Abstract
(See the article by Bejon et al, on pages 9–18, and Bousema et al, on pages 1–3.)
Background. Associations between antibody responses to Plasmodium falciparum antigens and protection against symptomatic malaria have been difficult to ascertain, in part because antibodies are potential markers of both exposure to P. falciparum and protection against disease.
Methods. We measured IgG responses to P. falciparum circumsporozoite protein, liver-stage antigen 1, apical-membrane antigen 1 (AMA-1), and merozoite surface proteins (MSP) 1 and 3, in children in Kampala, Uganda, and measured incidence of malaria before and after antibody measurement.
Results. Stronger responses to all 5 antigens were associated with an increased risk of clinical malaria (P < .01) because of confounding with prior exposure to P. falciparum. However, with use of another assessment, risk of clinical malaria once parasitemic, stronger responses to AMA-1, MSP-1, and MSP-3 were associated with protection (odds ratios, 0.34, 0.36, and 0.31, respectively, per 10-fold increase; P < .01). Analyses assessing antibodies in combination suggested that any protective effect of antibodies was overestimated by associations between individual responses and protection.
Conclusions. Using the risk of symptomatic malaria once parasitemic as an outcome may improve detection of associations between immune responses and protection from disease. Immunoepidemiology studies designed to detect mechanisms of immune protection should integrate prior exposure into the analysis and evaluate multiple immune responses.
A major impediment to malaria vaccine development has been a lack of understanding of which immunologic responses are important in protection. Naturally acquired antibodies to Plasmodium falciparum are known to play a key role in immunity against malaria [1, 2]. However, it is still unclear which antibody responses are important in protection from disease despite a number of immunoepidemiology studies that have attempted to answer this question.
Multiple studies have measured antibody responses to P. falciparum in individuals living in areas where malaria is endemic and prospectively assessed associations between antibody responses and the subsequent risk of malaria. However, associations between antibodies to parasite antigens and the risk of malaria have been inconsistent [3]. A limitation of this study design has been that it does not take into account variation in P. falciparum transmission intensity [4], which has consistently been observed to vary within a small geographical area [5–14]. Individuals living in microenvironments with greater transmission intensity may have a greater breadth and magnitude of antibody responses, because of greater exposure to plasmodial antigens [12, 13], but clinical benefits of these responses may be obscured by increased incidence of disease resulting from increased exposure. A proposed solution is to limit analysis to individuals with documented exposure [4], but this does not account for varied degrees of positive exposure. On the other hand, some measured antibodies may not offer protection, but rather are only surrogates of effective immune responses. These antibody responses may be higher in persons more exposed who therefore possess higher immunity, but play no causal role in protection. Thus, failure to take into account correlations between responses may lead to an overstatement of the causal effect of individual responses.
To address inconsistent associations between antibody responses to P. falciparum and protection against malaria, we measured responses to 5 P. falciparum antigens in a cohort of children in Kampala, Uganda, where heterogeneity in malaria incidence has been well defined [8]. We performed analyses with use of a standard outcome of protection (time to first malaria episode) and an outcome focused on blood-stage immunity, defined as protection from symptoms once parasitemic. By assessing parasitemia and malaria monthly, we were able to account for variation in P. falciparum exposure in our analysis and assess the impact of this variation on associations between antibodies and protection from malaria.
MATERIALS AND METHODS
Study Site and Participants
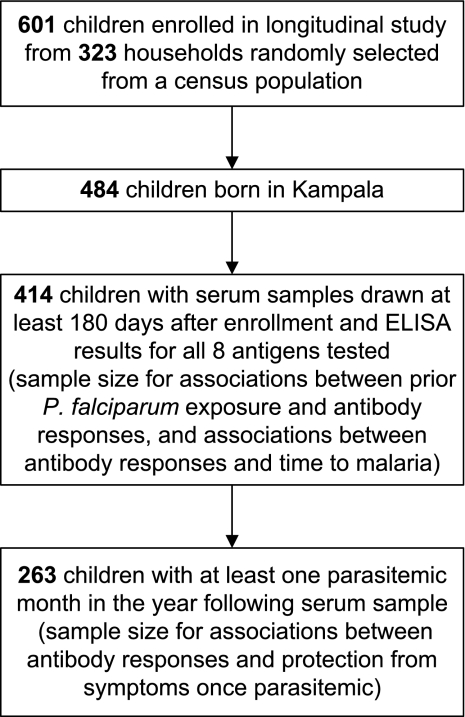
The study was conducted in a neighborhood of Kampala, Uganda, where we have previously shown that malaria incidence is heterogeneous, with those living near a swamp having 4 times the incidence of those living >200 meters away [8]. From November 2004 through April 2005, children aged 1–10 years were enrolled [15] in a randomized trial of antimalarial therapies [16, 17]. Children underwent monthly routine assessment, including blood smear. Malaria was diagnosed if a child had fever (tympanic temperature, ≥ 38.0°C) or history of fever in the previous 24 h and any parasitemia. Recrudescent cases of malaria, as determined by genotyping 6 loci [16], were excluded from the analysis. Parasitemia without fever was not treated. Serum samples were collected every 90 days. Serum samples tested for this study were those obtained closest in date to 1 year after study enrollment, to allow for assessment of malaria risk before and after antibody measurement. All persons born in Kampala who had available serum samples at least 180 days after enrollment were included in the analysis (Figure 1).
Figure 1.
Children from Kampala, Uganda, included in the study.
Antibody Testing by Enzyme-Linked Immunosorbent Assay (ELISA)
Antibodies to 8 antigens representing 5 different proteins were assessed. Antibodies to circumsporozoite protein (CSP) and liver-stage antigen 1 (LSA-1) were assessed using synthetic peptides [18]. Recombinant apical-membrane antigen 1 (AMA-1) from 3D7 and FVO strains (full-length ectodomain) [19]; recombinant merozoite surface protein 1 (MSP-142) from 3D7, FUP, and FVO strains [20, 21]; and MSP-3 from the FVO strain [22] were expressed in Escherichia coli.
Total IgG responses to antigens were measured by ELISA, as described elsewhere [18]. Antibody levels were expressed in arbitrary units (AUs), calculated by dividing the optical density (OD) of the sample by the mean OD plus 3 standard deviations (SD) of samples from 9 North Americans never exposed to malaria [23]. Positive control samples from individuals with known antibodies to these antigens were placed on each plate and values were similar on all test days. All analyses considered antibody levels as both dichotomous (values ≥ 1.0 AU considered to be positive) and continuous variables. For continuous variable analyses, we truncated minimum values at .01 AU and worked with log-transformed values.
Statistical Analysis
Our primary definition of malaria was fever and any parasitemia. A secondary definition using a parasite threshold of >5000 parasites/μL was also explored, but results were similar; therefore, only data using our primary definition are reported.
We hypothesized that prior exposure to P. falciparum may represent an important source of confounding when evaluating associations between antibody responses and subsequent malaria risk, because individuals with higher rates of exposure may develop more robust antibody responses but still develop more malaria if they continue to be more highly exposed. Therefore, we first assessed associations between factors related to prior P. falciparum exposure and antibody responses: age, distance from a swamp, and incidence of malaria before antibody measurement (Figure 1). Associations for dichotomous antibody responses were odds ratios (ORs), estimated with multivariate logistic regression; associations for continuous responses were relative levels, estimated with multivariate linear regression.
To assess associations between antibody responses and future malaria, we analyzed time to first malaria episode. Hazard ratios were estimated for each response using Cox proportional hazards regression with robust inference, adjusting for age. To account for heterogeneous exposure to P. falciparum, we performed a similar analysis additionally adjusting for distance from a swamp and prior malaria incidence.
We next assessed associations between antibody responses and the probability of developing malaria once parasitemic (Figure 1). We hypothesized that this analysis would be less susceptible to confounding by heterogeneous P. falciparum exposure because the outcome is conditional on being parasitemic. Outcomes were each month of person-time classified as containing at least 1 episode of malaria (no protection), no malaria but at least 1 episode of asymptomatic parasitemia (protection), or neither (no evidence for or against protection). Only person-months containing malaria or asymptomatic parasitemia were included in the analysis and were evaluated as binary outcomes. Outcomes were assessed monthly, because each child had at least 1 blood smear every month, and it was uncommon to have >1 new episode of malaria in a month. Asymptomatic parasitemia was defined as a positive blood smear result in the absence of fever at least 28 days after and 5 days before treatment for malaria [24]. The analysis was adjusted for age alone and age, distance from a swamp, and prior malaria incidence. Associations were estimated as ORs using generalized estimating equations with robust inference [25].
To assess the marginal effect of antibody responses in protection from malaria once parasitemic, including adjustment for age, distance from a swamp, prior malaria incidence, and all other measured antibody responses, we performed graphical-computation (G-computation) [26]. Using a linear additive model makes many assumptions about the relationships between covariates, and simply adding interaction terms with highly correlated variables may result in an unstable model (in this case, one which did not converge). Therefore, predictions for G-computation estimates were estimated using the SuperLearner algorithm [27]. To produce estimates which were robust to correlated antibody responses and did not assume linear or additive relationships, the SuperLearner library consisted of the following prediction algorithms: main term logistic regression, random forests [28], generalized additive regression with smoothing splines [29], k-nearest neighbor classification [30], and elastic net regression [31]. Twenty folds were used for cross-validation, and inference was obtained using 1000 bootstrap replicates.
To assess the accuracy of antibodies alone or in combination to predict protection, we generated predictions for all combinations of 1–5 antibodies plus age, using generalized estimating equations. Accuracy was assessed as the area under the receiver operating characteristic curve (AUROC) by using the predicted probabilities from the model and plotting the true positive rate versus the false positive rate.
Statistical analysis was performed using R, version 2.9.0. A P value of <.05 was considered to be statistically significant.
RESULTS
Characteristics of Patients and Samples
Of 601 children enrolled in the study, 414 (69%) had samples that met inclusion criteria (Figure 1). For these children, the prevalence of parasitemia was 19% at study enrollment, the mean age at the time of serum sample collection (∼12 months after enrollment) was 6.5 years (range, 1.8–11.2 years), 24% lived within 25 meters of a swamp, and the mean incidence of malaria before antibody measurement was .9 cases per person-year (range, 0–8.8 cases per person-year). Three percent of children were parasitemic at the time that the serum sample was obtained. The frequency of a positive response to an antigen ranged from 12% to 39%, and the median antibody level ranged from .21 to .68 AU, with the lowest responses to CSP and MSP-3 and the highest to MSP-1-FUP (Table 1). Responses to different variants of the same protein were very similar, with pairwise correlations ranging from .7 to .9 (P < .001 for all comparisons). Because of this high correlation, we simplified subsequent analyses to consider only responses to the 3D7 variants of AMA-1 and MSP-1.
Table 1.
Antibody Responses to Plasmodium falciparum Antigens
| Antigen | Antibody prevalencea | Median levelb (IQR) |
| AMA-1-3D7 | 29% | 0.26 (.12–1.26) |
| AMA-1-FVO | 30% | 0.27 (.10–1.59) |
| MSP-3 | 14% | 0.21 (.08–.49) |
| MSP-1-3D7 | 26% | 0.35 (.12–1.06) |
| MSP-1-FUP | 39% | 0.68 (.27–1.83) |
| MSP-1-FVO | 33% | 0.44 (.20–1.82) |
| CSP | 12% | 0.23 (.07–.52) |
| LSA-1 | 26% | 0.36 (.15–1.06) |
NOTE. IQR, interquartile range.
An AU ≥ 1 was considered a positive response, n = 414.
Levels are in arbitrary units (AU), calculated by dividing the optical density (OD) generated by the test sample by the mean OD plus 3 standard deviations of samples from 40 North Americans never exposed to malaria. Median level includes all individuals, including those with a negative response.
Higher Prior Plasmodium falciparum Exposure Was Associated With Stronger Antibody Responses
As expected, the level of responses to all 5 antigens evaluated increased with age, with responses to AMA-1 showing the greatest increase (Table 2, P < .05). Heterogeneity in exposure intensity was also associated with significant variation in antibody levels. Living near a swamp, where the incidence of malaria was higher [8], was a significant predictor of greater responses to all antigens (P < .05). Incidence of malaria before antibody measurement was positively associated with antibody levels (P < .05 for all responses except MSP-3). Similar associations were seen for dichotomous antibody responses, although fewer were statistically significant (data not shown). Of note, distance of residence from a swamp and incidence of malaria before antibody measurement were independently associated with incidence of malaria after antibody measurement (P < .001 for both), indicating that heterogeneous exposure may be an important confounder of associations between antibody levels and the risk of subsequent malaria.
Table 2.
Associations Between Prior Plasmodium falciparum Exposure and Antibody Levels
| CSP |
LSA-1 |
AMA-1 |
MSP-1 |
MSP-3 |
||||||
| Relative levela (95%CI) | P | Relative levela (95%CI) | P | Relative levela (95%CI) | P | Relative levela (95%CI) | P | Relative levela (95%CI) | P | |
| Age (per 5 year increase) | 1.38 (1.04–1.84) | .02 | 1.86 (1.39–2.50) | <.001 | 3.60 (2.65–4.90) | <.001 | 1.43 (1.02–2.02) | .04 | 2.06 (1.57–2.70) | <.001 |
| Living within 25m of swamp | 1.72 (1.19–2.50) | .004 | 1.86 (1.26–2.73) | .002 | 1.89 (1.27–2.83) | .002 | 1.68 (1.07–2.63) | .02 | 1.54 (1.08–2.19) | .02 |
| Prior malaria incidence (per case/person year) | 1.20 (1.07–1.35) | .003 | 1.38 (1.22–1.56) | <.001 | 1.53 (1.34–1.73) | <.001 | 1.23 (1.07–1.42) | .004 | 1.11 (.99–1.24) | .07 |
NOTE. CI, confidence interval; associations with log-transformed antibody responses were estimated with multivariate linear regression.
Relative level indicates fold change in antibody level, e.g. a relative level of 2 indicates a doubling of antibody levels.
Stronger Antibody Responses Were Associated With a Higher Risk of Subsequent Malaria
To determine whether responses to any of the 5 antigens tested were associated with protection from clinical malaria, we evaluated associations between responses and time until the first episode of malaria during the year after antibody measurement. After adjustment for age, higher antibody levels to all 5 antigens were significantly associated with a higher hazard of subsequent clinical malaria (P < .01) (Table 3). Results were similar when follow-up times were restricted to <1 year or were adjusted for parasitemia and season at the time of sampling (data not shown). Positive associations were also observed when restricting the analysis to only children who had at least 1 episode of parasitemia during follow-up (ie, only including persons with documented exposure [4]; data not shown). After adjustment for prior exposure to P. falciparum, as estimated by distance from a swamp and incidence of malaria before the antibody measurement, higher antibody levels still predicted a higher hazard of malaria, but associations were reduced and only remained significant for CSP, LSA-1, and AMA-1 (Table 3). Dichotomous antibody responses showed similar associations (data not shown). Thus, stronger antibody responses were associated with a higher risk of malaria, likely because of confounding by prior P. falciparum exposure.
Table 3.
Associations Between Antibody Levels and Time to First Malaria Episode
| CSP |
LSA-1 |
AMA-1 |
MSP-1 |
MSP-3 |
||||||
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| Adjusted for age | 1.64 (1.27–2.12) | <.001 | 1.77 (1.41–2.22) | <.001 | 1.69 (1.39–2.06) | <.001 | 1.29 (1.06–1.57) | .01 | 1.43 (1.11–1.85) | .006 |
| Adjusted for age and prior malaria incidence | 1.42 (1.12–1.81) | .004 | 1.48 (1.18–1.86) | <.001 | 1.35 (1.09–1.67) | .005 | 1.17 (.94–1.44) | .15 | 1.30 (1.01–1.68) | .04 |
| Adjusted for age, distance from swamp, and prior malaria incidence | 1.36 (1.07–1.74) | .01 | 1.32 (1.05–1.67) | .02 | 1.26 (1.01–1.56) | .04 | 1.11 (.90–1.37) | .33 | 1.23 (.94–1.60) | .13 |
NOTE. HR, hazard ratio; CI, confidence interval; associations are for every 10-fold increase in antibody level and were estimated using Cox proportional hazards with robust inference.
Stronger Antibody Responses to Blood-Stage Antigens Were Associated With Protection From Malaria After Parasitemic
Optimal assessment of protection from malaria would require measurement of both the number of parasite inoculations and the number of malaria episodes that result from these events. Direct measurement of mosquito inoculations was not possible, but it was possible to assess resultant parasitemia with regular blood smears. Therefore, we assessed blood-stage protection as the absence of malaria in a month once parasitemic. With adjustment for age, stronger antibody responses to the 3 blood-stage, but not the 2 pre-erythrocytic antigens, were significantly associated with protection from clinical malaria once parasitemic P < .01 for AMA-1, MSP-1, and MSP-3) (Table 4). Adjustment for exposure to P. falciparum based on distance from a swamp and prior malaria incidence did not affect these associations, suggesting that these antibody responses were better predictors of blood-stage immunity than were our measures of prior exposure. Dichotomous antibody responses showed similar associations, with positive responses to AMA-1 and MSP-3 associated with a lower risk of malaria once parasitemic (P < .05 for both). Thus, stronger antibody responses to blood-stage antigens were associated with protection from symptomatic malaria once parasitemic, without evidence of confounding by prior P. falciparum exposure.
Table 4.
Associations Between Antibody Levels and Probability of Developing Malaria in a Month if Parasitemic
| CSP |
LSA-1 |
AMA-1 |
MSP-1 |
MSP-3 |
||||||
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| Adjusted for agea | 0.68 (.28–1.69) | .41 | 0.64 (.38–1.08) | .10 | 0.34 (.18–.65) | .001 | 0.36 (.17–.76) | .008 | 0.31 (.15–.64) | .002 |
| Adjusted for age, distance from swamp, and prior malaria incidencea | 0.46 (.16–1.33) | .15 | 0.55 (.27–1.12) | .10 | 0.30 (.15–.63) | .001 | 0.37 (.18–.75) | .006 | 0.32 (.15–.67) | .003 |
| Marginal effect, adjusted for age, distance from swamp, prior malaria incidence, and other antibody responsesb | 0.89 (.43–1.43) | .68 | 0.97 (.77–1.34) | .72 | 0.70 (.46–.98) | .03 | 0.71 (.50–1.06) | .12 | 0.72 (.35–1.01) | .06 |
NOTE. OR, odds ratio; CI, confidence interval.
Associations are for every 10-fold increase in antibody level and were estimated using generalized estimating equations with robust inference accounting for repeated measures within individuals.
Associations are for every 10-fold increase in antibody level and were estimated using G-computation of SuperLearner predictions including parametric and nonparametric models, with inference obtained using 1000 bootstrap replications.
Analyses of Antibody Responses Individually Overestimated Any Causal Effect in Protection
Antibody responses to the 5 different antigens assayed were significantly correlated with each other (range, .2–.6; P < .001 for all pairwise comparisons). Therefore, some associations may have been attributable to antibodies acting as surrogates for other protective responses. To estimate the potential causal effect of each antibody response, we next performed an analysis of the marginal effect of each antibody, adjusting for the effect of other measured antibodies in addition to age and prior exposure to P. falciparum. Our statistical model did not assume that the effects of individual antibodies were independent and used methods designed to give stable results in the setting of correlated variables. With this analysis, associations between antibody responses and the probability of developing malaria once parasitemic were reduced, compared with the analysis including only a single antibody measurement (eg, the OR for AMA-1 was 0.3 when considered alone but 0.7 when considered in the context of other responses) (Table 4). Results were similar for dichotomous responses (data not shown). These data suggest that any causal protective effect of antibodies may have been overestimated in the analyses including only a single antibody response. Of interest, trends toward protection in those with higher levels of antibodies to the pre-erythrocytic antigens CSP and LSA-1 were completely abrogated in the marginal structural analysis, indicating that their association with protection from disease once parasitemic was likely attributable to associations with other protective responses.
The Ability of Combinations of Antibody Responses to Predict Protection
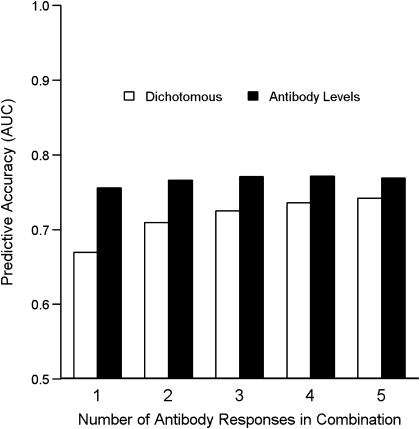
To determine whether responses to multiple antigens provided more information on protection than responses to a single antigen, we assessed the ability of combinations of antibody responses to predict protection from malaria once parasitemic. Accurate prediction of protection was evaluated using the AUROC, where a value of 0.5 indicated that prediction was no better than random and a value of 1.0 indicated a perfect predictor. For dichotomous antibody responses, AMA-1 and MSP-1 were the best single predictors of protection (AUROCs of 0.67 and 0.64, respectively). The combination of AMA-1 and MSP-1 offered better prediction (AUROC, 0.71) with combinations of 3, 4, or 5 antibodies showing marginal improvement (Figure 2). Antibody responses evaluated as levels provided better predictions than did dichotomous responses, with AMA-1 and MSP-3 being the best single predictors (AUROCs of 0.76 and 0.74, respectively). Using any combination of antibody levels offered no improvement in prediction over the response to AMA-1. These data indicate that antibody levels were better predictors of blood-stage protection than were dichotomous responses and that AMA-1 levels alone provided as much information about protection as any combination of the 5 responses measured.
Figure 2.
Accuracy of antibody responses alone and in combination in predicting protection from malaria once parasitemic. Hollow bars indicate predictive accuracy of dichotomous responses; solid bars indicate predictive accuracy of antibody levels. An area under the receiver operating characteristic curve (AUC) value of 0.5 indicates a prediction no better than random, and a value of 1.0 indicates a perfect predictor.
DISCUSSION
Numerous studies have assessed the relationship between antibodies to specific P. falciparum antigens and protection from malaria [3], but few have systematically assessed and controlled for prior exposure to P. falciparum. Thus, it has been difficult to distinguish antibodies serving as markers of prior exposure from their potential function as mediators of protection. In the present study, we found that increased prior exposure to P. falciparum was associated with both stronger antibody responses and an increased risk of subsequent malaria, with the result that stronger antibody responses were significantly associated with an increased risk of malaria. Adjustment for proximity of residence to a swamp and prior malaria incidence reduced but did not remove the confounding effect of prior exposure, likely because even these 2 detailed metrics did not perfectly capture variation in P. falciparum exposure. However, by using a novel method of assessment, evaluating the risk of clinical disease once parasitemic, we showed that stronger antibody responses to the blood-stage antigens AMA-1, MSP-1, and MSP-3, but not the pre-erythrocytic antigens CSP and LSA-1, were associated with blood-stage protection. Our study supports a role for antibodies to blood-stage antigens in antimalarial protection, but highlights the importance of considering varied P. falciparum exposure in assessing the importance of different antibodies.
Our analysis showed strong associations between antibodies to the blood-stage antigens AMA-1, MSP-1, and MSP-3 and protection from malaria. These findings are consistent with some findings from published cohort studies [32–37] but not others [35, 36, 38, 39]. Our data suggest that a reason for this discrepancy is that antibody-associated protection from malaria may be difficult to discern with use of traditional outcomes in areas where there is highly variable malaria exposure. Defining protection based on the incidence or hazard of malaria implicitly assumes that P. falciparum exposure is homogeneous and, thus, is subject to confounding. In contrast, assessing protection based on progression from asymptomatic parasitemia to symptomatic disease mitigates concerns about differential exposure because the exposure (parasitemia) is measured.
The strong associations that we observed between antibodies to AMA-1, MSP-1, and MSP-3 and protection from malaria remained unchanged after adjustment for prior P. falciparum exposure, as estimated by distance from a swamp and prior malaria incidence. This finding may suggest that these 3 antibody responses are truly protective. However, it is also possible that these antibody responses are simply better surrogate markers of protection than our estimates of prior exposure. Indeed, in our study, antibody responses were all correlated with each other, and when analyzed in combination, estimates of the causal effect for each antibody were less than that observed when analyzed individually. This finding should be considered in light of the fact that we measured responses to a limited subset of P. falciparum antigens. In addition, no combination of the 3 responses to blood-stage antigens predicted protection better than AMA-1 levels alone. Together, these findings suggest that there is no evidence of additive protection in our study and that antibodies to these 3 antigens trigger the same protective mechanisms or, alternatively, are all surrogate markers for different response(s) that mediated protection.
In the present study, protection was more strongly associated with antibody levels than dichotomous responses, which do not take into account the possibility of progressively increased protection with increased antibody levels. The present study supports the use of antibody levels as a finer tool to discriminate antibody-associated protection. Indeed, this may in part explain an apparent discrepancy between our study and one from Kenya, in which dichotomous antibody responses in combination provided more information about protection than individual responses [40]. Our data similarly demonstrated that multiple dichotomous responses provided more information than a single dichotomous response. However, a single antibody level provided more information than 5 dichotomous responses in combination.
Similar to responses to blood-stage antigens, antibodies to CSP and LSA-1 were associated with a higher risk of malaria, which persisted after adjustment for prior exposure. This lack of protection is consistent with some prior studies [41–44] but in contrast with others [18, 45]. The lack of protection seen in this study may reflect inadequate protection afforded by these responses in a relatively low transmission African setting or an inability to adequately control for prior exposure in our analysis. We also saw no association between antibody responses to the pre-erythrocytic antigens CSP and LSA-1 and protection from malaria once parasitemic. This finding is consistent with the parasite’s biology, because these antibodies should have no effect on the parasite after it has reached the blood stage. In contrast, antibodies to blood-stage antigens may have functional roles in inhibiting erythrocytic parasite development, such as blocking of merozoite invasion of erythrocytes [46] or opsonization of merozoites [47].
A limitation of our study is that analysis of protection from malaria once parasitemic does not provide information on protection from parasitemia once infected by a mosquito, such as may be induced by vaccines targeting pre-erythrocytic antigens [48, 49]. However, this approach remains appropriate for assessing protection associated with responses to blood-stage antigens. In addition, this type of analysis is likely to be useful in assessing naturally acquired immunity in general, especially in children, because protection against disease develops earlier and more completely than protection against parasitemia [50].
In conclusion, we demonstrated the usefulness of a novel clinical assessment, protection from malaria once parasitemic, in determining the association of antibody responses to blood-stage antigens with protection from malaria. Assessment of protection from malaria once parasitemic may be a particularly useful outcome measurement when evaluating protection against malaria in areas with heterogeneous malaria exposure. In addition, we showed that associations between individual immune responses and outcomes may overestimate the protective effect of these responses because of correlation with other protective responses. Immunoepidemiology studies designed to detect mechanisms of immune protection should integrate prior exposure into the analysis, either by estimating exposure or by performing analyses more robust to varied exposure, such as those used in this study, and evaluate multiple immune responses.
Funding
This work was supported by the National Institute of Allergy and Infectious Disease (NIAID) (AI052142, AI056270) and the Doris Duke Charitable Foundation (2004047). This work was funded in part by the Intramural Research Program of the National Institutes of Health, NIAID, Laboratory of Malaria Immunology and Vaccinology.
Acknowledgments
We thank all the parents and guardians for kindly giving their consent, the study participants for their cooperation, and all the members of the study team in Uganda.
References
- 1.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–7. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 2.Sabchareon A, Burnouf T, Ouattara D, et al. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 3.Fowkes FJ, Richards JS, Simpson JA, Beeson JG. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. PLoS Med. 2010;7:e1000218. doi: 10.1371/journal.pmed.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bejon P, Warimwe G, Mackintosh CL, et al. Analysis of immunity to febrile malaria in children that distinguishes immunity from lack of exposure. Infect Immun. 2009;77:1917–23. doi: 10.1128/IAI.01358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belizario VY, Saul A, Bustos MD, et al. Field epidemiological studies on malaria in a low endemic area in the Philippines. Acta Trop. 1997;63:241–56. doi: 10.1016/s0001-706x(96)00624-9. [DOI] [PubMed] [Google Scholar]
- 6.Smith DL, Dushoff J, Snow RW, Hay SI. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature. 2005;438:492–5. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreuels B, Kobbe R, Adjei S, et al. Spatial variation of malaria incidence in young children from a geographically homogeneous area with high endemicity. J Infect Dis. 2008;197:85–93. doi: 10.1086/524066. [DOI] [PubMed] [Google Scholar]
- 8.Clark TD, Greenhouse B, Njama-Meya D, et al. Factors determining the heterogeneity of malaria incidence in children in Kampala, Uganda. J infect dis. 2008;198:393–400. doi: 10.1086/589778. [DOI] [PubMed] [Google Scholar]
- 9.Brooker S, Clarke S, Njagi JK, et al. Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Trop Med Int Health. 2004;9:757–66. doi: 10.1111/j.1365-3156.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 10.Ernst KC, Adoka SO, Kowuor DO, Wilson ML, John CC. Malaria hotspot areas in a highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malar J. 2006;5:78. doi: 10.1186/1475-2875-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oesterholt MJ, Bousema JT, Mwerinde OK, et al. Spatial and temporal variation in malaria transmission in a low endemicity area in northern Tanzania. Malar J. 2006;5:98. doi: 10.1186/1475-2875-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson S, Booth M, Jones FM, et al. Age-adjusted Plasmodium falciparum antibody levels in school-aged children are a stable marker of microgeographical variations in exposure to Plasmodium infection. BMC Infect Dis. 2007;7:67. doi: 10.1186/1471-2334-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bejon P, Williams TN, Liljander A, et al. Stable and unstable malaria hotspots in longitudinal cohort studies in Kenya. PLoS Med. 2010;7:e1000304. doi: 10.1371/journal.pmed.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bousema T, Drakeley C, Gesase S, et al. Identification of hot spots of malaria transmission for targeted malaria control. J Infect Dis. 2010;201:1764–4. doi: 10.1086/652456. [DOI] [PubMed] [Google Scholar]
- 15.Davis JC, Clark TD, Kemble SK, et al. Longitudinal study of urban malaria in a cohort of Ugandan children: description of study site, census and recruitment. Malar J. 2006;5:18. doi: 10.1186/1475-2875-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorsey G, Staedke S, Clark TD, et al. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. JAMA. 2007;297:2210–9. doi: 10.1001/jama.297.20.2210. [DOI] [PubMed] [Google Scholar]
- 17.Clark TD, Njama-Meya D, Nzarubara B, et al. Incidence of malaria and efficacy of combination antimalarial therapies over 4 years in an urban cohort of Ugandan children. PLoS One. 2010;5:e11759. doi: 10.1371/journal.pone.0011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John CC, Moormann AM, Pregibon DC, et al. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am J Trop Med Hyg. 2005;73:222–8. [PubMed] [Google Scholar]
- 19.Dutta S, Lalitha PV, Ware LA, et al. Purification, characterization, and immunogenicity of the refolded ectodomain of the Plasmodium falciparum apical membrane antigen 1 expressed in Escherichia coli. Infect Immun. 2002;70:3101–0. doi: 10.1128/IAI.70.6.3101-3110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimp RL, Jr., Martin LB, Zhang Y, et al. Production and characterization of clinical grade Escherichia coli derived Plasmodium falciparum 42 kDa merozoite surface protein 1 (MSP1(42)) in the absence of an affinity tag. Protein Expr Purif. 2006;50:58–67. doi: 10.1016/j.pep.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Singh S, Kennedy MC, Long CA, Saul AJ, Miller LH, Stowers AW. Biochemical and immunological characterization of bacterially expressed and refolded Plasmodium falciparum 42-kilodalton C-terminal merozoite surface protein 1. Infect Immun. 2003;71:6766–4. doi: 10.1128/IAI.71.12.6766-6774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai CW, Duggan PF, Jin AJ, et al. Characterization of a protective Escherichia coli-expressed Plasmodium falciparum merozoite surface protein 3 indicates a non-linear, multi-domain structure. Mol Biochem Parasitol. 2009;164:45–56. doi: 10.1016/j.molbiopara.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.John CC, Ouma JH, Sumba PO, Hollingdale MR, Kazura JW, King CL. Lymphocyte proliferation and antibody responses to Plasmodium falciparum liver-stage antigen-1 in a highland area of Kenya with seasonal variation in malaria transmission. Am J Trop Med Hyg. 2002;66:372–8. doi: 10.4269/ajtmh.2002.66.372. [DOI] [PubMed] [Google Scholar]
- 24.Greenhouse B, Slater M, Njama-Meya D, et al. Decreasing efficacy of antimalarial combination therapy in Uganda is explained by decreasing host immunity rather than increasing drug resistance. J Infect Dis. 2009;199:758–65. doi: 10.1086/596741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 26.Robins JM, Blevins D, Ritter G, Wulfsohn M. G-estimation of the effect of prophylaxis therapy for Pneumocystis carinii pneumonia on the survival of AIDS patients. Epidemiology. 1992;3:319–36. doi: 10.1097/00001648-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 27.van der Laan MJ, Polley EC, Hubbard AE. Super learner. Stat Appl Genet Mol Biol. 2007;6 doi: 10.2202/1544-6115.1309. Article25. [DOI] [PubMed] [Google Scholar]
- 28.Breiman L. Random forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- 29.Hastie T, Tibshirani R. Generalized additive models. 1st ed. London, New York: Chapman and Hall; 1990. Monographs on statistics and applied probability 43. [Google Scholar]
- 30.Ripley BD. Pattern recognition and neural networks. Cambridge, New York: Cambridge University Press; 1996. [Google Scholar]
- 31.Friedman JH, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 32.Polley SD, Mwangi T, Kocken CH, et al. Human antibodies to recombinant protein constructs of Plasmodium falciparum Apical Membrane Antigen 1 (AMA1) and their associations with protection from malaria. Vaccine. 2004;23:718–28. doi: 10.1016/j.vaccine.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Polley SD, Tetteh KK, Lloyd JM, et al. Plasmodium falciparum merozoite surface protein 3 is a target of allele-specific immunity and alleles are maintained by natural selection. J infectious diseases. 2007;195:279–87. doi: 10.1086/509806. [DOI] [PubMed] [Google Scholar]
- 34.Osier FH, Polley SD, Mwangi T, Lowe B, Conway DJ, Marsh K. Naturally acquired antibodies to polymorphic and conserved epitopes of Plasmodium falciparum merozoite surface protein 3. Parasite Immunol. 2007;29:387–94. doi: 10.1111/j.1365-3024.2007.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dodoo D, Aikins A, Kusi KA, et al. Cohort study of the association of antibody levels to AMA1, MSP119, MSP3 and GLURP with protection from clinical malaria in Ghanaian children. Malar J. 2008;7:142. doi: 10.1186/1475-2875-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nebie I, Diarra A, Ouedraogo A, et al. Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infect Immun. 2008;76:759–66. doi: 10.1128/IAI.01147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meraldi V, Nebie I, Tiono AB, et al. Natural antibody response to Plasmodium falciparum Exp-1, MSP-3 and GLURP long synthetic peptides and association with protection. Parasite Immunol. 2004;26:265–72. doi: 10.1111/j.0141-9838.2004.00705.x. [DOI] [PubMed] [Google Scholar]
- 38.Gray JC, Corran PH, Mangia E, et al. Profiling the antibody immune response against blood stage malaria vaccine candidates. Clin chem. 2007;53:1244–53. doi: 10.1373/clinchem.2006.081695. [DOI] [PubMed] [Google Scholar]
- 39.Stanisic DI, Richards JS, McCallum FJ, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77:1165–74. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osier FH, Fegan G, Polley SD, et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun. 2008;76:2240–8. doi: 10.1128/IAI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Z, Xiao L, Branch OH, Kariuki S, Nahlen BL, Lal AA. Antibody responses to repetitive epitopes of the circumsporozoite protein, liver stage antigen-1, and merozoite surface protein-2 in infants residing in a Plasmodium falciparum-hyperendemic area of western Kenya. XIII. Asembo Bay Cohort Project. Am J Trop Med Hyg. 2002;66:7–12. doi: 10.4269/ajtmh.2002.66.7. [DOI] [PubMed] [Google Scholar]
- 42.Wongsrichanalai C, Webster HK, Permpanich B, Chuanak N, Ketrangsri S. Naturally acquired circumsporozoite antibodies and their role in protection in endemic falciparum and vivax malaria. Am J Trop Med Hyg. 1991;44:201–4. doi: 10.4269/ajtmh.1991.44.201. [DOI] [PubMed] [Google Scholar]
- 43.Kitua AY, Urassa H, Wechsler M, et al. Antibodies against Plasmodium falciparum vaccine candidates in infants in an area of intense and perennial transmission: relationships with clinical malaria and with entomological inoculation rates. Parasite Immunol. 1999;21:307–17. doi: 10.1046/j.1365-3024.1999.00230.x. [DOI] [PubMed] [Google Scholar]
- 44.John CC, Zickafoose JS, Sumba PO, King CL, Kazura JW. Antibodies to the Plasmodium falciparum antigens circumsporozoite protein, thrombospondin-related adhesive protein, and liver-stage antigen 1 vary by ages of subjects and by season in a highland area of Kenya. Infect Immun. 2003;71:4320–5. doi: 10.1128/IAI.71.8.4320-4325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.John CC, Tande AJ, Moormann AM, et al. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J Infect Dis. 2008;197:519–26. doi: 10.1086/526787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blackman MJ, Heidrich HG, Donachie S, McBride JS, Holder AA. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–82. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–18. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bejon P, Lusingu J, Olotu A, et al. Efficacy of RTS, S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359:2521–32. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffman SL, Goh LM, Luke TC, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–64. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 50.Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51–60. doi: 10.1111/j.1365-3024.2006.00808.x. [DOI] [PubMed] [Google Scholar]