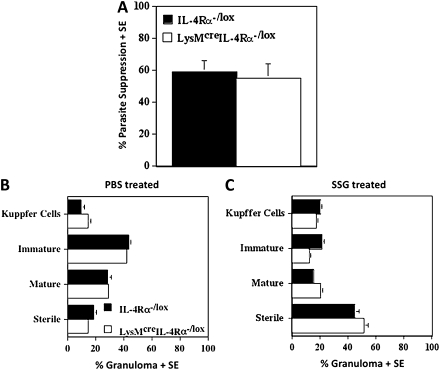
SSG drug-induced suppression of parasite burdens in the liver was equivalent in control (59%) and macrophage/neutrophil-specific IL-4Rα-/- (55%) mice at day 40 postinfection (Figure 6A), indicating that treatment was successful in both mouse strains. In agreement, compared with non-drug-treated mice (Figure 6B), both drug-treated control mice and macrophage/neutrophil-specific IL-4Rα-/- mice (Figure 6C) showed a significant increase (P < .01 and P < .001 respectively) in sterile granulomas (Figure 6B).
Figure 6.
The mean suppression (±standard error) of liver parasite burdens and granuloma induction in the livers of wild-type (IL-4Rα-/lox) and macrophage/neutrophil-specific IL-4Rα-/- (LysMcreIL-4Rα-/lox) mice at day 40 postinfection. These are representative data from 2 independent experiments: liver parasite burdens (A), and granuloma maturation in control (B) and drug-treated (C) L. donovani–infected IL-4Rα-/lox and LysMcreIL-4Rα-/lox mice at day 40 postinfection. Mice were treated with phosphate-buffered saline (controls) or 111mg Sbv/kg sodium stibogluconate at days 14 and 15 postinfection. A significant increase in sterile granulomas was observed between control and drug-treated mice with P < .01 and P < .001 for IL-4Rα-/lox and LysMcreIL-4Rα-/lox mice, respectively. *P < .05 drug-treated wild-type (IL-4Rα-/lox) versus drug-treated macrophage/neutrophil-specific IL-4Rα-/- (LysMcreIL-4Rα-/lox).
IL-13 Primes Dendritic Cells for IL-12 Production
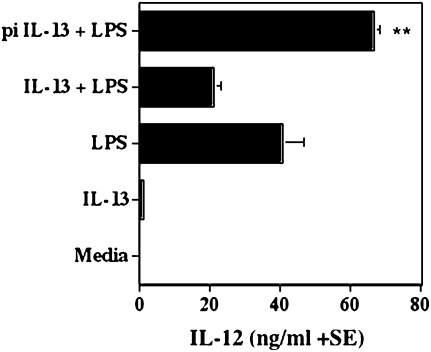
Previous studies have demonstrated that IL-4 can prime dendritic cells for IL-12 production and a protective immune response against Leishmania major [19, 20]. Because IL-13 also signals through the IL-4Rα chain and has similar functions to IL-4, we tested the possibility that IL-13 was also able to instruct dendritic cells for IL-12 production. We preincubated dendritic cells with rIL-13 either prior to or at the same time as LPS stimulation and analyzed the supernatants for IL-12p40 production by ELISA. Preincubation of dendritic cells with rIL-13 induced significantly increased levels of IL-12 production compared with levels from LPS stimulation alone (P < .0021, Figure 7). Indeed, preincubation of dendritic cells with rIL-13 was necessary to induce upregulation of IL-12 production following LPS stimulation because incubation of dendritic cells with rIL-13 and LPS together did not lead to significantly increased IL-12 levels compared with levels produced from LPS stimulation alone (P < .0625, Figure 7).
Figure 7.
Effect of recombinant interleukin 13 and lipopolysaccharide stimulation on interleukin 12 production by dendritic cells. Dendritic cells were either (a) incubated for 72 hours with lipopolysaccharide (LPS) alone (100 ng/mL), recombinant interleukin 13 (rIL-13) alone (100 ng/mL), or LPS and rIL-13 together, or (b) preincubated with rIL-13 overnight and then stimulated with LPS for a further 48 hours. Supernatants were frozen at −20°C before analysis for IL-12p40 by enzyme-linked immunosorbent assay. Preincubating with rIL-13 prior to LPS stimulation induced a significant increase in IL-12p40 production by dendritic cells compared with LPS stimulation alone (P < .0021) or incubation with rIL-13 and LPS together (P < .0001). pi = preincubated.
DISCUSSION
Our previous studies identified a protective role for IL-4 and IL-4Rα signaling during L. donovani infection [4, 5]. Furthermore, the increased susceptibility to L. donovani of IL-4Rα-/- mice compared with that of IL-4-/- BALB/c mice, as measured by hepatic parasite burdens [4], suggested a protective role for IL-13 also, because activities of both IL-4 and IL-13 are dependent on the expression of IL-4Rα. Whereas serum IFN-γ levels were similar in wild-type and IL-4-/- L. donovani–infected BALB/c mice, we measured no detectable IFN-γ in IL-4Rα-/- animals, which suggests that IL-13 alone or IL-13 and IL-4 together are essential for optimal development of type 1 responses and immunity against this parasite [4]. This study confirmed the suggestion by demonstrating that IL-13 promotes a protective response against visceral leishmaniasis, as parasite burdens were significantly enhanced and granuloma maturation was significantly inhibited in IL-13-/- BALB/c mice compared with those in wild-type mice. Concomitantly, type 1 responses, as measured by IFN-γ production, were significantly inhibited; type 2 responses, as measured by IL-4 and IL-10 levels, were significantly enhanced in L. donovani–infected IL-13-/- BALB/c mice compared with responses in their wild-type counterparts. This would suggest that IL-13 has a major role in regulating type 1 responses and resistance to L. donovani.
The Th2-potentiating role normally associated with IL-13 seems intuitively at odds with our results. However, some studies have demonstrated that IL-4 and IL-13 indirectly drive protective type 1 responses. For example, IL-13 has previously been shown to prime monocytes for IL-12 production [17] and drive a protective type 1 response during listeriosis [18]. Furthermore, a recent study on L. donovani that failed to demonstrate a protective role, as measured by hepatic parasite burdens, for IL-13 during L. donovani primary infection or following chemotherapy infection demonstrated that this cytokine promoted granuloma formation and IFN-γ production during primary infection [8]. Thus, this study, like our own, clearly identified a switch toward a type 1 immune response bias. Differences between the studies could reflect the different parasite strains used in the separate laboratories. However, we had similar results in our studies with 3 separate strains, including an SSG-resistant strain [15].
Previous studies on L. major infection have also demonstrated a protective role for IL-13, as IL-4Rα-/- BALB/c mice, unlike IL-4-/- mice, were unable to avoid disease dissemination and visceralization [13]. Because murine lymphocytes do not have receptors for IL-13, other cell populations must be instructed for a protective role by this cytokine in the absence of IL-4. Indeed, a recent study using CD4+ T-cell–specific IL-4Rα-/- mice has demonstrated that these mice, unlike global IL-4Rα-/- mice, are as resistant to L. major as C57BL/6 animals. Consequently, Radwanska et al concluded that IL-4/IL-13 responsive non-CD4 T cells induce protection against L. major in the absence of CD4+ T cells responding to IL-4 [19]. Because Flesch et al had previously shown that IL-13 primes monocytes for IL-12 production [17] and drives a protective type 1 response during listeriosis [18], we examined L. donovani disease progression in macrophage/neutrophil-specific IL-4Rα-/- BALB/c mice. We found macrophage/neutrophil-specific IL-4Rα-/- mice to be no more susceptible to infection with L. donovani than their wild-type littermates were. Over a number of experiments we noted an indication, although generally not significant, that granuloma maturation was more precocious in macrophage/neutrophil-specific IL-4Rα-/- L. donovani–infected BALB/c mice especially following chemotherapy. This clearly suggests that the protective role we have demonstrated for IL-13 during L. donovani infection is not due to the activity of this cytokine, or indeed IL-4, on macrophage/neutrophil populations. Hölscher et al found that L. major disease progression was inhibited in macrophage/neutrophil-specific IL-4Rα-/- BALB/c mice compared with progression in wild-type animals; their study indicated that IL-13/IL-4 functioned to enhance disease progression by the induction of alternative macrophage activation [21]. Our results in this study neither rule out nor confirm whether alternative macrophage activation contributes to susceptibility to L. donovani. However, alternative macrophage activation could explain the transiently elevated parasite burdens and impaired granuloma maturation in the presence of excess IL-13 in IL-13Rα2-/- L. donovani–infected mice [9]. Consequently, the protective effects, as opposed to disease-exacerbatory effects, of IL-4/IL-13 during both L. major and L. donovani infections are due to cell populations other than macrophages or neutrophils. Dendritic cells, for example, would be strong candidates because they play pivotal roles in granuloma formation [22], and an elegant study has previously shown IL-4 to induce dendritic cell IL-12 production and to generate a T-helper 1 (Th1) response and protection against L. major [20]. Furthermore, Ghosh et al found that IL-4–matured bone marrow–derived dendritic cell–based immunotherapy significantly enhances chemotherapy against murine visceral leishmaniasis [23]. They also found that IL-13 can induce dendritic cell IL-12 production in vitro when used to prime dendritic cells prior to LPS stimulation. The ability of IL-13 to influence dendritic cells in this way may play an important role in enhancing granuloma formation and reducing parasite burdens during L. donovani infection.
In addition to being more susceptible than wild-type mice to primary infection with L. donovani, IL-13-/- animals responded less well to SSG chemotherapy, as in fact did IL-4-/- BALB/c mice in a previous study [5]. This would again suggest that IL-4 and IL-13 are playing similar protective roles during L. donovani infection. However, as serum IL-4 levels in L. donovani–infected IL-13-/- BALB/c mice were significantly higher than levels in wild-type animals, this would suggest that IL-13 could be playing a role independently of IL-4. Using IL-4-/- mice, studies of asthma [24], schistosomes [25], and Nippostrongulas braziliensis [26], as well as L. major [10] have demonstrated that IL-13, which has the ability to substitute in part for IL-4, has independent properties or plays a more significant role than IL-4 in various functions. These functions include worm expulsion of N. braziliensis [26], effector activity in asthma [24, 27], and hepatic fibrosis and granuloma formation in the pathology associated with schistosomiasis [25]. Paradoxically, although IL-13 appears to be the main mediator of a type 2 immune response, our data suggest that this cytokine is also crucial in generating the granuloma-inducing type 1 immune response associated with protection against L. donovani. Nevertheless, in a previous coinfection study [28], schistosome egg granulomas were found to provide safe havens for the multiplication of L. donovani amastigotes, even in the presence of functional anti–L. donovani Th1 responses.
Together, our results demonstrate that IL-13 overall plays a beneficial role during L. donovani infection independent of a direct role for lymphocytes, macrophages, or neutrophils and is important in the generation of cell-mediated immunity. Preliminary evidence implicates the dendritic cell as a potential target for the IL-13 dependent protective response against L. donovani. However, final confirmation will be dependent on the generation of CD11c-specific IL-4Rα-/- mice.
Funding
This work was supported by grants from the Wellcome Trust (065308); the Royal Society; the UK Medical Research Council (G0400786); and the National Research Foundation, South Africa. There are no financial conflicts of interest.
References
- 1.Kaye PM, Svensson M, Ato M, et al. The immunopathology of experimental visceral leishmaniasis. Immunol Rev. 2004;201:239–53. doi: 10.1111/j.0105-2896.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 2.Murray HW, Montelibano C, Peterson R, Sypek JP. Interleukin-12 regulates the response to chemotherapy in experimental visceral leishmaniasis. J Infect Dis. 2000;182:1497–502. doi: 10.1086/315890. [DOI] [PubMed] [Google Scholar]
- 3.Murphy ML, Wille U, Villegas EN, Hunter CA, Farrell JP. IL-10 mediates susceptibility to Leishmania donovani infection. Eur J Immunol. 2001;31:2848–56. doi: 10.1002/1521-4141(2001010)31:10<2848::aid-immu2848>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 4.Stäger S, Alexander J, Carter KC, Brombacher F, Kaye PM. Both interleukin-4 (IL-4) and IL-4 receptor α signaling contribute to the development of hepatic granulomas with optimal antileishmanial activity. Infect Immun. 2003;71:4804–7. doi: 10.1128/IAI.71.8.4804-4807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander J, Carter KC, Al-Fasi N, Satoskar A, Brombacher F. Endogenous IL-4 is necessary for effective drug therapy against visceral leishmaniasis. Eur J Immunol. 2000;30:2935–43. doi: 10.1002/1521-4141(200010)30:10<2935::AID-IMMU2935>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 6.Roberts WL, Rainey PM. Antileishmanial activity of sodium stibogluconate fractions. Antimicrob Agents Chemother. 1993;37:1842–6. doi: 10.1128/aac.37.9.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray HW, Oca MJ, Granger AM, Schreiber RD. Requirement for T cells and effect of lymphokines in successful chemotherapy for an intracellular infection: J Clin Invest. 1989;83:1253–7. doi: 10.1172/JCI114009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray HW, Tsai CW, Liu J, Ma X. Visceral Leishmania donovani infection in interleukin-13-/- mice. Infect Immun. 2006;74:2487–90. doi: 10.1128/IAI.74.4.2487-2490.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray HW, Flanders KC, Donaldson DD, et al. Antagonizing deactivating cytokines to enhance host defense and chemotherapy in experimental visceral leishmaniasis. Infect Immun. 2005;73:3903–11. doi: 10.1128/IAI.73.7.3903-3911.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews DJ, Emson CL, McKenzie GJ, Jolin HE, Blackwell JM, McKenzie AN. IL-13 is a susceptibility factor for Leishmania major infection. J Immunol. 2000;164:1458–62. doi: 10.4049/jimmunol.164.3.1458. [DOI] [PubMed] [Google Scholar]
- 11.Herbert DR, Hölscher C, Mohrs M, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–35. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 12.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–77. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 13.Mohrs M, Ledermann B, Köhler G, Dorfmüller A, Gessner A, Brombacher F. Differences between IL-4- and IL-4 receptor α-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol. 1999;162:7302–8. [PubMed] [Google Scholar]
- 14.Carter KC, Sundar S, Spickett C, Pereira OC, Mullen AB. The in vivo susceptibility of Leishmania donovani to sodium stibogluconate is drug specific and can be reversed by inhibiting glutathione biosynthesis. Antimicrob Agents Chemother. 2003;47:1529–35. doi: 10.1128/AAC.47.5.1529-1535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter KC, Baillie AJ, Alexander J, Dolan TF. The therapeutic effect of sodium stibogluconate in BALB/c mice infected with Leishmania donovani is organ-dependent. J Pharm Pharmacol. 1988;40:370–3. doi: 10.1111/j.2042-7158.1988.tb05271.x. [DOI] [PubMed] [Google Scholar]
- 16.Murray HW. Tissue granuloma structure-function in experimental visceral leishmaniasis. Int J Exp Pathol. 2001;82:249–67. doi: 10.1046/j.1365-2613.2001.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minty A, Ferrara P, Caput D. Interleukin-13 effects on activated monocytes lead to novel cytokine secretion profiles intermediate between those induced by interleukin-10 and by interferon-γ. Eur Cytokine Netw. 1997;8:189–201. [PubMed] [Google Scholar]
- 18.Flesch IE, Wandersee A, Kaufmann SH. Effects of IL-13 on murine listeriosis. Int Immunol. 1997;9:467–74. doi: 10.1093/intimm/9.4.467. [DOI] [PubMed] [Google Scholar]
- 19.Radwanska M, Cutler AJ, Hoving JC, et al. Deletion of IL-4Rα on CD4 T cells renders BALB/c mice resistant to Leishmania major infection. PLoS Pathog. 2007;3:e68. doi: 10.1371/journal.ppat.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biedermann T, Zimmermann S, Himmelrich H, et al. IL-4 instructs Th1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat Immunol. 2001;2:1054–60. doi: 10.1038/ni725. [DOI] [PubMed] [Google Scholar]
- 21.Hölscher C, Arendse B, Schwegmann A, Myburgh E, Brombacher F. Impairment of alternative macrophage activation delays cutaneous leishmaniasis in nonhealing BALB/c mice. J Immunol. 2006;176:1115–21. doi: 10.4049/jimmunol.176.2.1115. [DOI] [PubMed] [Google Scholar]
- 22.Ato M, Maroof A, Zubairi S, Nakano H, Kakiuchi T, Kaye PM. Loss of dendritic cell migration and impaired resistance to Leishmania donovani infection in mice deficient in CCL19 and CCL21. J Immunol. 2006;176:5486–93. doi: 10.4049/jimmunol.176.9.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh M, Pal C, Ray M, Maitra S, Mandal L, Bandyopadhyay S. Dendritic cell-based immunotherapy combined with antimony-based chemotherapy cures established murine visceral leishmaniasis. J Immunol. 2003;170:5625–9. doi: 10.4049/jimmunol.170.11.5625. [DOI] [PubMed] [Google Scholar]
- 24.Wills-Karp M, Luyimbazi J, Xu X, et al. Interleukin-13: Central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 25.Chiaramonte MG, Cheever AW, Malley JD, Donaldson DD, Wynn TA. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology. 2001;34:273–82. doi: 10.1053/jhep.2001.26376. [DOI] [PubMed] [Google Scholar]
- 26.McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998;8:339–42. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- 27.Grünig G, Warnock M, Wakil AE, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassan MF, Zhang Y, Engwerda CR, Kaye PM, Sharp H, Bickle QD. The Schistosoma mansoni hepatic egg granuloma provides a favorable microenvironment for sustained growth of Leishmania donovani. Am J Pathol. 2006;169:943–53. doi: 10.2353/ajpath.2006.051319. [DOI] [PMC free article] [PubMed] [Google Scholar]