Abstract
ABCG2, or Breast Cancer Resistance Protein (BCRP), is an ABC transporter that has been the subject of intense study since its discovery a decade ago. With high normal tissue expression in the brain endothelium, gastrointestinal tract, and placenta, ABCG2 is believed to be important in protection from xenobiotics, regulating oral bioavailability, forming part of the blood-brain barrier, the blood-testis barrier, and the maternal-fetal barrier. Notably, ABCG2 is often expressed in stem cell populations, where it likely plays a role in xenobiotic protection. However, clues to its epigenetic regulation in various cell populations are only beginning to emerge. While ABCG2 overexpression has been demonstrated in cancer cells after in vitro drug treatment, endogenous ABCG2 expression in certain cancers is likely a reflection of the differentiated phenotype of the cell of origin and likely contributes to intrinsic drug resistance. Notably, research into the transporter’s role in cancer drug resistance and its development as a therapeutic target in cancer has lagged. Substrates and inhibitors of the transporter have been described, among them chemotherapy drugs, tyrosine kinase inhibitors, antivirals, HMG-CoA reductase inhibitors, carcinogens, and flavonoids. This broad range of substrates complements the efficiency of ABCG2 as a transporter in laboratory studies and suggests that, while there are redundant mechanisms of xenobiotic protection, the protein is important in normal physiology. Indeed, emerging studies in pharmacology and toxicology assessing polymorphic variants in man, in combination with murine knockout models have confirmed its dynamic role. Work in pharmacology may eventually lead us to a greater understanding of the physiologic role of ABCG2.
Keywords: ABCG2, BCRP, drug-resistance, ABC transporter, chemotherapy, pharmacology
1. INTRODUCTION
The efficacy of cancer chemotherapy can be limited by cellular mechanisms of resistance that result in increased drug efflux of chemotherapeutic agents thereby reducing intracellular drug levels and causing drug resistance. The ability of cells to acquire resistance to multiple compounds, termed multidrug resistance (MDR), is often mediated by overexpression of ATP-binding cassette (ABC) transporters that remove substrates out of the cell against a concentration gradient [1]. Of the 48 human ABC transporters, three are most often associated with MDR: the multidrug resistance protein, P-glycoprotein (P-gp), encoded by the ABCB1 (or MDR-1) gene; the multidrug resistance-associated protein-1 (MRP-1) encoded by the ABCC1 (or MRP-1) gene; and the breast cancer resistance protein (BCRP or ABCG2) encoded by the ABCG2 gene [1]. Other ABC transporters have been implicated in drug resistance, but these other transporters play highly specialized roles in normal physiology and are less likely to be usurped to play a role in drug resistance in a cancer cell.
Before the first transporter genes were cloned, it had long been known that incubating cancer cell lines with chemotherapy agents resulted in sublines that were not only resistant to the selecting drug, but also to other, structurally different agents [2–4]. Juliano and Ling in 1976 were the first to note that a particular 170 kD glycoprotein was associated with this resistance [5] and over a decade later the gene encoding P-gp, then termed mdr1 (and later called MDR-1), was cloned [6]. Early interest in P-gp focused on its role in drug resistance since it was responsible for the transport of a wide variety of chemotherapeutic agents such as anthracyclines, vinca alkaloids, taxanes and etoposide [1]. Today, the importance of P-gp is understood to go well beyond drug resistance, since the high levels of expression in epithelial cells of the gastrointestinal tract and brain capillary endothelium have led to experiments showing that P-gp mediates oral absorption and forms part of the blood-brain barrier (BBB) [7, 8]. P-gp expression in the proximal tubules of the kidney suggests it plays a role in drug excretion [9]. Thus, the significance of P-gp has gone beyond that of a multidrug resistance transporter.
The adriamycin-selected leukemia subline, HL-60/AR, was reported to have a cross-resistance profile slightly different from that observed for cells expressing the MDR-1 gene [10], but was not found to overexpress MDR-1 compared to parental cells [11]. Additionally, a doxorubicin-selected, small-cell lung cancer cell line, H69/AR, and a doxorubicin-selected fibrosarcoma cell line, HT1080/DR4, were also found to exhibit a pattern of drug resistance similar to that of the HL-60/AR cells; a pattern nonetheless distinct from that conferred by expression of P-glycoprotein [12]. A new drug resistance gene, the multidrug resistance-associated protein gene, or MRP (later renamed MRP1), was later cloned by Cole and colleagues from the H69/AR subline [13]. Later studies revealed MRP1 conferred resistance to drugs that were also transported by P-gp: anthracyclines, vinca alkaloids, mitoxantrone and etoposide [14]. Much like P-gp, the importance of MRP1 is believed to extend beyond conferring drug resistance, as it is also an organic ion transporter, transporting compounds conjugated to glutathione, glucuronide, or sulfate [15].
Still another phenotype, similar but distinct from that found in cells expressing P-gp or MRP1, was reported in cells selected with mitoxantrone [16, 17]. These cells lacked MDR-1 and MRP1 expression and were highly cross resistant to mitoxantrone as well as topotecan, camptothecin, 9-aminocamptothecin, and SN-38, but lacked cross-resistance to vinblastine [18]. A nearly identical phenotype was described in a breast cancer cell line selected by the Fojo lab with doxorubicin in the presence of verapamil to prevent overexpression of P-gp [19]. These cells, MCF-7 Adr/Vp, also displayed ATP-dependent transport of doxorubicin and the fluorescent substrate rhodamine 123 in the absence of P-gp or MRP1 [20].
It was from the MCF-7 Adr/Vp subline that Doyle and colleagues first cloned the gene responsible for the novel resistance phenotype [21]. They named the gene BCRP for breast cancer resistance protein since it was cloned from a breast cancer subline. Soon after, Allikmets et al. reported a nearly identical transporter, termed ABCP for ABC transporter highly expressed in placenta, after searching an expressed sequence tag database [22]. Our laboratory also cloned a gene from the mitoxantrone-selected colon carcinoma cell line S1-M1-80 [23], derived from the S1-M1-3.2 cell line reported by Rabindran et al [24]. We called the gene MXR, or mitoxantrone resistance gene, since it appeared to be responsible for the high levels of resistance to mitoxantrone observed in cell lines expressing the gene. When the sequences for the genes became available, they proved to be nearly identical. The BCRP/ABCP/MXR gene was later placed in the “G” subfamily of ABC transporters, which includes only of half-transporters, and was assigned the name ABCG2.
2. THE ABC TRANSPORTER SUPERFAMILY
The ABC transporters are one of the largest families of active transport molecules [25, 26]. These transporters are abundant in the genomes of all organisms, and are nearly always import pumps in prokaryotes and involved in efflux in eukaryotic cells [26–28]. The well characterized eukaryotic transporters all transport substances from the cytoplasm or plasma membrane out of the cell, or into organelles such as the peroxisome, endoplasmic reticulum, and lysosomes. In addition, four transporters are localized to the mitochondria. Except for CFTR, a chloride ion channel, all ABC transporters are believed to efflux molecules by an energy dependent process involving ATP binding and hydrolysis.
The structure of ABC transporters consists of two sets of hydrophobic segments that span the membrane and are thought to confer all or most of the specificity of the transporter, and a pair of ATP-binding domains or nucleotide-binding folds (NBFs). ABC genes either encode a full transporter encoding all four domains, or a half-transporter with a single TM domain and a single NBF. Half-transporter proteins must dimerize as either homo- or heterodimers to form a complete transporter complex.
Because of the diverse substrates that can be transported, ABC proteins are found expressed in a number of specialized cell types and carry out varied functions. A large number of ABC genes have been found to be mutated in Mendelian genetic diseases such as cystic fibrosis, adrenoleukodystrophy, macular degeneration, sterol transport defects, mitochondrial abnormalities, and respiratory failure [29]. In fact, it is from these diseases that we have learned most of what we know about the normal function and substrates of the transporters.
There are 48 ABC genes in the human genome and they are dispersed mostly on different chromosomes, with a few clusters of 2–5 genes [26]. The transporters can be grouped into subfamilies based on the conservation of the NBF amino acid sequence. Eukaryotes have eight ABC subfamilies (A–H) with seven of these (A–G) present in the human genome.
The ABCG2 gene is highly conserved and has been found in all sequenced vertebrates to date, including birds, reptiles, and fish. In most species there is a single gene present [28]. The exceptions to this are the rodents which contain one or more copies of a closely related gene, Abcg3; and fish which have 3 or more ABCG2 genes [30].
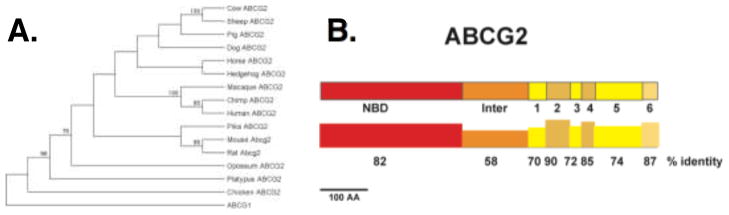
An alignment of 14 vertebrate and one bird (chicken) ABCG2 protein sequences was generated and used to build a phylogenetic tree (Figure 1A). The primate proteins (macaque, chimp, and human) all cluster together as do the rodent sequences. Figure 2B shows the domain structure of ABCG2 and the conservation of each domain. Interestingly the TM domains are nearly as conserved across all vertebrates as are the NBF sequences. This suggests that the function of ABCG2 has remained relatively conserved across vertebrate evolution.
Figure 1.
Evolutionary analysis of ABCG2. A) Full length sequences of all full-length mammalian genes, chicken and ABCG1 were aligned used to build a phylogenetic tree using the Minimal Evolution method. Numbers represent the percent of bootstraps supporting that node. B) The domains of the ABCG2 protein are shown to scale along with the percent identity of each domain. Inter, inter-domain region; 1=TM domain and loop 1.
Figure 2.
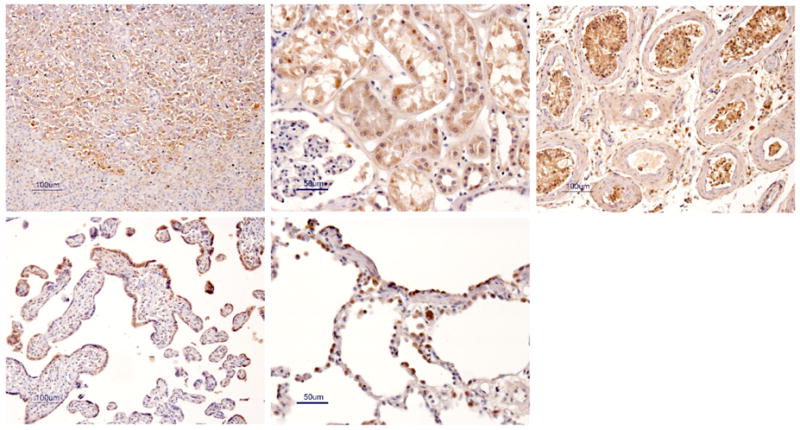
Expression of ABCG2 in normal tissues. Formalin-fixed, paraffin embedded tissue sections were stained with the polyclonal anti-ABCG2 antibody 87405. Hematoxylin and 3,3′-diaminobenzidene counterstain. Tissues shown are the adrenal gland (A), kidney (B), testis (C), placenta (D), and lung (E).
3. GENETICS AND GENE REGULATION
The human ABCG2 gene is located on chromosome 4, band 4q21-4q22, and extends over 66 kb containing 16 exons and 15 introns. Exons range in size from 60 to 532 bp, with the translational start site in the second exon, the Walker A site in exon 3 and the ABC signature motif in exon 6 [31]. The ABCG2 promoter is TATA-less with multiple Sp1, AP1 and AP2 sites as has been described for other ABC transporter genes, with the basal promoter located approximately 312 bp from the transcriptional start site [31]. While the molecular mechanisms controlling ABCG2 expression are not well understood, we have found previously that cell lines with high levels of ABCG2 expression harbor multiple rearrangements involving chromosome 4, including gene amplification as well as translocations [32, 33].
To date, the majority of studies examining the regulation of ABCG2 are focused at the transcriptional level. Two functional cis elements in the ABCG2 promoter, the estrogen [34] and hypoxia [35] response elements, and a peroxisome proliferator-activated receptor γ (PPARγ) response element upstream of the ABCG2 gene [36] have been shown to control ABCG2 expression. It has also been found that progesterone upregulates ABCG2 expression via a progesterone response element in the promoter [37]. Ebert and colleagues proposed that aryl hydrocarbon receptor (AhR) upregulates ABCG2 expression but the response element has not been identified [38]. While the sex hormones estrogen, progesterone, and testosterone have been shown to impact ABCG2 expression [34, 39–41], the data are controversial. In particular, the effect of estradiol on ABCG2 expression appears cell-dependent [34, 39]. We and others have independently reported that DNA methylation can repress ABCG2 expression in human renal carcinoma [42] as well as in multiple myleoma cell lines [43].
Other mechanisms that have been suggested to be responsible for upregulation of ABCG2 include the use of alternative 5′ promoters due to differential expression of splice variants at the 5′-untranslated region (5′UTR) of ABCG2 as reported by Nakanishi et al. [44]. Recently, we reported that overexpression of ABCG2 in resistant cells was correlated with increased binding of a set of permissive histone modification marks, RNA polymerase II and a chromatin remodeling factor Brg-1, but decreased association of a repressive histone mark, HDAC-1, and Sp1 with the proximal ABCG2 promoter [45]. While these changes were similar to those found in some cell lines when treated with the histone deacetylase inhibitor, romidepsin, a repressive histone mark, trimethylated histone H3 lysine 9 (Me3-K9 H3), was found in untreated parental cells and cells that did not respond to HDAC inhibition with ABCG2 upregulation [45]. These results suggest that removal of the repressive histone mark is necessary for cells to upregulate ABCG2 in response to treatment with a histone deacetylase inhibitor. We also identified a putative microRNA (miRNA) binding site in a portion of the 3′UTR that was found to be absent from ABCG2 mRNA isolated from a number of drug-selected cell lines, suggesting that a putative miRNA binds at this site and can suppress expression of ABCG2 [46].
Cytokines and growth factors have also been reported to alter the expression of the ABCG2 gene. When ABCG2 positive side population cells were isolated from MCF-7 cells and treated with transforming growth factor-beta, Yin and colleagues reported a decrease in ABCG2 gene expression [47]. Treatment of primary term trophoblasts with tumor necrosis factor-alpha or interleukin 1 beta decreased mRNA and protein expression of ABCG2, while treatment with insulin-like growth factor II increased expression [48]. Further studies are warranted to accurately characterize the mechanisms controlling ABGC2 expression.
4. PROTEIN STRUCTURE
ABCG2 is a 72-kDa protein composed of 665 amino acids. It has an N-terminal ATP-binding domain (NBF) and a C-terminal transmembrane domain (TMD), a structure half the size and in reverse configuration to most other ABC proteins comprising two NBFs and two TMDs. Since ABCG2 is a half-transporter, it is believed to dimerize, or possibly oligomerize in order to function, since transfection of Sf9 insect cells with human ABCG2 results in a functional protein [49] and coimmunoprecipitation experiments have supported this theory as well [50, 51]. This is in contrast to other members of the G subfamily of transporter, ABCG5 and ABCG8, which heterodimerize to form a functional transporter [52].
Relatively little is known with certainty about the structure of ABCG2. The transmembrane domain of ABCG2 (residues 361 to 655) is predicted to have six transmembrane segments and an extracellular loop between segments five and six. Of the three putative N-linked glycosylation sites, only asparagine 596 in the extracellular loop was shown to be glycosylated, but this did not appear to be critical for proper localization or function [53]. Also in the extracellular loop is cysteine 603, which was reported to form a symmetrical intermolecular disulfide bond in the homodimer, but was not found to be essential for localization or function. Cysteines 592 and 608 are also in the extracellular loop and reportedly form intramolecular bonds and appear to impact the ability of the protein to traffic and transport properly if mutated [54]. A GXXXG motif, shown to be involved in the dimerization of other membrane proteins such as glycophorin A [55], was found in the ABCG2 protein (residues 406 to 410) and subsequent mutation of the glycines to leucines resulted in impaired function but not expression of ABCG2 [56]. We reported that mutation of glycine 553 in a conserved region in transmembrane segment 5 to a leucine or glutamic acid caused impaired trafficking of the protein [57]. Xu and colleagues also reported that a region between the fifth and sixth transmembrane segments, residues 528 to 655, may be responsible for homooligomerization of the protein [58]. Work by McDevitt and colleagues suggests ABCG2 forms a tetrameric structure made up of four homodimer complexes [59]. It is hoped that insights into the structure of ABCG2 will translate into a better understanding of the function of the protein, yielding potent and specific inhibitors.
It is interesting to note that several studies have reported that the protein kinase AKT is involved in regulating surface expression of the ABCG2 protein. Mogi and colleagues were the first to report that Akt1-deficient mice displayed a reduced number of cells in the side-population [60], a distinct population of Abcg2-positive hematopoietic stem cells that become visible when bone marrow cells are incubated with the DNA dye Hoechst 33342. When side-population cells from normal mice were incubated with the phosphotidylinositol 3-kinase inhibitor LY294002, Abcg2 was translocated from the plasma membrane to the cytoplasm, although total protein expression did not appear to be affected [60]. When bone marrow cells were transfected with Akt1, an increase in the number of cells in the side population was observed [60]. Takada et al subsequently reported that, in ABCG2-transfected LLC-PK1 polarized kidney cells, the phosphotidylinositol 3-kinase inhibitors LY294002 and wortmanin both caused a shift of ABCG2 expression from the apical membrane to the intracellular space and that the shift correlated with the phosphorylation state of Akt [61]. The exact mechanism by which Akt controls surface expression of ABCG2 has yet to be elucidated.
5. TISSUE LOCALIZATION AND PREDICTED FUNCTION
With the discovery of ABCG2 came lines of inquiry to determine the location, expression and possible physiologic role of ABCG2. By northern blot analysis, Doyle et al reported high levels of ABCG2 expression in placenta, as well as lower levels in the brain, prostate, small intestine, testis, ovary and liver [21]. ABCG2 expression was absent in the heart, lung, skeletal muscle, kidney, pancreas, spleen, thymus and peripheral blood leukocytes [21]. We also found high levels of ABCG2 in the central nervous system, liver, adrenal gland, placenta, prostate, testes, and uterus as well as lower levels in the small and large intestine, stomach, lung, kidney, and pancreas as determined by northern blot [62].
Maliepaard et al examined ABCG2 expression by immunohistochemistry using the BXP-21 and BXP-34 antibodies and reported high levels in the placenta, specifically in the syncytiotrophoblasts [63]. High expression was identified in the colon, as well as tissue from the small intestine, biliary caniliculi, breast, venous endothelium and capillaries [64]. Using a polyclonal rabbit anti-ABCG2 antibody (87405) as well as the monoclonal 5D3 antibody, we noted high expression in the syncytiotrophoblasts of the placenta, alveolar pneumocytes of the lung, sebaceous glands, small and large intestine, bile caniliculi and blood vessels as well as the endothelium of the nervous system [62]. Figure 1 shows immunohistochemical data localizing ABCG2 to the zona reticularis of the adrenal gland (Figure 2A), the proximal tubule of the kidney (Figure 2B), Sertoli/Leydig cells of the testis (Figure 2C), syncytiotrophoblasts of the placenta (Figure 2D) and alveolar pneumocytes of the lung (Figure 2E). Many of the cells that stained positively for ABCG2 were found to have a secretory role, suggesting ABCG2 may have a role beyond xenobiotic protection in these cells. The location and level of expression of ABCG2 lends clues to determining its likely role in normal physiology. Further research into the likely role of ABCG2 in specific tissues is described below.
5.1 Placenta
Given its high expression in the placenta in the syncitiotrophoblasts of the chorionic villus, ABCG2 is believed to protect the developing fetus from the possible transmission of toxins as well as remove toxins from the fetal space. Jonker and colleagues were the first to demonstrate the ability of ABCG2 to protect the fetus. When Abcb1/2-deficient, pregnant mice were administered topotecan along with the ABCG2 inhibitor elacridar (GF120918), fetal plasma levels of topotecan were twice as high as those measured in maternal plasma, supporting the theory that ABCG2 protects the developing fetus from possible toxins in the maternal space [65]. Similarly, Zhang et al reported that, after IV administration of the ABCG2 substrate nitrofurantoin, the fetal concentration of nitrofurantoin was 5-fold higher in Abcg2-deficient pregnant mice compared to wild-type mice pregnant mice [66]. Several reports have also demonstrated that ABCG2 serves to limit fetal penetration of the antidiabetic drug, glyburide [67–70].
The data showing transport from the fetal to the maternal space are compelling. In placental perfusion studies in the rat, Staud and colleagues demonstrated transport of cimetidine from the fetal to maternal space, against a concentration gradient [71]. Myllynen et al reported similar results in perfused human placenta, demonstrating that ABCG2 mediated transfer of 14C-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) from the fetal to maternal space could be decreased with the addition of the ABCG2 inhibitor Ko143 [72]. Both studies suggested that ABCG2 serves to remove potential toxins from the fetal space.
5.2 Brain and Blood-Brain Barrier
ABC transporters, such as Pgp, have previously been shown to form part of the blood-brain barrier [8], leading to studies seeking to determine the contribution of ABCG2 in limiting brain penetration of substrate compounds. Cooray and colleagues reported ABCG2 to be localized to the the microvessel endothelium of the brain [73]. Immunohistochemical analysis revealed that rat ABCG2 is expressed on the luminal side of rat brain capillaries, suggestive of brain-to-blood transport [74]. Additionally, ABCG2 was found to be more highly expressed in porcine brain endothelium than ABCB1 or ABCC1 based on mRNA analysis [75]. This was also found to be true for human brain vessels [76]. Similarly, Cisternino et al reported an almost 700-fold higher level of Abcg2 mRNA in wild-type mouse brain capillaries compared to the cortex [77]. They also noted a 3-fold higher level of Abcg2 mRNA in the capillaries of Abcb1/2-deficient mice compared to wild-type mice [77], suggesting that Abcg2 might compensate for the loss of Pgp at the blood-brain barrier.
Mounting evidence suggests ABCG2 restricts brain penetration of substrate compounds. Breedveld and colleagues demonstrated that inhibition of both Pgp and ABCG2 resulted in the highest brain penetration of imatinib in mice [78], a finding confirmed in a later study using mice in which both Pgp and ABCG2 genes were deleted [79]. Bihorel et al showed that brain uptake of the imatinib metabolite, CGP74588, was 1.5 times higher in Abcg2-deficient mice compared to wild-type mice [80], and that coadministration of the dual Pgp and ABCG2 inhibitor elacridar with imatinib increased brain penetration of imatinib in wild-type mice [81]. This latter study implies that inhibition of both ABCG2 and P-gp provided the greatest brain penetration of imatinib. Brain penetration of topotecan was also found to be 3.7-fold higher in Mdr1a/b/Abcg2-deficient mice compared to wild-type mice [82].
The importance of ABCG2 for brain penetration of any given substrate will depend upon the relevance of other constituents of the blood brain barrier. As noted above, both P-gp and ABCG2 contribute to the blood brain barrier for imatinib and topotecan. Thus, deletion or inhibition of ABCG2 alone will have a reduced effect in the presence of P-gp. Similarly, deletion or inhibition of ABCG2 alone will have a reduced effect for an ABCG2 substrate for which the tight junctions in the blood brain barrier are very important. Thus, the determination of which ABCG2 substrates are limited predominantly by ABCG2 will be a painstaking process and almost require study compound by compound. From the P-gp literature we have the early example of ivermectin, where P-gp knockout alone provoked a 87-fold increase in ivermectin accumulation, suggesting that there was minimal contribution from brain barrier elements other than P-gp [83]. Most other substrates tested demonstrated much less impact of P-gp alone. Pronounced blood brain barrier effects like that observed for P-gp and ivermectin have not yet been reported for any ABCG2 substrate. Perhaps the most pronounced effect for ABCG2 to date was noted in the research of Enokizono et al., who showed a 9.2-fold increase in brain penetration of genistein in Abcg2−/− mice compared to wild-type [84].
5.3 Mammary gland
In contrast to the proposed protective role in the placenta outlined above, several surprising studies demonstrated that ABCG2 in the mammary gland served to concentrate toxins into breast milk. Jonker and colleagues reported that ABCG2 expression was upregulated in the lactating mammary glands of mice, cows and humans, and reported higher levels of the ABCG2 substrates topotecan and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in the milk of lactating mice compared to Abcg2-deficient mice [85]. Similarly, ABCG2 has been shown to concentrate dietary carcinogens [86] as well as the antibiotics nitrofurantonin [87], ciprofloxacin [88], and enrofloxacin [89] in animal milk. Subsequently, van Herwaarden and colleagues shed some light on the possible normal physiological role of ABCG2 in the lactating breast, demonstrating that ABCG2 secretes the B vitamin riboflavin, required for the metabolism of fats, into milk [90]. Interestingly, ABCG2 has been liked to alterations in fat and protein content in milk as well as milk production in dairy cattle [91, 92].
5.4 Testis
ABCG2 may serve to protect germinal stem cells from genotoxic mutagens, as high levels of ABCG2 have been reported in the interstitial cells of the normal testis as well as in Sertoli/Leydig cells [62]. Bart and colleagues have also reported high ABCG2 expression levels in myoid cells and cells of the luminal capillary endothelial wall of the normal testis [93]. Enokizono et al found that tissue/plasma ratios in the testis were higher in Abcg2-deficient mice compared to wild type for the ABCG2 substrates PhIP, N-hydroxyl PhIP, MeIQx, dantrolene, and prazosin, confirming a protective role for ABCG2 expression in the testis [94].
5.5 Gastrointestinal tract
Initial studies of ABCG2 expression revealed high levels of the transporter in the intestine. Specific examination of ABCG2 expression along the human gastrointestinal tract (GI) showed that levels of ABCG2 were highest in the duodenum, and then decreased along the GI tract from terminal ileum, ascending colon, transverse colon, descending colon, sigmoid colon to the rectum, where the lowest levels of ABCG2 were found [95]. The expression of ABCG2 in the GI tract suggested that it plays a role in limiting the oral absorption of substrates.
Jonker and colleagues were the first to confirm this role, noting more than 6-fold higher plasma levels of topotecan in Abcb1/2-deficient mice when topotecan was administered in the presence of the ABCG2 inhibitor elacridar compared to drug administered in the absence of the inhibitor [65]. Similarly, oral administration of topotecan to Abcb1/2-deficient mice in the presence of the inhibitor Ko143 resulted in a 4–6 fold increase in plasma topotecan levels compared to levels in the absence of Ko143 [96]. Marchetti and colleagues also found that ABC transporters affected oral absorption of elotinib, reporting that oral bioavailability of the drug increased from 40% in wild-type mice to 60.4% in Mdr1a/Mdr1b/Abcg2-deficient mice [97]. Intestinal uptake of antibiotics [87, 88]; quercetin [98]; the glycoprotein antagonist ME3229 [99]; the CDK inhibitor JNJ-7706621 [100]; sulfasalazine [101] and dietary carcinogens such as PhIP and aflatoxin B1 [86, 102] was increased in Abcg2−/− mice compared to wild-type mice, providing strong evidence for the role of ABCG2 in oral drug absorption.
The importance of ABCG2 in determining the overall bioavailability of a substrate drug can be very complex. The contribution of ABCG2 would be expected to be greater when an oral route of administration is used. However, this is not always the case, as in a recent report examining the pharmacokinetics of oral and IV administration of imatinib [79]. Unlike an earlier report [78], this study demonstrated no increase in CNS uptake in Abcg2-deficient mice following an oral dose of imatinib. One possible explanation for this failure was the high dose of oral imatinib administered (100 mg/kg) compared to the IV dose of (12.5 mg/kg). Since imatinib, like any substrate, can inhibit the transporter at high concentrations, the high oral dose may have limited the ability of the wild type transporter to reduce CNS accumulation in this study.
5.6 Kidney
In the kidney, we localized ABCG2 to the cortical tubule [62], and subsequent studies have reported expression at the proximal tubule brush border membrane [103], suggesting the potential involvement of ABCG2 in renal drug excretion. In support of this theory, impaired renal excretion of 6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl) benzothiazole sulfate [104], and edaravone sulfate [105] was noted in Abcg2−/− mice compared to wild-type mice.
5.7 Liver and biliary tract
Maliepaard and colleagues reported expression of ABCG2 in the liver canalicular membrane [63] and we also noted ABCG2 staining in hepatocytes [62]. Similarly, ABCG2 expression has been reported in the bile ducts, reactive bile ductules and blood vessel endothelium of human liver [106]. ABCG2 was also found in the luminal membrane of gall bladder epithelial cells [107]. Merino and colleagues found that biliary excretion of the antibiotic nitrofurantoin was nearly absent in Abcg2-deficient mice compared to wild-type animals [87] and Hirano and colleagues reported that biliary excretion of pitavistatin in Abcg2-deficient mice was once-tenth that of wild-type mice [108]. The role of ABCG2 in the biliary excretion of the fluoroquinolone antibiotics ciprofloxacin, grepafloxacin, ofloxacin, ulifloxacin in a mouse model was demonstrated as well [109]. Abcg2 has also been shown to play a role in the biliary transport of 4-methylumbelliferyl sulfate in a rat model [110], as well as acetaminophen sulfate and harmol sulfate in a mouse model [111]. Abcg2 was found to be responsible for the biliary excretion of troglitazone sulfate, the major metabolite of the antidiabetic drug troglitazone, in a mouse model [112]. Thus, ABCG2 may also play a role in the biliary excretion of drug, xenobiotic or endogenous compound conjugates.
5.8 Hematopoietic stem cells
While using the dye Hoechst 33342 to stain mouse bone marrow cells, Goodell and colleagues noted that a distinct subpopulation of cells, side-population or “SP” cells, accumulated less dye and were enriched for their ability to reconstitute bone marrow in irradiated mice [113]. While it was initially suggested that a transporter might be associated with this low accumulation of Hoechst 33342 [113], it was not until the work of Zhou that it was made clear that Abcg2 was responsible for the SP in mouse bone marrow [114]. Scharenberg and colleagues subsequently demonstrated ABCG2 to be responsible for the SP in human bone marrow as well [115]. The ABCG2 protein does not appear to be necessary for normal hematopoiesis, as Abcg2-deficient mice are viable with normal numbers of stem cells when detected by other surface markers, despite the complete absence of SP cells [116]. It has been postulated that ABCG2 may play a protective role for stem cells, as studies by Zhou and colleagues demonstrated that stem cells derived from Abcg2-deficient mice were more sensitive to the chemotherapy agent mitoxantrone [117].
6. SUBSTRATES AND INHIBITORS
The list of substrates and inhibitors of ABCG2 has been steadily expanding since its discovery. The first reported substrates of ABCG2 were predominantly chemotherapy agents, due to its initial discovery in drug-resistant cells. Mitoxantrone transport is the hallmark of cells expressing ABCG2, but other chemotherapeutic substrates include flavopiridol; the camptothecins 9-aminocamptothecin, topotecan, irinotecan and its active metabolite SN-38; the indolocarbazoles J-107088, NB-506, compound A and UCN-01; antifolates such as methotrexate; porphyrins such as 2-(1-hexyloxethyl)-2-devinylpyropheophorbide a (HPPH), pyropheophorbide a methyl ester and chlorin e6; and the tyrosine kinase inhibitors imatinib, gefitinib, and erlotinib [118]. If the amino acid at position 482 is mutated, mitoxantrone transport is more efficient and ABCG2 can additionally transport rhodamine 123 and anthracyclines such as doxorubicin and bisantrene [119–122]. Several other substrate classes have been described including antivirals, HMG-CoA reductase inhibitors, carcinogens, and flavonoids [118]. A partial list of non-chemotherapy substrates is provided in Table 1.
Table 1.
Selected non-chemotherapy substrates of ABCG2
| Antivirals: |
| Zidovudine (AZT) |
| Lamivudine |
| Abacavir |
| HMG-CoA reductase inhibitors: |
| Rosuvastatin |
| Pitavastatin |
| Cerivastatin |
| Carcinogens: |
| Aflatoxin B1 |
| 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) |
| 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) |
| 2-amino-3-methylimidazo[4,5-f]quinoline(IQ) |
| 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1) |
| Antibiotics: |
| Ciprofloxacin |
| Ofloxacin |
| Norfloxacin |
| Erythromycin |
| Nitrofurantoin |
| Calcium Channel Blockers: |
| Azidopine |
| Dipyridamole |
| Nitrendipene |
| Other compounds: |
| Sulfasalazine |
| Cimetidine |
| Riboflavin |
| Vitamin K3 |
| Glyburide |
| d-Luciferin |
Fumitremorgin C was the first ABCG2 inhibitor described and was reported even before the ABCG2 gene had been cloned [123]. Some of the first inhibitors reported were also inhibitors of P-gp or MRP1 such as cyclosporine a [124], elacridar (GF120918) [125], tariquidar (XR-9576) [126] and biricodar (VX-710) [127]. Additional classes of inhibitors include pyridines and dihydropyridines such as nimodipene and nicardipene; tyrosine kinase inhibitors (TKIs) such as imatinib, nilotinib and erlotinib; flavonoids such as quercetin, gensitein, chrysin and tectochrysin; taxane derivatives and as well as bisindolylmaleimides and indolcarbazole kinase inhibitors [118]. No doubt the list of substrates and inhibitors will continue to grow.
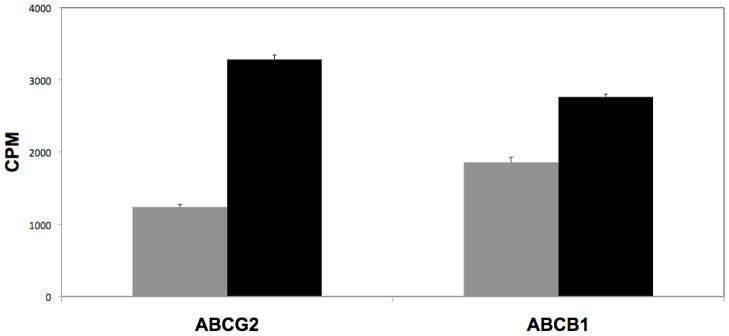
The number of different substrates and inhibitors that have been described to date is remarkable. And yet, no clear structure-function relationship has been identified that can explain the definitive requirements for a substrate or for an inhibitor. Perhaps the most interesting and intensively studied groups of interacting compounds are the TKIs. A number continue in clinical development and several have been approved for specific indications. In many cases it has been difficult to determine whether or not the compound is a substrate for transport by ABCG2 (or by P-glycoprotein). The first TKI to be approved, imatinib for CML, has been the subject of several papers examining its interaction with ABCG2 or with P-gp. Several investigators report that imatinib is an inhibitor of one or both transporters in in vitro studies [128–131]; but reports differ greatly on whether or not imatinib is a substrate for transport [129, 132–134]. This may be due to the conditions of the transport assay. In the case of ABCG2, incubating ABCG2 positive cells in high concentrations (1 μM or greater) of imatinib appeared to mask transport that was observed at lower levels of imatinib [135], suggesting that imatinib has a high affinity for ABCG2. As shown in Figure 3, when ABCG2- or ABCB1-transfected HEK293 cells are incubated in 200 nM radiolabeled imatinib, intracellular imatinib concentrations are significantly lower compared to transfected cells incubated in the presence of the inhibitors tariquidar or fumitremorgin C, respectively. Our results support the idea that imatinib is indeed a substrate for both P-gp and ABCG2.
Figure 3.
ABC transporter expression decreases intracellular imatinib accumulation. ABCG2-and ABCB1-transfected HEK 293 cells were incubated for 2 with 0.2 μM 14C-imatinib in the presence (balck bars) or absence (grey bars) of 10 μM FTC or 200 nM tariquidar, respectively. Inhibition of transporter activity significantly increased intracellular imatinib concentration as measured by liquid scintillation counting compared to cells incubated in the absence of inhibitor.
Although the compounds interact with the ATP binding pocket of the tyrosine kinases, they do not appear to bind at the ATP site of ABCG2 or Pgp [136]. Instead, imatinib, as with other TKIs, is able to prevent IAAP binding [136], indicating that the compound interacts with ABCG2 at the drug binding site.Such studies have served to prove that most of the TKIs are able to inhibit ABCG2 and Pgp. Because ABCG2 inhibitory activity of the TKIs manifests at concentrations that cause cell growth inhibition, convincing data showing that a TKI is a substrate has been more difficult to obtain without radiolabeled drug. In vivo studies in mice lacking the murine orthologue of ABCG2 or P-gp have provided definitive evidence that imatinib is a substrate for transport by both proteins [78–80]. In vivo studies have also confirmed that other TKIs such as erlotinib are substrates based on altered drug pharmokinetics when either or both orthologues are deleted [97].
7. SINGLE NUCLEOTIDE POLYMORPHISMS
Over 80 naturally occurring sequence variations have been reported in the ABCG2 gene [137]. Of these, the nonsynonymous Q141K single nucleotide polymorphism (SNP) has been studied most extensively. The Q141K SNP has been linked to decreased plasma membrane expression of ABCG2, decreased drug transport or reduced ATPase activity [138–141]. Some small studies have shown that the Q141K SNP alters the pharmacokinetics of chemotherapeutic agents such as topotecan, diflomotecan, and 9-aminocamptothecin [142–144]. This variant is found with low frequency in people of African-American (2–5%), Europeans (11–14%), Hispanic (10%) or Middle Eastern (13%) descent, but is found in high levels in people of Chinese (35%) or Japanese (35%) descent [145]. Thus, the Q141K SNP may lead to higher drug toxicity in some patient populations. Rudin and colleagues recently reported that diplotypes of two linked polymorphisms in the ABCG2 gene were associated with higher AUC and Cmax of erlotinib [146]. This will need to be confirmed in a larger cohort of patients but their observation does highlight the point that definitive studies of ABCG2 variants require large patient data sets and analysis of haplotypes as well as individual polymorphisms.
8. ABCG2 EXPRESSION IN CANCER
Since ABCG2 expression in cancer cells has been shown to confer a drug-resistant phenotype, considerable study has been devoted to determining the role of ABCG2 in drug resistance in cancer. One of the earliest studies suggested that ABCG2 may play a role in drug resistance in leukemia [147]; however, this has proved a point of controversy, as some studies have shown that ABCG2 expression has an effect on outcome or survival, while others have not [148]. Still, some large scale studies have demonstrated that ABCG2 does play a role in drug resistance in leukemia [149, 150].
In solid tumors, data are lacking. Diestra et al studied ABCG2 expression in paraffin-embedded tumor samples with the BXP-21 antibody and reported frequent expression in tumors of the digestive tract, endometrium, lung and melanoma [64]. Breast cancer has been most extensively studied, with most reports concluding that ABCG2 expression is generally low in the disease [148]. Clearly, larger studies are needed before the contribution of ABCG2 to drug resistance in cancer can accurately be determined.
9. CONCLUSION
ABCG2 was discovered a decade ago and has been studied in laboratories around the globe, yielding a welth of knowledge akin to that gathered for P-gp. While the preceding 20 years of work with P-gp set the stage for rapid basic science discoveries about ABCG2, it also brought a certain “baggage” that has shaped our translational studies in ABCG2. When P-glycoprotein was discovered, our understanding of the cell was relatively primitive. Membrane proteins signaled to the nucleus without dozens of interlocking intermediaries. P-glycoprotein, it was thought, was going to be the definitive mechanism of drug resistance – far outweighing glutathione conjugation as a mechanism of cellular protection. We worried whether we should prevent drug resistance rather than reverse drug resistance. At a time when clinical trials were largely about how best to combine different cytotoxic drugs, the idea that we could target P-glycoprotein was welcome news. This led to a flurry of drug resistance reversal trials that we have since labeled the first generation trials. These trials used drugs that were easily adapted for use in the clinic based on approved medical indications and were determined to be P-gp inhibitors in the laboratory. These drugs were not particularly potent P-gp inhibitors and the non-randomized, home-run nature of the trial design meant that it was never clear whether the observed responses were due to inhibition of P-gp-mediated resistance or not. As second and third generation P-gp inhibitors were developed, many of them were found to inhibit cytochrome P450 and impair drug clearance [151]. This prompted decreases in drug dosing to prevent excess toxicity, thus undermining any benefit that would be obtained from having a drug efflux inhibitor. Because good antibodies weren’t -- and still aren’t – available for diagnostics, patients weren’t selected for studies based on tumor expression of P-gp. Ultimately, all the trials failed to prove the P-gp hypothesis. In many ways, these studies foreshadowed many trials over the last decade in which targeted therapies have been used without selection of patients for target expression in the cancer. These trials also came of age at a time when we still expected major clinical benefit and before the notion of treating cancer as a chronic disease and incremental benefit in clinical trials had become well accepted. Altogether, these forces led to the indelible conclusion that the P-gp hypothesis was no longer of interest and that drug resistance reversal could not be achieved by this means.
This was the enviroment in which ABCG2 was discovered. While ABCG2 is overexpressed in drug resistant cell lines and is apparently as effective a transporter as P-gp in terms of its ability to transport drug against a concentration gradient, clinical trials attempting to exploit ABCG2 as a therpauetic target have not come to fruition. Instead of developing ABCG2 as a clinical target, we and others have added exponentially to the list of compounds known to be substrates and inhibitors through basic science studies. We have studied protein structure, function, and gene regulation. This unwillingness to look at cancer drug resistance has meant that, almost 40 years since the discovery of P-gp and 10 years since the discovery of ABCG2, we still do not know in which cancers these transporters are important.
It was interesting for those laboring in this field to observe the intense interest in ABCG2 that spiked after expression was discovered in stem cells in both normal tissue and malignant settings. The notion that any expression of ABCG2 was indicative of a stem cell population was eventually dispelled and ABCG2 became just another stem cell marker. It is likely that, as for P-gp, ABCG2 is expressed in cancers as part of their differentiated phenotype, so discriminating the putative stem cell from the more differentiated counterpart will require other markers.
At this writing, it seems that field of pharmacology will lead the charge in translating ABCG2 to the clinic. The evaluation of polymorphic variants is being intensely pursued. Since ABCG2 plays an important role in normal tissue protection, the existence of variants that would provide more or less normal tissue protection will be very important in terms of carcinogenesis, toxicology, and pharmacology. However, we must be cautious and not repeat mistakes of the past by designing overly simplistic experiments in the hope that one gene or one polymorphism will really matter. Extensive use of this type of study runs the risk that random associations and publication bias will result in more confusion than light. Rather, it is likely that genes combine to create increased risk. This is probably also true for drug resistance. Genome-wide associate studies, currently under investigation for cancer risk [152], should also be carried out for identifying modifiers of drug resistance risk.
Some groups have suggested that SNPs in the ABCB1 gene that have been linked to impaired protein function are predictors of response to chemotherapy [153–155]. The Q141K SNP offers the possibility to do similar studies with ABCG2. As noted, this SNP has been associated with gefitinib toxicity [156]. Might it also be associated with increased gefitinib efficacy? In such an example, it may be hard to differentiate between somewhat higher drug exposure levels and increased tumor exposure. Then, pharmacogenetics and drug resistance research has to some extent merged. Imatinib resistance in chronic myelogenous leukemia offers a pertinent example. Numerous reports have appeared demonstrating that imatinib resistance is due to mutations in the ATP binding site of BCR-ABL. While some such as T315I undoubtedly confer high levels of drug resistance, there are other mutations that confer much lower levels [157] and might not even be clinically relevant. Picard et al noted that imatinib trough levels correlated with the efficacy of therapy. Of 68 patients examined, 56 who achieved complete cytogenetic response were found to have higher trough imatinib lavels than those patients who did not and a plasma threshold of 1002 ng/ml or higher was found to strongly associate with major molecular response [158]. Consistent with this is another report that increasing the imatinib dose improves responses in some patients [159]. These data suggest that exposure to imatinib is important in determining the depth and duration of response. The unknown in this is whether P-gp or ABCG2 expression could be influencing the exposure to imatinib in the patient, and locally, in the cancer cell.
These examples highlight the fact that we are still a long way from being able to exploit our knowledge of ABCG2 in the clinic. It is good that the failed first generation P-gp studies limited enthusiasm for repeating such study designs for ABCG2. However, it is not good that the subsequent disappointments have so thoroughly limited translational studies. Until we can apply our considerable knowledge of ABCG2 in the clinical setting, we will never be able to determine whether ABCG2 is a major limiting factor in the outcome of cancer chemotherapy.
References
- 1.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 2.Biedler JL, Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res. 1970;30:1174–1184. [PubMed] [Google Scholar]
- 3.Akiyama S, Fojo AT, Hanover JA, Gottesman MM. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Molec Genet. 1985;11:117–126. doi: 10.1007/BF01534700. [DOI] [PubMed] [Google Scholar]
- 4.Kartner N, Riordan JR, Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983;221:1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- 5.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 6.Roninson IB, Chin JE, Choi K, Gros P, Housman DE, Fojo A, Shen D-W, Gottesman MM, Pastan I. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci USA. 1986;83:4538–4542. doi: 10.1073/pnas.83.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Benet LZ. The gut as a barrier to drug absorption: combined role of cytochrome P450 3A and P-glycoprotein. Clin Pharmacokinet. 2001;40:159–168. doi: 10.2165/00003088-200140030-00002. [DOI] [PubMed] [Google Scholar]
- 8.Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 9.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Bhalla K, Hindenburg A, Taub RN, Grant S. Isolation and characterization of an anthracycline-resistant human leukemic cell line. Cancer Res. 1985;45:3657–3662. [PubMed] [Google Scholar]
- 11.Gervasoni JE, Jr, Fields SZ, Krishna S, Baker MA, Rosado M, Thuraisamy K, Hindenburg AA, Taub RN. Subcellular distribution of daunorubicin in P-glycoprotein-positive and -negative drug-resistant cell lines using laser-assisted confocal microscopy. Cancer Res. 1991;51:4955–4963. [PubMed] [Google Scholar]
- 12.Cole SP, Downes HF, Slovak ML. Effect of calcium antagonists on the chemosensitivity of two multidrug-resistant human tumour cell lines which do not overexpress P-glycoprotein. Br J Cancer. 1989;59:42–46. doi: 10.1038/bjc.1989.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole SPC, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 14.Bakos E, Homolya L. Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1) Pflugers Arch. 2007;453:621–641. doi: 10.1007/s00424-006-0160-8. [DOI] [PubMed] [Google Scholar]
- 15.Leslie EM, Deeley RG, Cole SP. Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters. Toxicology. 2001;167:3–23. doi: 10.1016/s0300-483x(01)00454-1. [DOI] [PubMed] [Google Scholar]
- 16.Dietel M, Arps H, Lage H, Niendorf A. Membrane vesicle formation due to acquired mitoxantrone resistance in human gastric carcinoma cell line EPG85–257. Cancer Res. 1990;50:6100–6106. [PubMed] [Google Scholar]
- 17.Nakagawa M, Schneider E, Dixon KH, Horton J, Kelley K, Morrow C, Cowan KH. Reduced intracellular drug accumulation in the absence of P-glycoprotein (mdr1) overexpression in mitoxantrone-resistant human MCF-7 human breast cancer cells. Cancer Res. 1992;52:6175–6181. [PubMed] [Google Scholar]
- 18.Yang CJ, Horton JK, Cowan KH, Schneider E. Cross-resistance to camptothecin analogues in a mitoxantrone-resistant human breast carcinoma cell line is not due to DNA topoisomerase I alterations. Cancer Res. 1995;55:4004–4009. [PubMed] [Google Scholar]
- 19.Chen Y-N, Mickley LA, Schwartz AM, Acton EM, Hwang J, Fojo AT. Characterization of Adriamycin-resistant human breast cancer cells which display overexpression of a novel resistance-related membrane protein. J Biol Chem. 1990;265:10073–10080. [PubMed] [Google Scholar]
- 20.Lee JS, Scala S, Matsumoto Y, Dickstein B, Robey R, Zhan Z, Altenberg G, Bates SE. Reduced drug accumulation and multidrug resistance in human breast cancer cells without associated P-glycoprotein or MRP overexpression. J Cell Biochem. 1997;65:513–526. [PubMed] [Google Scholar]
- 21.Doyle LA, Yang W, Abruzzo LE, Krogmann T, Gao Y, Rishi AK, Ross DD. Cloning and characterization of breast cancer resistance protein (BCRP), a novel ATP-binding cassette (ABC) transporter that may contribute to the multidrug-resistance phenotype of MCF-7/AdrVp breast cancer cells. Proc Amer Assoc Cancer Res. 1998;39 [Google Scholar]
- 22.Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- 23.Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, Bates SE. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 24.Rabindran SK, He H, Singh M, Brown E, Collins KI, Annable T, Greenberger LM. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998;58:5850–5858. [PubMed] [Google Scholar]
- 25.Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 26.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 27.Davidson AL, Chen J. ATP-binding cassette transporters in bacteria. Annu Rev Biochem. 2004;73:241–268. doi: 10.1146/annurev.biochem.73.011303.073626. [DOI] [PubMed] [Google Scholar]
- 28.Annilo T, Chen ZQ, Shulenin S, Costantino J, Thomas L, Lou H, Stefanov S, Dean M. Evolution of the vertebrate ABC gene family: analysis of gene birth and death. Genomics. 2006;88:1–11. doi: 10.1016/j.ygeno.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123–142. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- 30.Mickley L, Jain P, Miyake K, Schriml LM, Rao K, Fojo T, Bates S, Dean M. An ATP-binding cassette gene (ABCG3) closely related to the multidrug transporter ABCG2 (MXR/ABCP) has an unusual ATP-binding domain. Mamm Genome. 2001;12:86–88. doi: 10.1007/s003350010237. [DOI] [PubMed] [Google Scholar]
- 31.Bailey-Dell KJ, Hassel B, Doyle LA, Ross DD. Promoter characterization and genomic organization of the human breast cancer resistance protein (ATP-binding cassette transporter G2) gene. Biochim Biophys Acta. 2001;1520:234–241. doi: 10.1016/s0167-4781(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 32.Knutsen T, Rao VK, Ried T, Mickley L, Schneider E, Miyake K, Ghadimi BM, Padilla-Nash H, Pack S, Greenberger L, Cowan K, Dean M, Fojo T, Bates S. Amplification of 4q21-q22 and the MXR gene in independently derived mitoxantrone-resistant cell lines. Genes Chromosomes Cancer. 2000;27:110–116. [PubMed] [Google Scholar]
- 33.Rao VK, Wangsa D, Robey RW, Huff L, Honjo Y, Hung J, Knutsen T, Ried T, Bates SE. Characterization of ABCG2 gene amplification manifesting as extrachromosomal DNA in mitoxantrone-selected SF295 human glioblastoma cells. Cancer Genet Cytogenet. 2005;160:126–133. doi: 10.1016/j.cancergencyto.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Ee PL, Kamalakaran S, Tonetti D, He X, Ross DD, Beck WT. Identification of a novel estrogen response element in the breast cancer resistance protein (ABCG2) gene. Cancer Res. 2004;64:1247–1251. doi: 10.1158/0008-5472.can-03-3583. [DOI] [PubMed] [Google Scholar]
- 35.Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 36.Szatmari I, Vamosi G, Brazda P, Balint BL, Benko S, Szeles L, Jeney V, Ozvegy-Laczka C, Szanto A, Barta E, Balla J, Sarkadi B, Nagy L. Peroxisome proliferator-activated receptor gamma-regulated ABCG2 expression confers cytoprotection to human dendritic cells. J Biol Chem. 2006;281:23812–23823. doi: 10.1074/jbc.M604890200. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Lee EW, Zhou L, Leung PC, Ross DD, Unadkat JD, Mao Q. Progesterone receptor (PR) isoforms PRA and PRB differentially regulate expression of the breast cancer resistance protein in human placental choriocarcinoma BeWo cells. Mol Pharmacol. 2008;73:845–854. doi: 10.1124/mol.107.041087. [DOI] [PubMed] [Google Scholar]
- 38.Ebert B, Seidel A, Lampen A. Identification of BCRP as transporter of benzo[a]pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. Carcinogenesis. 2005;26:1754–1763. doi: 10.1093/carcin/bgi139. [DOI] [PubMed] [Google Scholar]
- 39.Imai Y, Ishikawa E, Asada S, Sugimoto Y. Estrogen-mediated post transcriptional down-regulation of breast cancer resistance protein/ABCG2. Cancer Res. 2005;65:596–604. [PubMed] [Google Scholar]
- 40.Wang H, Zhou L, Gupta A, Vethanayagam RR, Zhang Y, Unadkat JD, Mao Q. Regulation of BCRP/ABCG2 expression by progesterone and 17beta-estradiol in human placental BeWo cells. Am J Physiol Endocrinol Metab. 2006;290:E798–807. doi: 10.1152/ajpendo.00397.2005. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda S, Itagaki S, Hirano T, Iseki K. Effects of sex hormones on regulation of ABCG2 expression in the placental cell line BeWo. J Pharm Pharm Sci. 2006;9:133–139. [PubMed] [Google Scholar]
- 42.To KK, Zhan Z, Bates SE. Aberrant promoter methylation of the ABCG2 gene in renal carcinoma. Mol Cell Biol. 2006 doi: 10.1128/MCB.00650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner JG, Gump JL, Zhang C, Cook JM, Marchion D, Hazlehurst L, Munster P, Schell MJ, Dalton WS, Sullivan DM. ABCG2 expression, function and promoter methylation in human multiple myeloma. Blood. 2006 doi: 10.1182/blood-2005-10-009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakanishi T, Bailey-Dell KJ, Hassel BA, Shiozawa K, Sullivan DM, Turner J, Ross DD. Novel 5′ untranslated region variants of BCRP mRNA are differentially expressed in drug-selected cancer cells and in normal human tissues: implications for drug resistance, tissue-specific expression, and alternative promoter usage. Cancer Res. 2006;66:5007–5011. doi: 10.1158/0008-5472.CAN-05-4572. [DOI] [PubMed] [Google Scholar]
- 45.To KK, Polgar O, Huff LM, Morisaki K, Bates SE. Histone modifications at the ABCG2 promoter following treatment with histone deacetylase inhibitor mirror those in multidrug-resistant cells. Mol Cancer Res. 2008;6:151–164. doi: 10.1158/1541-7786.MCR-07-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.To KK, Zhan Z, Litman T, Bates SE. Regulation of ABCG2 expression at the 3′ untranslated region of its mRNA through modulation of transcript stability and protein translation by a putative microRNA in the S1 colon cancer cell line. Mol Cell Biol. 2008;28:5147–5161. doi: 10.1128/MCB.00331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin L, Castagnino P, Assoian RK. ABCG2 expression and side population abundance regulated by a transforming growth factor beta-directed epithelial-mesenchymal transition. Cancer Res. 2008;68:800–807. doi: 10.1158/0008-5472.CAN-07-2545. [DOI] [PubMed] [Google Scholar]
- 48.Evseenko DA, Paxton JW, Keelan JA. Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab Dispos. 2007;35:595–601. doi: 10.1124/dmd.106.011478. [DOI] [PubMed] [Google Scholar]
- 49.Ozvegy C, Litman T, Szakacs G, Nagy Z, Bates S, Varadi A, Sarkadi B. Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem Biophys Res Commun. 2001;285:111–117. doi: 10.1006/bbrc.2001.5130. [DOI] [PubMed] [Google Scholar]
- 50.Henriksen U, Gether U, Litman T. Effect of Walker A mutation (K86M) on oligomerization and surface targeting of the multidrug resistance transporter ABCG2. J Cell Sci. 2005;118:1417–1426. doi: 10.1242/jcs.01729. [DOI] [PubMed] [Google Scholar]
- 51.Kage K, Tsukahara S, Sugiyama T, Asada S, Ishikawa E, Tsuruo T, Sugimoto Y. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int J Cancer. 2002;97:626–630. doi: 10.1002/ijc.10100. [DOI] [PubMed] [Google Scholar]
- 52.Graf GA, Li WP, Gerard RD, Gelissen I, White A, Cohen JC, Hobbs HH. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J Clin Invest. 2002;110:659–669. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diop NK, Hrycyna CA. N-Linked glycosylation of the human ABC transporter ABCG2 on asparagine 596 is not essential for expression, transport activity, or trafficking to the plasma membrane. Biochemistry. 2005;44:5420–5429. doi: 10.1021/bi0479858. [DOI] [PubMed] [Google Scholar]
- 54.Henriksen U, Fog JU, Litman T, Gether U. Identification of intra- and intermolecular disulfide bridges in the multidrug resistance transporter ABCG2. J Biol Chem. 2005;280:36926–36934. doi: 10.1074/jbc.M502937200. [DOI] [PubMed] [Google Scholar]
- 55.Russ WP, Engelman DM. The GxxxG motif: a framework for transmembrane helix-helix association. J Mol Biol. 2000;296:911–919. doi: 10.1006/jmbi.1999.3489. [DOI] [PubMed] [Google Scholar]
- 56.Polgar O, Robey RW, Morisaki K, Dean M, Michejda C, Sauna ZE, Ambudkar SV, Tarasova N, Bates SE. Mutational analysis of ABCG2: role of the GXXXG motif. Biochemistry. 2004;43:9448–9456. doi: 10.1021/bi0497953. [DOI] [PubMed] [Google Scholar]
- 57.Polgar O, Ozvegy-Laczka C, Robey RW, Morisaki K, Okada M, Tamaki A, Koblos G, Elkind NB, Ward Y, Dean M, Sarkadi B, Bates SE. Mutational Studies of G553 in TM5 of ABCG2: a Residue Potentially Involved in Dimerization. Bichemistry. 2006 doi: 10.1021/bi0521590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J, Peng H, Chen Q, Liu Y, Dong Z, Zhang JT. Oligomerization domain of the multidrug resistance-associated transporter ABCG2 and its dominant inhibitory activity. Cancer Res. 2007;67:4373–4381. doi: 10.1158/0008-5472.CAN-06-3169. [DOI] [PubMed] [Google Scholar]
- 59.McDevitt CA, Collins RF, Conway M, Modok S, Storm J, Kerr ID, Ford RC, Callaghan R. Purification and 3D structural analysis of oligomeric human multidrug transporter ABCG2. Structure. 2006;14:1623–1632. doi: 10.1016/j.str.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 60.Mogi M, Yang J, Lambert JF, Colvin GA, Shiojima I, Skurk C, Summer R, Fine A, Quesenberry PJ, Walsh K. Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J Biol Chem. 2003;278:39068–39075. doi: 10.1074/jbc.M306362200. [DOI] [PubMed] [Google Scholar]
- 61.Takada T, Suzuki H, Gotoh Y, Sugiyama Y. Regulation of the cell surface expression of human BCRP/ABCG2 by the phosphorylation state of Akt in polarized cells. Drug Metab Dispos. 2005;33:905–909. doi: 10.1124/dmd.104.003228. [DOI] [PubMed] [Google Scholar]
- 62.Fetsch PA, Abati A, Litman T, Morisaki K, Honjo Y, Mittal K, Bates SE. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 2005 doi: 10.1016/j.canlet.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 63.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- 64.Diestra JE, Scheffer GL, Catala I, Maliepaard M, Schellens JH, Scheper RJ, Germa-Lluch JR, Izquierdo MA. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol. 2002;198:213–219. doi: 10.1002/path.1203. [DOI] [PubMed] [Google Scholar]
- 65.Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH, Schinkel AH. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92:1651–1656. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Wang H, Unadkat JD, Mao Q. Breast cancer resistance protein 1 limits fetal distribution of nitrofurantoin in the pregnant mouse. Drug Metab Dispos. 2007;35:2154–2158. doi: 10.1124/dmd.107.018044. [DOI] [PubMed] [Google Scholar]
- 67.Pollex E, Lubetsky A, Koren G. The Role of Placental Breast Cancer Resistance Protein in the Efflux of Glyburide across the Human Placenta. Placenta. 2008;29:743–747. doi: 10.1016/j.placenta.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Zhou L, Naraharisetti SB, Wang H, Unadkat JD, Hebert MF, Mao Q. The breast cancer resistance protein (Bcrp1/Abcg2) limits fetal distribution of glyburide in the pregnant mouse: an Obstetric-Fetal Pharmacology Research Unit Network and University of Washington Specialized Center of Research Study. Mol Pharmacol. 2008;73:949–959. doi: 10.1124/mol.107.041616. [DOI] [PubMed] [Google Scholar]
- 69.Gedeon C, Behravan J, Koren G, Piquette-Miller M. Transport of glyburide by placental ABC transporters: implications in fetal drug exposure. Placenta. 2006;27:1096–1102. doi: 10.1016/j.placenta.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 70.Gedeon C, Anger G, Piquette-Miller M, Koren G. Breast cancer resistance protein: mediating the trans-placental transfer of glyburide across the human placenta. Placenta. 2008;29:39–43. doi: 10.1016/j.placenta.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 71.Staud F, Vackova Z, Pospechova K, Pavek P, Ceckova M, Libra A, Cygalova L, Nachtigal P, Fendrich Z. Expression and Transport Activity of Breast Cancer Resistance Protein (Bcrp/Abcg2) in Dually Perfused Rat Placenta and HRP-1 Cell Line. J Pharmacol Exp Ther. 2006;319:53–62. doi: 10.1124/jpet.106.105023. [DOI] [PubMed] [Google Scholar]
- 72.Myllynen P, Kummu M, Kangas T, Ilves M, Immonen E, Rysa J, Pirila R, Lastumaki A, Vahakangas KH. ABCG2/BCRP decreases the transfer of a food-born chemical carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in perfused term human placenta. Toxicol Appl Pharmacol. 2008 doi: 10.1016/j.taap.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 73.Cooray HC, Blackmore CG, Maskell L, Barrand MA. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport. 2002;13:2059–2063. doi: 10.1097/00001756-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 74.Hori S, Ohtsuki S, Tachikawa M, Kimura N, Kondo T, Watanabe M, Nakashima E, Terasaki T. Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s) J Neurochem. 2004;90:526–536. doi: 10.1111/j.1471-4159.2004.02537.x. [DOI] [PubMed] [Google Scholar]
- 75.Eisenblatter T, Huwel S, Galla HJ. Characterisation of the brain multidrug resistance protein (BMDP/ABCG2/BCRP) expressed at the blood-brain barrier. Brain Res. 2003;971:221–231. doi: 10.1016/s0006-8993(03)02401-6. [DOI] [PubMed] [Google Scholar]
- 76.Zhang W, Mojsilovic-Petrovic J, Andrade MF, Zhang H, Ball M, Stanimirovic DB. The expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB J. 2003;17:2085–2087. doi: 10.1096/fj.02-1131fje. [DOI] [PubMed] [Google Scholar]
- 77.Cisternino S, Mercier C, Bourasset F, Roux F, Scherrmann JM. Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood-brain barrier. Cancer Res. 2004;64:3296–3301. doi: 10.1158/0008-5472.can-03-2033. [DOI] [PubMed] [Google Scholar]
- 78.Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, Schellens JH. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65:2577–2582. doi: 10.1158/0008-5472.CAN-04-2416. [DOI] [PubMed] [Google Scholar]
- 79.Oostendorp RL, Buckle T, Beijnen JH, van Tellingen O, Schellens JH. The effect of P-gp (Mdr1a/1b), BCRP (Bcrp1) and P-gp/BCRP inhibitors on the in vivo absorption, distribution, metabolism and excretion of imatinib. Invest New Drugs. 2008 doi: 10.1007/s10637-008-9138-z. [DOI] [PubMed] [Google Scholar]
- 80.Bihorel S, Camenisch G, Lemaire M, Scherrmann JM. Influence of breast cancer resistance protein (Abcg2) and p-glycoprotein (Abcb1a) on the transport of imatinib mesylate (Gleevec) across the mouse blood-brain barrier. J Neurochem. 2007;102:1749–1757. doi: 10.1111/j.1471-4159.2007.04808.x. [DOI] [PubMed] [Google Scholar]
- 81.Bihorel S, Camenisch G, Lemaire M, Scherrmann JM. Modulation of the brain distribution of imatinib and its metabolites in mice by valspodar, zosuquidar and elacridar. Pharm Res. 2007;24:1720–1728. doi: 10.1007/s11095-007-9278-4. [DOI] [PubMed] [Google Scholar]
- 82.de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13:6440–6449. doi: 10.1158/1078-0432.CCR-07-1335. [DOI] [PubMed] [Google Scholar]
- 83.Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP, Berns AJ, Borst P. Disruption of mouse mdr-1a p-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 84.Enokizono J, Kusuhara H, Sugiyama Y. Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens. Mol Pharmacol. 2007;72:967–975. doi: 10.1124/mol.107.034751. [DOI] [PubMed] [Google Scholar]
- 85.Jonker JW, Merino G, Musters S, van Herwaarden AE, Bolscher E, Wagenaar E, Mesman E, Dale TC, Schinkel AH. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med. 2005;11:127–129. doi: 10.1038/nm1186. [DOI] [PubMed] [Google Scholar]
- 86.van Herwaarden AE, Wagenaar E, Karnekamp B, Merino G, Jonker JW, Schinkel AH. Breast cancer resistance protein (Bcrp1/Abcg2) reduces systemic exposure of the dietary carcinogens aflatoxin B1, IQ and Trp-P-1 but also mediates their secretion into breast milk. Carcinogenesis. 2006;27:123–130. doi: 10.1093/carcin/bgi176. [DOI] [PubMed] [Google Scholar]
- 87.Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol Pharmacol. 2005;67:1758–1764. doi: 10.1124/mol.104.010439. [DOI] [PubMed] [Google Scholar]
- 88.Merino G, Alvarez AI, Pulido MM, Molina AJ, Schinkel AH, Prieto JG. Breast Cancer Resistance Protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics and milk secretion. Drug Metab Dispos. 2006 doi: 10.1124/dmd.105.008219. [DOI] [PubMed] [Google Scholar]
- 89.Pulido MM, Molina AJ, Merino G, Mendoza G, Prieto JG, Alvarez AI. Interaction of enrofloxacin with breast cancer resistance protein (BCRP/ABCG2): influence of flavonoids and role in milk secretion in sheep. J Vet Pharmacol Ther. 2006;29:279–287. doi: 10.1111/j.1365-2885.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 90.van Herwaarden AE, Wagenaar E, Merino G, Jonker JW, Rosing H, Beijnen JH, Schinkel AH. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol Cell Biol. 2007;27:1247–1253. doi: 10.1128/MCB.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Olsen HG, Nilsen H, Hayes B, Berg PR, Svendsen M, Lien S, Meuwissen T. Genetic support for a quantitative trait nucleotide in the ABCG2 gene affecting milk composition of dairy cattle. BMC Genet. 2007;8:32. doi: 10.1186/1471-2156-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ron M, Cohen-Zinder M, Peter C, Weller JI, Erhardt G. Short communication: a polymorphism in ABCG2 in Bos indicus and Bos taurus cattle breeds. J Dairy Sci. 2006;89:4921–4923. doi: 10.3168/jds.S0022-0302(06)72542-5. [DOI] [PubMed] [Google Scholar]
- 93.Bart J, Hollema H, Groen HJ, de Vries EG, Hendrikse NH, Sleijfer DT, Wegman TD, Vaalburg W, van der Graaf WT. The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood-testis barrier and in primary testicular tumours. Eur J Cancer. 2004;40:2064–2070. doi: 10.1016/j.ejca.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 94.Enokizono J, Kusuhara H, Ose A, Schinkel AH, Sugiyama Y. Quantitative investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) in limiting brain and testis penetration of xenobiotic compounds. Drug Metab Dispos. 2008;36:995–1002. doi: 10.1124/dmd.107.019257. [DOI] [PubMed] [Google Scholar]
- 95.Gutmann H, Hruz P, Zimmermann C, Beglinger C, Drewe J. Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem Pharmacol. 2005;70:695–699. doi: 10.1016/j.bcp.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 96.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JHM, Koomen G-J, Schinkel AH. Potent and Specific Inhibition of the Breast Cancer Resistance Protein Multidrug Transporter in Vitro and in Mouse Intestine by a Novel Analogue of Fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
- 97.Marchetti S, de Vries NA, Buckle T, Bolijn MJ, van Eijndhoven MA, Beijnen JH, Mazzanti R, van Tellingen O, Schellens JH. Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1−/−/Mdr1a/1b−/− (triple-knockout) and wild-type mice. Mol Cancer Ther. 2008;7:2280–2287. doi: 10.1158/1535-7163.MCT-07-2250. [DOI] [PubMed] [Google Scholar]
- 98.Sesink AL, Arts IC, de Boer VC, Breedveld P, Schellens JH, Hollman PC, Russel FG. Breast cancer resistance protein (Bcrp1/Abcg2) limits net intestinal uptake of quercetin in rats by facilitating apical efflux of glucuronides. Mol Pharmacol. 2005;67:1999–2006. doi: 10.1124/mol.104.009753. [DOI] [PubMed] [Google Scholar]
- 99.Kondo C, Onuki R, Kusuhara H, Suzuki H, Suzuki M, Okudaira N, Kojima M, Ishiwata K, Jonker JW, Sugiyama Y. Lack of improvement of oral absorption of ME3277 by prodrug formation is ascribed to the intestinal efflux mediated by breast cancer resistant protein (BCRP/ABCG2) Pharm Res. 2005;22:613–618. doi: 10.1007/s11095-005-2487-9. [DOI] [PubMed] [Google Scholar]
- 100.Seamon JA, Rugg CA, Emanuel S, Calcagno AM, Ambudkar SV, Middleton SA, Butler J, Borowski V, Greenberger LM. Role of the ABCG2 drug transporter in the resistance and oral bioavailability of a potent cyclin-dependent kinase/Aurora kinase inhibitor. Mol Cancer Ther. 2006;5:2459–2467. doi: 10.1158/1535-7163.MCT-06-0339. [DOI] [PubMed] [Google Scholar]
- 101.Zaher H, Khan AA, Palandra J, Brayman TG, Yu L, Ware JA. Breast cancer resistance protein (Bcrp/abcg2) is a major determinant of sulfasalazine absorption and elimination in the mouse. Mol Pharm. 2006;3:55–61. doi: 10.1021/mp050113v. [DOI] [PubMed] [Google Scholar]
- 102.van Herwaarden AE, Jonker JW, Wagenaar E, Brinkhuis RF, Schellens JH, Beijnen JH, Schinkel AH. The breast cancer resistance protein (Bcrp1/Abcg2) restricts exposure to the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res. 2003;63:6447–6452. [PubMed] [Google Scholar]
- 103.Huls M, Brown CD, Windass AS, Sayer R, van den Heuvel JJ, Heemskerk S, Russel FG, Masereeuw R. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73:220–225. doi: 10.1038/sj.ki.5002645. [DOI] [PubMed] [Google Scholar]
- 104.Mizuno N, Suzuki M, Kusuhara H, Suzuki H, Takeuchi K, Niwa T, Jonker JW, Sugiyama Y. Impaired renal excretion of 6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl) benzothiazole (E3040) sulfate in breast cancer resistance protein (BCRP1/ABCG2) knockout mice. Drug Metab Dispos. 2004;32:898–901. [PubMed] [Google Scholar]
- 105.Mizuno N, Takahashi T, Kusuhara H, Schuetz JD, Niwa T, Sugiyama Y. Evaluation of the role of breast cancer resistance protein (BCRP/ABCG2) and multidrug resistance-associated protein 4 (MRP4/ABCC4) in the urinary excretion of sulfate and glucuronide metabolites of edaravone (MCI-186; 3-methyl-1-phenyl-2-pyrazolin-5-one) Drug Metab Dispos. 2007;35:2045–2052. doi: 10.1124/dmd.107.016352. [DOI] [PubMed] [Google Scholar]
- 106.Vander Borght S, Libbrecht L, Katoonizadeh A, van Pelt J, Cassiman D, Nevens F, Van Lommel A, Petersen BE, Fevery J, Jansen PL, Roskams TA. Breast cancer resistance protein (BCRP/ABCG2) is expressed by progenitor cells/reactive ductules and hepatocytes and its expression pattern is influenced by disease etiology and species type: possible functional consequences. J Histochem Cytochem. 2006;54:1051–1059. doi: 10.1369/jhc.5A6912.2006. [DOI] [PubMed] [Google Scholar]
- 107.Aust S, Obrist P, Jaeger W, Klimpfinger M, Tucek G, Wrba F, Penner E, Thalhammer T. Subcellular localization of the ABCG2 transporter in normal and malignant human gallbladder epithelium. Lab Invest. 2004;84:1024–1036. doi: 10.1038/labinvest.3700127. [DOI] [PubMed] [Google Scholar]
- 108.Hirano M, Maeda K, Matsushima S, Nozaki Y, Kusuhara H, Sugiyama Y. Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol Pharmacol. 2005;68:800–807. doi: 10.1124/mol.105.014019. [DOI] [PubMed] [Google Scholar]
- 109.Ando T, Kusuhara H, Merino G, Alvarez AI, Schinkel AH, Sugiyama Y. Involvement of breast cancer resistance protein (ABCG2) in the biliary excretion mechanism of fluoroquinolones. Drug Metab Dispos. 2007;35:1873–1879. doi: 10.1124/dmd.107.014969. [DOI] [PubMed] [Google Scholar]
- 110.Zamek-Gliszczynski MJ, Hoffmaster KA, Humphreys JE, Tian X, Nezasa K, Brouwer KL. Differential involvement of Mrp2 (Abcc2) and Bcrp (Abcg2) in biliary excretion of 4-methylumbelliferyl glucuronide and sulfate in the rat. J Pharmacol Exp Ther. 2006;319:459–467. doi: 10.1124/jpet.106.101840. [DOI] [PubMed] [Google Scholar]
- 111.Zamek-Gliszczynski MJ, Nezasa K, Tian X, Kalvass JC, Patel NJ, Raub TJ, Brouwer KL. The important role of Bcrp (Abcg2) in the biliary excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in mice. Mol Pharmacol. 2006;70:2127–2133. doi: 10.1124/mol.106.026955. [DOI] [PubMed] [Google Scholar]
- 112.Enokizono J, Kusuhara H, Sugiyama Y. Involvement of breast cancer resistance protein (BCRP/ABCG2) in the biliary excretion and intestinal efflux of troglitazone sulfate, the major metabolite of troglitazone with a cholestatic effect. Drug Metab Dispos. 2007;35:209–214. doi: 10.1124/dmd.106.012567. [DOI] [PubMed] [Google Scholar]
- 113.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 115.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 116.Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, Beijnen JH, Schinkel AH. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A. 2002;99:15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 2002;99:12339–12344. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Polgar O, Robey RW, Bates SE. ABCG2: structure, function and role in drug response. Expert Opin Drug Metab Toxicol. 2008;4:1–15. doi: 10.1517/17425255.4.1.1. [DOI] [PubMed] [Google Scholar]
- 119.Honjo Y, Hrycyna CA, Yan QW, Medina-Perez WY, Robey RW, van de Laar A, Litman T, Dean M, Bates SE. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 2001;61:6635–6639. [PubMed] [Google Scholar]
- 120.Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, Poruchynsky MS, Bates SE. Mutations at amino acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–1978. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ejendal KF, Diop NK, Schweiger LC, Hrycyna CA. The nature of amino acid 482 of human ABCG2 affects substrate transport and ATP hydrolysis but not substrate binding. Protein Sci. 2006;15:1597–1607. doi: 10.1110/ps.051998406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miwa M, Tsukahara S, Ishikawa E, Asada S, Imai Y, Sugimoto Y. Single amino acid substitutions in the transmembrane domains of breast cancer resistance protein (BCRP) alter cross resistance patterns in transfectants. Int J Cancer. 2003;107:757–763. doi: 10.1002/ijc.11484. [DOI] [PubMed] [Google Scholar]
- 123.Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]
- 124.Qadir M, O’Loughlin KL, Fricke SM, Williamson NA, Greco WR, Minderman H, Baer MR. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11:2320–2326. doi: 10.1158/1078-0432.CCR-04-1725. [DOI] [PubMed] [Google Scholar]
- 125.de Bruin M, Miyake K, Litman T, Robey R, Bates SE. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett. 1999;146:117–126. doi: 10.1016/s0304-3835(99)00182-2. [DOI] [PubMed] [Google Scholar]
- 126.Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004;64:1242–1246. doi: 10.1158/0008-5472.can-03-3298. [DOI] [PubMed] [Google Scholar]
- 127.Minderman H, O’Loughlin KL, Pendyala L, Baer MR. VX-710 (biricodar) increases drug retention and enhances chemosensitivity in resistant cells overexpressing P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Clin Cancer Res. 2004;10:1826–1834. doi: 10.1158/1078-0432.ccr-0914-3. [DOI] [PubMed] [Google Scholar]
- 128.Mukai M, Che XF, Furukawa T, Sumizawa T, Aoki S, Ren XQ, Haraguchi M, Sugimoto Y, Kobayashi M, Takamatsu H, Akiyama S. Reversal of the resistance to STI571 in human chronic myelogenous leukemia K562 cells. Cancer Sci. 2003;94:557–563. doi: 10.1111/j.1349-7006.2003.tb01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Houghton PJ, Germain GS, Harwood FC, Schuetz JD, Stewart CF, Buchdunger E, Traxler P. Imatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitro. Cancer Res. 2004;64:2333–2337. doi: 10.1158/0008-5472.can-03-3344. [DOI] [PubMed] [Google Scholar]
- 130.Liu W, Baer MR, Bowman MJ, Pera P, Zheng X, Morgan J, Pandey RA, Oseroff AR. The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clin Cancer Res. 2007;13:2463–2470. doi: 10.1158/1078-0432.CCR-06-1599. [DOI] [PubMed] [Google Scholar]
- 131.Ozvegy-Laczka C, Hegedus T, Varady G, Ujhelly O, Schuetz JD, Varadi A, Keri G, Orfi L, Nemet K, Sarkadi B. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol. 2004;65:1485–1495. doi: 10.1124/mol.65.6.1485. [DOI] [PubMed] [Google Scholar]
- 132.Mahon FX, Belloc F, Lagarde V, Chollet C, Moreau-Gaudry F, Reiffers J, Goldman JM, Melo JV. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood. 2003;101:2368–2373. doi: 10.1182/blood.V101.6.2368. [DOI] [PubMed] [Google Scholar]
- 133.Ferrao PT, Frost MJ, Siah SP, Ashman LK. Overexpression of P-glycoprotein in K562 cells does not confer resistance to the growth inhibitory effects of imatinib (STI571) in vitro. Blood. 2003;102:4499–4503. doi: 10.1182/blood-2003-01-0083. [DOI] [PubMed] [Google Scholar]
- 134.Jordanides NE, Jorgensen HG, Holyoake TL, Mountford JC. Functional ABCG2 is over-expressed on primary CML CD34+ cells and is inhibited by imatinib mesylate. Blood. 2006 doi: 10.1182/blood-2006-02-003145. [DOI] [PubMed] [Google Scholar]
- 135.Brendel C, Scharenberg C, Dohse M, Robey RW, Bates SE, Shukla S, Ambudkar SV, Wang Y, Wennemuth G, Burchert A, Boudriot U, Neubauer A. Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia. 2007;21:1267–1275. doi: 10.1038/sj.leu.2404638. [DOI] [PubMed] [Google Scholar]
- 136.Shukla S, Sauna ZE, Ambudkar SV. Evidence for the interaction of imatinib at the transport-substrate site(s) of the multidrug-resistance-linked ABC drug transporters ABCB1 (P-glycoprotein) and ABCG2. Leukemia. 2008;22:445–447. doi: 10.1038/sj.leu.2404897. [DOI] [PubMed] [Google Scholar]
- 137.Tamura A, Onishi Y, An R, Koshiba S, Wakabayashi K, Hoshijima K, Priebe W, Yoshida T, Kometani S, Matsubara T, Mikuriya K, Ishikawa T. In vitro evaluation of photosensitivity risk related to genetic polymorphisms of human ABC transporter ABCG2 and inhibition by drugs. Drug Metab Pharmacokinet. 2007;22:428–440. doi: 10.2133/dmpk.22.428. [DOI] [PubMed] [Google Scholar]
- 138.Imai Y, Nakane M, Kage K, Tsukahara S, Ishikawa E, Tsuruo T, Miki Y, Sugimoto Y. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther. 2002;1:611–616. [PubMed] [Google Scholar]
- 139.Mizuarai S, Aozasa N, Kotani H. Single nucleotide polymorphisms result in impaired membrane localization and reduced atpase activity in multidrug transporter ABCG2. Int J Cancer. 2004;109:238–246. doi: 10.1002/ijc.11669. [DOI] [PubMed] [Google Scholar]
- 140.Morisaki K, Robey RW, Ozvegy-Laczka C, Honjo Y, Polgar O, Steadman K, Sarkadi B, Bates SE. Single nucleotide polymorphisms modify the transporter activity of ABCG2. Cancer Chemother Pharmacol. 2005;56:161–172. doi: 10.1007/s00280-004-0931-x. [DOI] [PubMed] [Google Scholar]
- 141.Tamura A, Watanabe M, Saito H, Nakagawa H, Kamachi T, Okura I, Ishikawa T. Functional validation of the genetic polymorphisms of human ATP-binding cassette (ABC) transporter ABCG2: identification of alleles that are defective in porphyrin transport. Mol Pharmacol. 2006;70:287–296. doi: 10.1124/mol.106.023556. [DOI] [PubMed] [Google Scholar]
- 142.Sparreboom A, Loos WJ, Burger H, Sissung TM, Verweij J, Figg WD, Nooter K, Gelderblom H. Effect of ABCG2 Genotype on the Oral Bioavailability of Topotecan. Cancer Biol Ther. 2005;4:650–658. doi: 10.4161/cbt.4.6.1731. [DOI] [PubMed] [Google Scholar]
- 143.Sparreboom A, Gelderblom H, Marsh S, Ahluwalia R, Obach R, Principe P, Twelves C, Verweij J, McLeod HL. Diflomotecan pharmacokinetics in relation to ABCG2 421C>A genotype. Clin Pharmacol Ther. 2004;76:38–44. doi: 10.1016/j.clpt.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 144.Zamboni WC, Ramanathan RK, McLeod HL, Mani S, Potter DM, Strychor S, Maruca LJ, King CR, Jung LL, Parise RA, Egorin MJ, Davis TA, Marsh S. Disposition of 9-nitrocamptothecin and its 9-aminocamptothecin metabolite in relation to ABC transporter genotypes. Invest New Drugs. 2006;24:393–401. doi: 10.1007/s10637-006-6335-5. [DOI] [PubMed] [Google Scholar]
- 145.Lepper ER, Nooter K, Verweij J, Acharya MR, Figg WD, Sparreboom A. Mechanisms of resistance to anticancer drugs: the role of the polymorphic ABC transporters ABCB1 and ABCG2. Pharmacogenomics. 2005;6:115–138. doi: 10.1517/14622416.6.2.115. [DOI] [PubMed] [Google Scholar]
- 146.Rudin CM, Liu W, Desai A, Karrison T, Jiang X, Janisch L, Das S, Ramirez J, Poonkuzhali B, Schuetz E, Fackenthal DL, Chen P, Armstrong DK, Brahmer JR, Fleming GF, Vokes EE, Carducci MA, Ratain MJ. Pharmacogenomic and pharmacokinetic determinants of erlotinib toxicity. J Clin Oncol. 2008;26:1119–1127. doi: 10.1200/JCO.2007.13.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ross DD, Karp JE, Chen TT, Doyle LA. Expression of breast cancer resistance protein in blast cells from patients with acute leukemia. Blood. 2000;96:365–368. [PubMed] [Google Scholar]
- 148.Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26:39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- 149.Benderra Z, Faussat AM, Sayada L, Perrot JY, Chaoui D, Marie JP, Legrand O. Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin Cancer Res. 2004;10:7896–7902. doi: 10.1158/1078-0432.CCR-04-0795. [DOI] [PubMed] [Google Scholar]
- 150.Wilson CS, Davidson GS, Martin SB, Andries E, Potter J, Harvey R, Ar K, Xu Y, Kopecky KJ, Ankerst DP, Gundacker H, Slovak ML, Mosquera-Caro M, Chen IM, Stirewalt DL, Murphy M, Schultz FA, Kang H, Wang X, Radich JP, Appelbaum FR, Atlas SR, Godwin J, Willman CL. Gene expression profiling of adult acute myeloid leukemia identifies novel biologic clusters for risk classification and outcome prediction. Blood. 2006;108:685–696. doi: 10.1182/blood-2004-12-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leonard GD, Polgar O, Bates SE. ABC transporters and inhibitors: new targets, new agents. Curr Opin Investig Drugs. 2002;3:1652–1659. [PubMed] [Google Scholar]
- 152.Garcia-Closas M, Chanock SJ. Genetic susceptibility loci for beast cancer by esgtrogen reseptor (ER) status. Clin Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-08-0975. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pan JH, Han JX, Wu JM, Sheng LJ, Huang HN, Yu QZ. MDR1 single nucleotide polymorphisms predict response to vinorelbine-based chemotherapy in patients with non-small cell lung cancer. Respiration. 2008;75:380–385. doi: 10.1159/000108407. [DOI] [PubMed] [Google Scholar]
- 154.Kafka A, Sauer G, Jaeger C, Grundmann R, Kreienberg R, Zeillinger R, Deissler H. Polymorphism C3435T of the MDR-1 gene predicts response to preoperative chemotherapy in locally advanced breast cancer. Int J Oncol. 2003;22:1117–1121. [PubMed] [Google Scholar]
- 155.Jamroziak K, Mlynarski W, Balcerczak E, Mistygacz M, Trelinska J, Mirowski M, Bodalski J, Robak T. Functional C3435T polymorphism of MDR1 gene: an impact on genetic susceptibility and clinical outcome of childhood acute lymphoblastic leukemia. Eur J Haematol. 2004;72:314–321. doi: 10.1111/j.1600-0609.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- 156.Cusatis G, Gregorc V, Li J, Spreafico A, Ingersoll RG, Verweij J, Ludovini V, Villa E, Hidalgo M, Sparreboom A, Baker SD. Pharmacogenetics of ABCG2 and adverse reactions to gefitinib. J Natl Cancer Inst. 2006;98:1739–1742. doi: 10.1093/jnci/djj469. [DOI] [PubMed] [Google Scholar]
- 157.Apperley JF. Part II: management of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8:1116–1128. doi: 10.1016/S1470-2045(07)70379-0. [DOI] [PubMed] [Google Scholar]
- 158.Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA, Lassalle R, Marit G, Reiffers J, Begaud B, Moore N, Molimard M, Mahon FX. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2007;109:3496–3499. doi: 10.1182/blood-2006-07-036012. [DOI] [PubMed] [Google Scholar]
- 159.Kantarjian H, Pasquini R, Hamerschlak N, Rousselot P, Holowiecki J, Jootar S, Robak T, Khoroshko N, Masszi T, Skotnicki A, Hellmann A, Zaritsky A, Golenkov A, Radich J, Hughes T, Countouriotis A, Shah N. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]