Abstract
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide. Although considerable progress has been made in elucidating the etiology of the disease, the prognosis for individuals diagnosed with HNSCC remains poor, underscoring the need for development of additional treatment modalities. HNSCC is characterized by the upregulation of a large number of proteolytic enzymes, including urokinase plasminogen activator (uPA) and an assortment of matrix metalloproteinases (MMPs) that may be expressed by tumor cells, by tumor-supporting stromal cells or by both. Here we explored the use of an intercomplementing anthrax toxin that requires combined cell surface uPA and MMP activities for cellular intoxication and specifically targets the ERK/MAPK pathway for the treatment of HNSCC. We found that this toxin displayed strong systemic anti-tumor activity towards a variety of xenografted human HNSCC cell lines by inducing apoptotic and necrotic tumor cell death, and by impairing tumor cell proliferation and angiogenesis. Interestingly, the human HNSCC cell lines were insensitive to the intercomplementing toxin when cultured ex vivo, suggesting that either the toxin targets the tumor-supporting stromal cell compartment or that the tumor cell requirement for ERK/MAPK signaling differs in vivo and ex vivo. This intercomplementing toxin warrants further investigation as an anti-HNSCC agent.
Introduction
With more than 500,000 new cases diagnosed each year, head and neck squamous cell carcinoma (HNSCC) represents the sixth most common cancer worldwide [1]. Although the risk factors and the molecular pathways that underlie HNSCC development are by now well-known, the five-year survival rate after diagnosis is low and has remained unchanged for many decades [2]–[6].
The overexpression, prognostic significance, and causal involvement of extracellular/pericellular proteases in the progression of HNSCC have been extensively studied (reviewed in [7]–[10]). The multiple proteases that are expressed at very high levels by either tumor cells, stromal cells or both include urokinase plasminogen activator (uPA), tissue plasminogen activator, matrix metalloproteinase (MMP)-1, -2, -3, -7, -9, -10, -11, -13, and -14, cathepsins B, D, H, and L, kallikreins 5, 7, 8, and 10, and matriptase [11]–[32].
Anthrax toxin is a three-component toxin secreted by Bacillus anthracis that consists of protective antigen (PA, 83 kDa), lethal factor (LF, 90 kDa), and edema factor (EF, 90 kDa) [33]. To intoxicate cells, PA binds to either of two widely-expressed cell surface receptors, tumor endothelial marker 8 (TEM8, also “ANTXR1”) or capillary morphogenesis gene 2 product (CMG2, also “ANTXR2”) [34]–[37], and subsequently is cleaved at the sequence 164RKKR167 by cell-surface furin or furin-like proteases, which are ubiquitously expressed by cells [38], [39]. This cleavage is essential for all subsequent steps of intoxication. The newly generated C-terminal 63-kDa fragment of PA remains bound to its cell surface receptor and forms a heptamer that binds and translocates up to three molecules of LF or EF into the cytosol to induce their cytotoxic effects. EF is a potent adenylate cyclase that intoxicates cells by raising cAMP levels, whereas LF is a metalloproteinase that cleaves and inactivates mitogen-activated protein kinase kinases (MEKs), thereby blocking the extracellular signal-regulated kinase (ERK)/mitogen activated protein kinase (MAPK) pathway [40]–[45].
Strategies to therapeutically exploit the signature overexpression of proteolytic enzymes in cancer have mostly focused on inhibiting their enzymatic activity so as to blunt invasive and metastatic processes [46]–[48]. More recently, however, strategies have been devised to generate tumor cytotoxic pro-drugs that are activated by specific tumor-expressed proteases [49]–[51]. In this regard, we previously generated a reengineered intercomplementing anthrax toxin that requires the combined activities of both cell surface uPA and MMP for cytotoxicity, and showed that this toxin has a greatly diminished off-target cytotoxicity when compared to native anthrax toxin [52]. The intercomplementing toxin consists of PA-U2-R200A, which is a reengineered PA that is activated by uPA and PA-L1-I210A, which is a reengineered PA that is activated by MMPs. PA-U2-R200A and PA-L1-I210A also each harbor additional, but different, mutations in the LF/EF binding site that make heptamers composed of PA-U2-R200A alone or of PA-L1-I210A alone unable to bind EF or LF and support their translocation to the cytoplasm. However, heptamers that are composed of a mixture of PA-U2-R200A and PA-L1-I210A can form functional EF/LF binding sites in by intermolecular complementation.
Because of the consistent expression of both uPA and several MMPs by human HNSCC, here we explored the potential use of this toxin as a novel targeted treatment for this disease. Indeed, we found that systemic administration of the intercomplementing toxin caused regression of several xenografted human HNSCC. Interestingly, the in vivo efficacy of the toxin was independent of the sensitivity of the cultured tumor cells to the toxin, suggesting that the intercomplementing toxin may inhibit tumor growth by targeting both the tumor cell compartment and the stromal cell compartment.
Methods
Ethics statement
All animal work was performed in accordance with protocols approved by the National Institute of Dental and Craniofacial Research Animal Care and Use Committee (Animal Study Proposal Numbers: 09-523 and 10-585).
Protein purification
Recombinant PA, PA-U2-R200A, PA-L1-I210A, LF, and FP59 were generated and purified as previously described [52]-[54]. The LF used here is a recombinant protein having a non-native N-terminal sequence of HMAGG [55].
Cell culture
The human HNSCC cell lines Cal27, Hep2, HN6, HN12, HN30 [56]–[58], the cervical carcinoma cell line HeLa [59], and the human colon carcinoma cell line HT29 [60] have been described. All cell lines were cultured in a humidified 5% CO2 environment at 37°C. Cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA).
In vitro cytotoxicity assays
The cytotoxicity of PA-U2-R200A and PA-L1-I210A, with LF or FP59, was assessed using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in 96-well plates [61]. Cells exhibiting ∼40% confluence were incubated with serial dilutions of PA (0–10 nM) or PA-U2-R200A + PA-L1-I210A (0–10 nM) and either FP59 (1.9 nM) or LF (5.5 nM) to a final volume of 200 µl per well. Cell viability was determined after 48 h.
Animals
Female Hsd:Athymic Nude-Foxn1nu mice (Harlan Laboratories Inc., Indianapolis, IN) between 4 and 6 weeks of age were used in this study. Animals were housed in a pathogen-free environment certified by the Association for Assessment and Accreditation of Laboratory Animal Care International.
In vivo tumor xenograft model
Cells (9×105 per mouse) were injected intradermally in the mid-scapular dorsal region of each mouse as described [62]. When tumors reached a volume of 50–100 mm3, the mice were divided into two groups of ten mice with equivalent median tumor sizes. Treatment was initiated on day 0. Mice received intraperitoneal (I.P.) injections of either 100 or 500 µl PBS or PA-U2-R200A + PA-L1-I210A + LF in 100 or 500 µl PBS, respectively. Mice bearing HN12 xenografts were administered 25 µg PA-U2-R200A +25 µg PA-L1-I210A +17 µg LF, while mice bearing Cal27, HN6 and Hep2 xenografts were treated with 20 µg PA-U2-R200A +20 µg PA-L1-I210A +13 µg LF. Mice received five injections total using a Monday, Wednesday, Friday dosing schedule. An investigator unaware of the treatment group measured the longest and shortest tumor diameters with digital calipers (FV Fowler Company, Inc., Newton, MA). Tumor volume was estimated using the equation V = (length in mm * (width in mm)2)/2 [63]. The statistical significance of differences in tumor sizes and mouse weight was determined by the two-tailed Student's t-test using Microsoft Excel software. During the 11-day treatment period, no animals in either the control or treatment groups met the criteria for early euthanasia: frank tumor ulceration or tumor mass exceeding 10% of body weight.
Histopathological analysis
HN12 (n = 5) and Hep2 (n = 4) tumor-bearing nude mice were treated I.P. with 100 or 500 µl PBS or with 15 µg PA-U2-R200A +15 µg PA-L1-I210A +10 µg LF in 100 or 500 µl PBS on days 0, 2 and 4. The mice were euthanized by CO2 inhalation 24 h after the last injection. Tumors were excised, fixed in 4% paraformaldehyde for 24 h, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Sections with identifiable carcinoma cells in the H&E sections were also stained with a monoclonal rabbit anti-mouse PECAM-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and a polyclonal rabbit anti-human Ki67 (Novocastra Laboratories, Ltd., Newcastle, UK). TUNEL staining was performed by Histoserv, Inc. (Germantown, MD). Images were captured using an Aperio T3 Scanscope (Aperio Technologies, Vista, CA) and were quantified using Aperio Imagescope Software (Aperio Technologies, Vista, CA) by a blinded investigator. Statistical significance of differences for apoptosis, cellular proliferation and tumor vascularization were determined using the Student's t-test, two-tailed, using GraphPad Prism software. Statistical significance of differences for necrosis was determined using the Mann-Whitney U-test using GraphPad Prism software.
Results
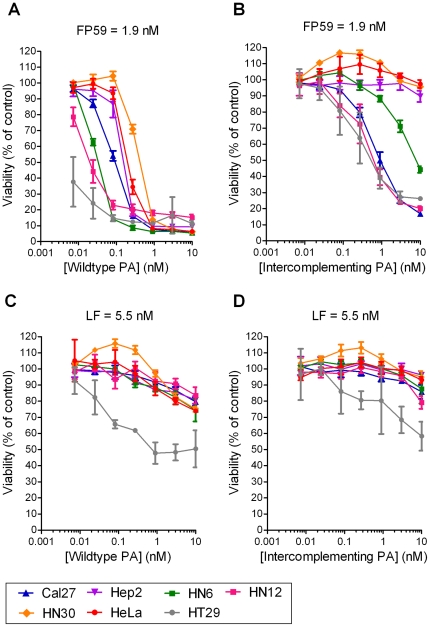
We first explored if HNSCC cells express functional anthrax toxin receptors by exposing the five human HNSCC cell lines, Cal27, Hep2, HN6, HN12, and HN30 to increasing concentrations of PA in combination with 1.9 nM FP59 (Figure 1A). FP59 is a fusion protein consisting of LF residues 1–254 with the ADP-ribosylation domain of Pseudomonas exotoxin A. When translocated to the cytoplasm via PA, FP59 efficiently kills all cells by ADP-ribosylation and inhibition of translation elongation factor 2 [64]. PA in combination with FP59 killed all HNSCC cell lines with an LD50 ranging from less than 7 to 400 pM demonstrating the presence of functional anthrax toxin receptors. To assess if these HNSCC cell lines also express both functional uPA and MMP cell surface proteolytic activity, we next exposed the five HNSCC cell lines to the same concentration of FP59 in combination with increasing concentrations of intercomplementing PA (PA-U2-R200A + PA-L1-I210A) (Figure 1B). Three of the HNSCC cell lines (Cal27, HN6, HN12) were sensitive to the intercomplementing toxin (LD50, 0.5 nM to 8 nM), demonstrating functional uPA and MMP expression, while the two other cell lines, Hep2 and HN30 were resistant, indicating the absence of either functional uPA or MMP activity, or both. We next determined if the five HNSCC cell lines were dependent on a functional MEK/MAPK pathway for growth in culture by incubating them with 5.5 nM LF in combination with increasing concentrations of either wildtype PA (Figure 1C) or intercomplementing PA (Figure 1D). None of the HNSCC cell lines were sensitive to the two toxin combinations, showing that MEK activity is dispensable for HNSCC cell growth in culture.
Figure 1. Cytotoxicity of intercomplementing anthrax lethal toxins to human HNSCC cell lines.
Cal27 (blue triangles), Hep2 (purple triangles), HN6 (green squares), HN12 (purple squares), and HN30 (yellow diamonds) cells were incubated with increasing concentrations of wildtype PA in combination with FP59 (A), intercomplementing PA (PA-U2-R200A + PA-L1-I210A) in the presence of FP59 (B), wildtype PA with LF (C) or intercomplementing PA with LF (D) for 48 h. The cell viability was then measured using an MTT assay. HT29 colon carcinoma (grey circles) and HeLa (red circles) cells were used as a positive and negative controls, respectively [52], [74]. Cell survival is expressed as mean viability ± standard deviation of the mean.
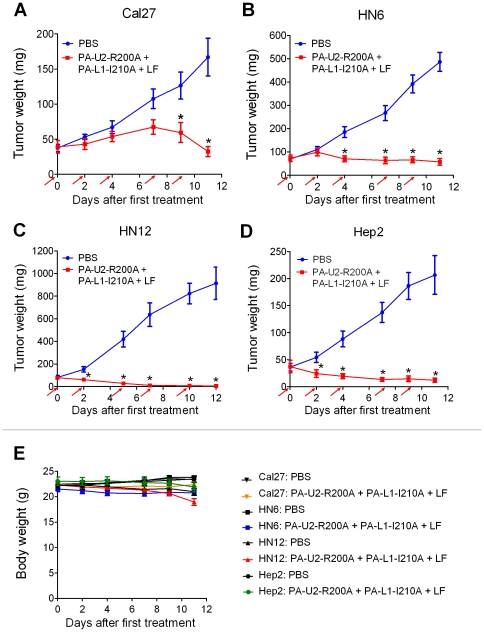
Cal27, Hep2, HN6, and HN12 HNSCC cell lines form solid tumors when xenografted to immunocompromized mice and were therefore suitable for assessing the efficacy of the intercomplementing toxin for HNSCC treatment in vivo. The four cell lines were transplanted intradermally to nude mice, and solid tumor nodules constituting 0.25 to 0.5 percent of the total body weight were allowed to form. The mice thereafter were treated three times per week with intraperitoneal injections of either intercomplementing toxin in combination with LF or with PBS as a control (Figure 2). Cal27, HN6, and HN12 tumors were all efficiently treated with the intercomplementing toxin, consistent with their expression of functional cell surface uPA and MMP activities in cell culture (Figure 2A-C). Average tumor sizes in toxin-treated mice ranged from 0.6 to 26 percent of the average tumor sizes of PBS-injected mice at treatment cessation. Interestingly, although Hep2 cells did not express uPA or MMP activity sufficient for intercomplementing toxin activation in culture (see above), the Hep2 tumors were treated efficiently in vivo, with the average tumor size of toxin-treated mice being just six percent of the average tumor sizes of PBS-injected mice at treatment cessation (Figure 2D). The greatest response to the intercomplementing toxin was observed with HN12-bearing mice, with 40 percent of the mice remaining tumor-free when observed for up to one year after treatment cessation.
Figure 2. Tumoricidal activity of intermolecular complementing PA to human HNSCC.
Nude mice bearing intradermal Cal27 (A), HN6 (B), HN12 (C), and Hep2 (D) HNSCC xenografts were injected intraperitoneally with either PBS (blue lines) or PA-U2-R200A + PA-L1-I210A + LF (red lines) at the time points indicated by the red arrows. (E) Body weight change over time in all groups. The data are expressed as mean tumor weight ± standard error of the mean; *, P<0.01. Ten mice were used per tumor and treatment group.
The toxin was generally well tolerated. Two of 40 (five percent) of the toxin-treated mice died within the treatment period, whereas no death was observed in PBS-treated groups (P = N. S., Chi-square test, two tailed). The largest weight loss in toxin-treated mice as compared to PBS-treated mice (Figure 2E) was observed at day 11 in all trials. Excluding the mass attributed to tumor burden, the average body weight of all toxin treated mice was 8.3 percent lower than the average body weight for all mice in PBS-treatment groups (P<0.0004, Student's t-test, two-tailed).
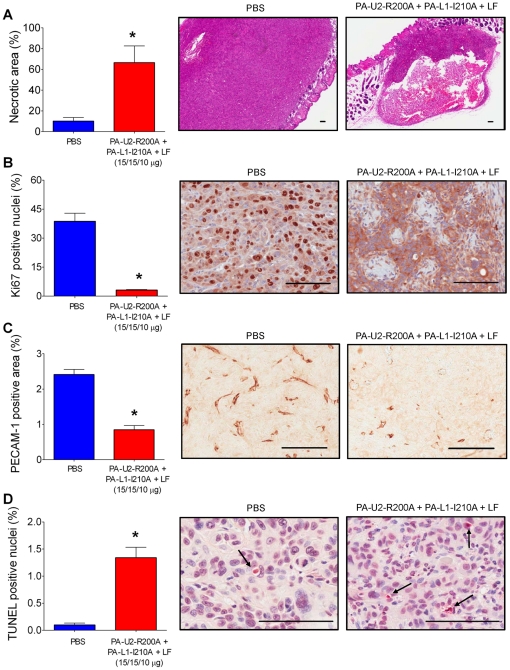
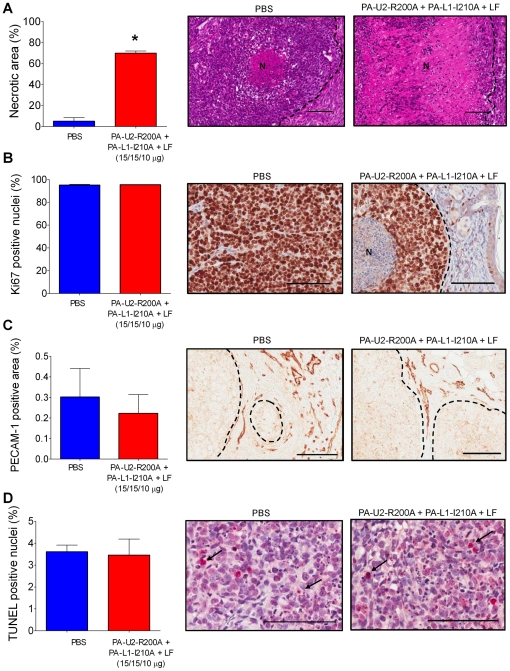
We next examined the mechanistic basis for the potent systemic anti-HNSCC activity of the intercomplementing toxin. For this purpose HN12 (sensitive to intercomplementing toxin administered with FP59 in vitro) and Hep2 cells (insensitive to intercomplementing toxin administered with FP59 in vitro) were transplanted to mice, and established tumors were treated three times with toxin or PBS. The tumors were then excised (at day 5, as per Figure 2), and histological sections were generated, scanned, and subjected to an unbiased quantitative histomorphometric analysis. No tumor tissue was identified in histological sections from two of five examined HN12 toxin-treated tumors, indicating complete tumor regression. The three remaining toxin-treated HN12 tumors presented with a four-fold increase in the area of tumor necrosis, as compared to untreated tumors (Figure 3A). Cell proliferation in the remaining viable areas of these toxin-treated tumors was more than twelve-fold reduced, as determined by staining of the cell proliferation marker, Ki67 (Figure 3B). Vessel density was likewise reduced by three-fold (Figure 3C), while the apoptotic index was increased 13-fold (Figure 3D). Interestingly, unlike the case for the HN12 tumors examined above, the intercomplementing toxin primarily targeted Hep2 tumors by inducing necrosis (Figure 4). Thus, necrosis was increased by more than 13-fold (Figure 4A), while cell proliferation, vessel densities, and apoptotic indices in the remaining viable areas of the xenografted Hep2 tumors were all unaffected by treatment with intercomplementing toxin (Figure 4B-D). The retention of these latter tumor properties was notable given the striking decrease in tumor mass (Figure 2D, day 5).
Figure 3. Increased necrosis and apoptosis and decreased proliferation and vessel density of human HN12 xenografts in intercomplementing toxin-treated mice.
Necrosis (A), proliferation (B), tumor vascularization (C), and apoptosis (D) of HN12 xenografts 5 days after initiation of systemic treatment with either PBS (blue bars and left panels) or intercomplementing toxin (red bars and right panels). A, hematoxylin and eosin staining. B, Ki67 staining. C, PECAM-1 staining. D, TUNEL staining. The arrows in D highlight examples of TUNEL positive cells. Columns, mean; bars, standard error of the mean, *, P<0.05. In all cases, representative images are shown. Scale Bars; 100 µM.
Figure 4. Intercomplementing toxin induces massive tumor necrosis in xenografted human Hep2 tumors, but does not affect proliferation, vascularization or apoptosis in viable tumor areas.
Necrosis (A), proliferation (B), tumor vascularization (C), and apoptosis (D) of Hep2 xenografts five days after initiation of systemic treatment with either PBS (blue bars and left panels) or intercomplementing toxin (red bars and right panels). A, hematoxylin and eosin staining. B, Ki67 staining. C, PECAM-1 staining. D, TUNEL staining. N in A and B indicates necrotic area. Tumor margins in A–C are indicated with dotted lines. Arrows in D highlight examples of TUNEL positive cells. Columns, mean; bars, standard error of the mean, *, P<0.05. In all cases, representative images are shown. Scale Bars; 100 µM.
Discussion
Research over the last decade has led to the generation of several modified anthrax toxin-based candidate anti-tumor compounds that exploit the signature overexpression of extracellular proteases by tumor cells and the cellular components of the tumor stroma to achieve tumor selectivity [52], [53], [65]–[74]. In the current study we performed a comprehensive analysis of the suitability of one of these compounds: an intercomplementing toxin for the treatment of HNSCC. A unique property of this toxin is its inclusion of no less than three specificity determinants: the requirement for both cell surface uPA activity and cell surface MMP activity, combined with selective toxicity to cells dependent on the MEK/MAPK kinase pathway for survival [42], [52].
Xenograft studies revealed that the intercomplementing toxin displayed excellent systemic antitumor activity towards all of the four analyzed HNSCC cell lines. By using a treatment regimen consisting of five intraperitoneal toxin injections, we achieved effects ranging from tumor stasis to complete tumor eradication, as defined by the absence of relapse in mice that were followed for up to one year after treatment cessation.
A notable observation in the current study was that sensitivity to LF or expression of functional cell surface uPA and MMP activity by cultured HNSCC cell lines were poor predictors of the in vivo sensitivity of the tumor to the intercomplementing toxin. Thus, although all of the four tumors resulting from the HNSCC cell lines were efficiently treated with the intercomplementing toxin when xenografted to mice, none of the cell lines displayed significant sensitivity to LF in culture (wildtype PA with LF, Figure 1C), and one cell line did not express sufficient cell surface uPA and MMP activity for functional PA heptamer formation (Hep2 cells, intercomplementing toxin with FP59, Figure 1B). Two possible, and not mutually exclusive, explanations for this observation can be offered. First, the repertoire of cell surface proteases expressed by the tumor cells or their requirement for the ERK/MAPK pathway for proliferation and survival may differ in culture and in vivo. Secondly, the intercomplementing toxin may exert its anti-tumor activity by targeting the cellular component of the HNSCC tumor stroma (tumor-associated inflammatory cells, fibroblasts, endothelial cells) [75]. In direct support of the latter, we have previously shown that LF can efficiently impair the growth of a xenografted immortalized ovarian cell line that was made genetically deficient in anthrax toxin receptors [69]. It also remains a possibility that activation of the intercomplementing toxin by proteases different from uPA and MMPs account for the in vivo efficacy of the intercomplementing toxin. This, however, is unlikely to be the case. First, cleavage of MMP-activated PA and subsequent cellular intoxication can be blocked completely by the synthetic MMP inhibitors BB94 (Batimastat), BB2516 (Marimastat), and GM6001, as well as tissue inhibitor of matrix metalloproteinases-2. Cleavage of the uPA-activated PA and cellular intoxication on the other hand can be prevented by plasminogen activator inhibitor-1 and by antibodies and also by protein fragments that block the binding of uPA to its cellular receptor. Finally, using mice genetically deficient in components of the plasminogen activation system, we have previously shown that the anti-tumor activity of the uPA-activated PA in vivo is dependent on cell surface uPA activity [52], [53], [73], [74], [76].
Histological analysis of HN12 and Hep2 xenografts revealed markedly different effects of systemic intercomplementing toxin treatment on the two tumors. While toxin-treated HN12 xenografts displayed decreased proliferation and an increase in both apoptotic and necrotic cell death, neither proliferation or apoptosis was affected by toxin treatment of Hep2 xenografts. Rather, the potent anti-tumor activity of the intercomplementing toxin was caused exclusively by the induction of tumor necrosis. In light of the inability of cultured Hep2 cells to express sufficient uPA and MMP activity for cellular intoxication, it is tempting to speculate that tumor necrosis in Hep2 xenografts is induced by a vascular collapse caused by direct targeting of the tumor vasculature or other essential cellular components of the tumor stroma.
The anti-tumor efficacy of the intercomplementing toxin and tolerability at administered doses highlight the therapeutic potential of this agent. Procedures for recombinant expression and purification of large quantities of anthrax toxins in avirulent strains of Bacillus anthracis and Eschericia coli are already established and will not represent an impediment to the therapeutic development of the intercomplementing toxin for treatment of HNSCC. The pharmacology and pharmacokinetics of wildtype PA and LF are well studied, and the intercomplementing PA could be expected to display pharmacological and pharmacokinetic properties similar to or even more favorable that those of wildtype PA due to enhanced plasma stability [69], [77]–[79]. Systematic animal toxicity studies required for the clinical introduction of the intercomplementing toxin are in progress (D. E. P., S. H. L., and T. H. B., unpublished data).
The absence of correlation between in vivo efficacy and sensitivity of cultured HNSCC cell lines to the intercomplementing toxin discussed above raise interesting questions as to the potential for prescreening HNSCC patients for treatment. Based on our findings, assaying toxin-sensitivity of cultured primary tumor cell explants may be of limited value. However, determination of tumor cell and stromal cell uPA and MMP protein expression or enzymatic activity in resected tumors or needle biopsies may be a clinically useful predictor of treatment efficacy.
In summary, we have shown that an intercomplementing toxin specifically targeting ERK/MAPK-dependent cells with high cell surface uPA and MMP activity holds promise as a novel candidate drug for the treatment of HNSCC. Future research towards the clinical development of this toxin is warranted.
Acknowledgments
We thank Drs. Mary Jo Danton and Silvio Gutkind for critically reviewing this manuscript, and Rasem Fattah for preparation of toxins.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by NIAID and NIDCR Intramural Research Programs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 4.Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5:311–316. doi: 10.1016/s1535-6108(04)00090-x. [DOI] [PubMed] [Google Scholar]
- 5.Molinolo AA, Amornphimoltham P, Squarize CH, Castilho RM, Patel V, et al. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol. 2009;45:324–334. doi: 10.1016/j.oraloncology.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leemans CR, Braakhuis BJ, Brakenhoff RH. Nat Rev Cancer In press; 2010. The molecular biology of head and neck cancer. [DOI] [PubMed] [Google Scholar]
- 7.Bugge TH. Proteolysis in carcinogenesis. In: Ensley JF, Gutkind, J. S Jacob, J R, Lippman, S M, editors. Head and Neck Cancer. San Diego: Academic Press; 2003. pp. 137–149. [Google Scholar]
- 8.Emami N, Diamandis EP. Utility of kallikrein-related peptidases (KLKs) as cancer biomarkers. Clin Chem. 2008;54:1600–1607. doi: 10.1373/clinchem.2008.105189. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal EL, Matrisian LM. Matrix metalloproteases in head and neck cancer. Head Neck. 2006;28:639–648. doi: 10.1002/hed.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Z, Stack MS. Urinary-type plasminogen activator (uPA) and its receptor (uPAR) in squamous cell carcinoma of the oral cavity. Biochem J. 2007;407:153–159. doi: 10.1042/BJ20071037. [DOI] [PubMed] [Google Scholar]
- 11.Szabo R, Rasmussen AL, Moyer AB, Kosa P, Schafer J, et al. Oncogene In press; 2011. c-Met-induced epithelial carcinogenesis is initiated by the serine protease matriptase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettus JR, Johnson JJ, Shi Z, Davis JW, Koblinski J, et al. Multiple kallikrein (KLK 5, 7, 8, and 10) expression in squamous cell carcinoma of the oral cavity. Histol Histopathol. 2009;24:197–207. doi: 10.14670/hh-24.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franchi A, Santucci M, Masini E, Sardi I, Paglierani M, et al. Expression of matrix metalloproteinase 1, matrix metalloproteinase 2, and matrix metalloproteinase 9 in carcinoma of the head and neck. Cancer. 2002;95:1902–1910. doi: 10.1002/cncr.10916. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Schler G, Gruensfelder P, Muller J, Hoppe F. Urokinase receptor up-regulation in head and neck squamous cell carcinoma. Head Neck. 2000;22:498–504. doi: 10.1002/1097-0347(200008)22:5<498::aid-hed9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Curino A, Patel V, Nielsen BS, Iskander AJ, Ensley JF, et al. Detection of plasminogen activators in oral cancer by laser capture microdissection combined with zymography. Oral Oncol. 2004;40:1026–1032. doi: 10.1016/j.oraloncology.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Romer J, Pyke C, Lund LR, Ralfkiaer E, Dano K. Cancer cell expression of urokinase-type plasminogen activator receptor mRNA in squamous cell carcinomas of the skin. J Invest Dermatol. 2001;116:353–358. doi: 10.1046/j.1523-1747.2001.01241.x. [DOI] [PubMed] [Google Scholar]
- 17.Okada A, Bellocq JP, Rouyer N, Chenard MP, Rio MC, et al. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci U S A. 1995;92:2730–2734. doi: 10.1073/pnas.92.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyajima Y, Nakano R, Morimatsu M. Analysis of expression of matrix metalloproteinases-2 and -9 in hypopharyngeal squamous cell carcinoma by in situ hybridization. Ann Otol Rhinol Laryngol. 1995;104:678–684. doi: 10.1177/000348949510400902. [DOI] [PubMed] [Google Scholar]
- 19.Bjorlin G, Ljungner H, Wennerberg J, Astedt B. Plasminogen activators in human xenografted oro-pharyngeal squamous cell carcinomas. Acta Otolaryngol. 1987;104:568–572. [PubMed] [Google Scholar]
- 20.Itaya T, Suzuki K, Takagi I, Motai H, Baba S. Relationship between head and neck squamous cell carcinomas and fibrinolytic factors. Immunohistological study. Acta. 1996;Otolaryngol(Suppl 525):113–119. [PubMed] [Google Scholar]
- 21.Yasuda T, Sakata Y, Kitamura K, Morita M, Ishida T. Localization of plasminogen activators and their inhibitor in squamous cell carcinomas of the head and neck. Head Neck. 1997;19:611–616. doi: 10.1002/(sici)1097-0347(199710)19:7<611::aid-hed8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt M, Hoppe F. Increased levels of urokinase receptor in plasma of head and neck squamous cell carcinoma patients. Acta Otolaryngol. 1999;119:949–953. doi: 10.1080/00016489950180342. [DOI] [PubMed] [Google Scholar]
- 23.Johansson N, Airola K, Grenman R, Kariniemi AL, Saarialho-Kere U, et al. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am J Pathol. 1997;151:499–508. [PMC free article] [PubMed] [Google Scholar]
- 24.Budihna M, Strojan P, Smid L, Skrk J, Vrhovec I, et al. Prognostic value of cathepsins B, H, L, D and their endogenous inhibitors stefins A and B in head and neck carcinoma. Biol Chem Hoppe Seyler. 1996;377:385–390. doi: 10.1515/bchm3.1996.377.6.385. [DOI] [PubMed] [Google Scholar]
- 25.Muller D, Wolf C, Abecassis J, Millon R, Engelmann A, et al. Increased stromelysin 3 gene expression is associated with increased local invasiveness in head and neck squamous cell carcinomas. Cancer Res. 1993;53:165–169. [PubMed] [Google Scholar]
- 26.Kusukawa J, Sasaguri Y, Shima I, Kameyama T, Morimatsu M. Expression of matrix metalloproteinase-2 related to lymph node metastasis of oral squamous cell carcinoma. A clinicopathologic study. Am J Clin Pathol. 1993;99:18–23. doi: 10.1093/ajcp/99.1.18. [DOI] [PubMed] [Google Scholar]
- 27.Birkedal-Hansen B, Pavelic ZP, Gluckman JL, Stambrook P, Li YQ, et al. MMP and TIMP gene expression in head and neck squamous cell carcinomas and adjacent tissues. Oral Dis. 2000;6:376–382. doi: 10.1111/j.1601-0825.2000.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 28.Imanishi Y, Fujii M, Tokumaru Y, Tomita T, Kanke M, et al. Clinical significance of expression of membrane type 1 matrix metalloproteinase and matrix metalloproteinase-2 in human head and neck squamous cell carcinoma. Hum Pathol. 2000;31:895–904. doi: 10.1053/hupa.2000.9756. [DOI] [PubMed] [Google Scholar]
- 29.Strojan P, Budihna M, Smid L, Vrhovec I, Skrk J. Urokinase-type plasminogen activator, plasminogen activator inhibitor type 1 and cathepsin D: analysis of their prognostic significance in squamous cell carcinoma of the head and neck. Anticancer Res. 2000;20:3975–3981. [PubMed] [Google Scholar]
- 30.Yoshizaki T, Maruyama Y, Sato H, Furukawa M. Expression of tissue inhibitor of matrix metalloproteinase-2 correlates with activation of matrix metalloproteinase-2 and predicts poor prognosis in tongue squamous cell carcinoma. Int J Cancer. 2001;95:44–50. doi: 10.1002/1097-0215(20010120)95:1<44::aid-ijc1008>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Kos J, Smid A, Krasovec M, Svetic B, Lenarcic B, et al. Lysosomal proteases cathepsins D, B, H, L and their inhibitors stefins A and B in head and neck cancer. Biol Chem Hoppe Seyler. 1995;376:401–405. doi: 10.1515/bchm3.1995.376.7.401. [DOI] [PubMed] [Google Scholar]
- 32.Kawada A, Hara K, Kominami E, Kobayashi T, Hiruma M, et al. Cathepsin B and D expression in squamous cell carcinoma. Br J Dermatol. 1996;135:905–910. doi: 10.1046/j.1365-2133.1996.d01-1093.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Schubert RL, Bugge TH, Leppla SH. Anthrax toxin: structures, functions and tumour targeting. Expert Opin Biol Ther. 2003;3:843–853. doi: 10.1517/14712598.3.5.843. [DOI] [PubMed] [Google Scholar]
- 34.Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- 35.Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2003;100:5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Crown D, Miller-Randolph S, Moayeri M, Wang H, et al. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc Natl Acad Sci U S A. 2009;106:12424–12429. doi: 10.1073/pnas.0905409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S, Miller-Randolph S, Crown D, Moayeri M, Sastalla I, et al. Anthrax toxin targeting of myeloid cells through the CMG2 receptor is essential for establishment of Bacillus anthracis infections in mice. Cell Host Microbe. 2010;8:455–462. doi: 10.1016/j.chom.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molloy SS, Bresnahan PA, Leppla SH, Klimpel KR, Thomas G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem. 1992;267:16396–16402. [PubMed] [Google Scholar]
- 39.Klimpel KR, Molloy SS, Thomas G, Leppla SH. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc Natl Acad Sci U S A. 1992;89:10277–10281. doi: 10.1073/pnas.89.21.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mogridge J, Cunningham K, Collier RJ. Stoichiometry of anthrax toxin complexes. Biochemistry. 2002;41:1079–1082. doi: 10.1021/bi015860m. [DOI] [PubMed] [Google Scholar]
- 41.Mogridge J, Cunningham K, Lacy DB, Mourez M, Collier RJ. The lethal and edema factors of anthrax toxin bind only to oligomeric forms of the protective antigen. Proc Natl Acad Sci U S A. 2002;99:7045–7048. doi: 10.1073/pnas.052160199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duesbery NS, Webb CP, Leppla SH, Gordon VM, Klimpel KR, et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 43.Vitale G, Bernardi L, Napolitani G, Mock M, Montecucco C. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem J 352 Pt. 2000;3:739–745. [PMC free article] [PubMed] [Google Scholar]
- 44.Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, et al. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem Biophys Res Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- 45.Leppla SH. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982;79:3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 47.Johnsen M, Lund LR, Romer J, Almholt K, Dano K. Cancer invasion and tissue remodeling: common themes in proteolytic matrix degradation. Curr Opin Cell Biol. 1998;10:667–671. doi: 10.1016/s0955-0674(98)80044-6. [DOI] [PubMed] [Google Scholar]
- 48.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 49.Wong L, Suh DY, Frankel AE. Toxin conjugate therapy of cancer. Semin Oncol. 2005;32:591–595. doi: 10.1053/j.seminoncol.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Williams SA, Merchant RF, Garrett-Mayer E, Isaacs JT, Buckley JT, et al. A prostate-specific antigen-activated channel-forming toxin as therapy for prostatic disease. J Natl Cancer Inst. 2007;99:376–385. doi: 10.1093/jnci/djk065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olson ES, Aguilera TA, Jiang T, Ellies LG, Nguyen QT, et al. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr Biol (Camb) 2009;1:382–393. doi: 10.1039/b904890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu S, Redeye V, Kuremsky JG, Kuhnen M, Molinolo A, et al. Intermolecular complementation achieves high-specificity tumor targeting by anthrax toxin. Nat Biotechnol. 2005;23:725–730. doi: 10.1038/nbt1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu S, Netzel-Arnett S, Birkedal-Hansen H, Leppla SH. Tumor cell-selective cytotoxicity of matrix metalloproteinase-activated anthrax toxin. Cancer Res. 2000;60:6061–6067. [PubMed] [Google Scholar]
- 54.Liu S, Leung HJ, Leppla SH. Characterization of the interaction between anthrax toxin and its cellular receptors. Cell Microbiol. 2007;9:977–987. doi: 10.1111/j.1462-5822.2006.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta PK, Moayeri M, Crown D, Fattah RJ, Leppla SH. Role of N-terminal amino acids in the potency of anthrax lethal factor. PLoS One. 2008;3:e3130. doi: 10.1371/journal.pone.0003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gioanni J, Fischel JL, Lambert JC, Demard F, Mazeau C, et al. Two new human tumor cell lines derived from squamous cell carcinomas of the tongue: establishment, characterization and response to cytotoxic treatment. Eur J Cancer Clin Oncol. 1988;24:1445–1455. doi: 10.1016/0277-5379(88)90335-5. [DOI] [PubMed] [Google Scholar]
- 57.Cardinali M, Pietraszkiewicz H, Ensley JF, Robbins KC. Tyrosine phosphorylation as a marker for aberrantly regulated growth-promoting pathways in cell lines derived from head and neck malignancies. Int J Cancer. 1995;61:98–103. doi: 10.1002/ijc.2910610117. [DOI] [PubMed] [Google Scholar]
- 58.Moore AE, Sabachewsky L, Toolan HW. Culture characteristics of four permanent lines of human cancer cells. Cancer Res. 1955;15:598–602. [PubMed] [Google Scholar]
- 59.Scherer WF, Syverton JT, Gey GO. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J Exp Med. 1953;97:695–710. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Kleist S, Chany E, Burtin P, King M, Fogh J. Immunohistology of the antigenic pattern of a continuous cell line from a human colon tumor. J Natl Cancer Inst. 1975;55:555–560. doi: 10.1093/jnci/55.3.555. [DOI] [PubMed] [Google Scholar]
- 61.Liu S, Leppla SH. Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J Biol Chem. 2003;278:5227–5234. doi: 10.1074/jbc.M210321200. [DOI] [PubMed] [Google Scholar]
- 62.Bugge TH, Kombrinck KW, Xiao Q, Holmback K, Daugherty CC, et al. Growth and dissemination of Lewis lung carcinoma in plasminogen- deficient mice. Blood. 1997;90:4522–4531. [PubMed] [Google Scholar]
- 63.Sugiura K, Stock CC. Studies in a tumor spectrum. III. The effect of phosphoramides on the growth of a variety of mouse and rat tumors. Cancer Res. 1955;15:38–51. [PubMed] [Google Scholar]
- 64.Arora N, Leppla SH. Residues 1-254 of anthrax toxin lethal factor are sufficient to cause cellular uptake of fused polypeptides. J Biol Chem. 1993;268:3334–3341. [PubMed] [Google Scholar]
- 65.Alfano RW, Leppla SH, Liu S, Bugge TH, Ortiz JM, et al. Inhibition of tumor angiogenesis by the matrix metalloproteinase-activated anthrax lethal toxin in an orthotopic model of anaplastic thyroid carcinoma. Mol Cancer Ther. 2010;9:190–201. doi: 10.1158/1535-7163.MCT-09-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alfano RW, Leppla SH, Liu S, Bugge TH, Meininger CJ, et al. Matrix metalloproteinase-activated anthrax lethal toxin inhibits endothelial invasion and neovasculature formation during in vitro morphogenesis. Mol Cancer Res. 2009;7:452–461. doi: 10.1158/1541-7786.MCR-08-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alfano RW, Leppla SH, Liu S, Bugge TH, Herlyn M, et al. Cytotoxicity of the matrix metalloproteinase-activated anthrax lethal toxin is dependent on gelatinase expression and B-RAF status in human melanoma cells. Mol Cancer Ther. 2008;7:1218–1226. doi: 10.1158/1535-7163.MCT-08-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alfano RW, Leppla SH, Liu S, Bugge TH, Duesbery NS, et al. Potent inhibition of tumor angiogenesis by the matrix metalloproteinase-activated anthrax lethal toxin: implications for broad anti-tumor efficacy. Cell Cycle. 2008;7:745–749. doi: 10.4161/cc.7.6.5627. [DOI] [PubMed] [Google Scholar]
- 69.Liu S, Wang H, Currie BM, Molinolo A, Leung HJ, et al. Matrix metalloproteinase-activated anthrax lethal toxin demonstrates high potency in targeting tumor vasculature. J Biol Chem. 2008;283:529–540. doi: 10.1074/jbc.M707419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Su Y, Ortiz J, Liu S, Bugge TH, Singh R, et al. Systematic urokinase-activated anthrax toxin therapy produces regressions of subcutaneous human non-small cell lung tumor in athymic nude mice. Cancer Res. 2007;67:3329–3336. doi: 10.1158/0008-5472.CAN-06-4642. [DOI] [PubMed] [Google Scholar]
- 71.Abi-Habib RJ, Singh R, Leppla SH, Greene JJ, Ding Y, et al. Systemic anthrax lethal toxin therapy produces regressions of subcutaneous human melanoma tumors in athymic nude mice. Clin Cancer Res. 2006;12:7437–7443. doi: 10.1158/1078-0432.CCR-06-2019. [DOI] [PubMed] [Google Scholar]
- 72.Abi-Habib RJ, Singh R, Liu S, Bugge TH, Leppla SH, et al. A urokinase-activated recombinant anthrax toxin is selectively cytotoxic to many human tumor cell types. Mol Cancer Ther. 2006;5:2556–2562. doi: 10.1158/1535-7163.MCT-06-0315. [DOI] [PubMed] [Google Scholar]
- 73.Liu S, Aaronson H, Mitola DJ, Leppla SH, Bugge TH. Potent antitumor activity of a urokinase-activated engineered anthrax toxin. Proc Natl Acad Sci U S A. 2003;100:657–662. doi: 10.1073/pnas.0236849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu S, Bugge TH, Leppla SH. Targeting of tumor cells by cell surface urokinase plasminogen activator-dependent anthrax toxin. J Biol Chem. 2001;276:17976–17984. doi: 10.1074/jbc.M011085200. [DOI] [PubMed] [Google Scholar]
- 75.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 76.Connolly BM, Choi EY, Gardsvoll H, Bey AL, Currie BM, et al. Selective abrogation of the uPA-uPAR interaction in vivo reveals a novel role in suppression of fibrin-associated inflammation. Blood. 2010;116:1593–1603. doi: 10.1182/blood-2010-03-276642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leppla SH. Anthrax toxin. In: Aktories K, Just I, editors. Bacterial Protein Toxins. Berlin: Springer; 2000. [Google Scholar]
- 78.Park S, Leppla SH. Optimized production and purification of Bacillus anthracis lethal factor. Protein Expr Purif. 2000;18:293–302. doi: 10.1006/prep.2000.1208. [DOI] [PubMed] [Google Scholar]
- 79.Frankel AE, Bugge TH, Liu S, Vallera DA, Leppla SH. Peptide toxins directed at the matrix dissolution systems of cancer cells. Protein Pept Lett. 2002;9:1–14. doi: 10.2174/0929866023409048. [DOI] [PubMed] [Google Scholar]