Abstract
The conventional design of high affinity drugs targeted to a single molecule has not resulted in clinically useful therapies for pain relief. Recent reviews have suggested that newly designed analgesic drugs should incorporate multiple targets. The distributions of cholecystokinin (CCK) and CCK receptors in the central nervous system (CNS) overlap significantly with endogenous opioid systems and can be dually targeted. CCK has been shown to act as an endogenous “anti-analgesic” peptide and neuropathic pain conditions promote endogenous CCK release in CNS regions of pain modulation. Administration of CCK into nuclei of the rostral ventromedial medulla induces pronociceptive behaviors in rats. RSA 504 and RSA 601 are novel bifunctional compounds developed to target neuropathic pain by simultaneously acting as agonists at two distinct opioid receptors and antagonizing CCK receptors in the CNS. RSA 504 and RSA 601 demonstrate agonist activity in vitro and antihypersensitivity to mechanical and thermal stimuli in vivo using the spinal nerve ligation model of neuropathic pain. Intrathecal administration of RSA 504 and RSA 601 did not demonstrate antinociceptive tolerance over 7 days of administration and did not display motor impairment or sedation using a rotarod. These are the first behavioral studies that demonstrate how multi-targeted molecule design can address the pathology of neuropathic pain. These compounds with δ and μ opioid agonist activity and CCK antagonist activity within one molecule offer a novel approach with efficacy for neuropathic pain while lacking the side effects typically caused by conventional opioid therapies.
Keywords: neuropathic pain, spinal nerve ligation, cholecystokinin, opioids
1. Introduction
Chronic pain affects 78 million people in the United States and at least 75 million Europeans (Breivik et al., 2006; Loeser and Bonica, 2001). For many patients chronic pain is untreated or undermanaged due to a lack of novel medications that are tolerable. Recently, the National Institutes for Health described the need for designing medications that may have multiple sites of action to better address the pathology of chronic and/or neuropathic pain (Woodcock et al., 2007), stimulating the development of mechanism-based, individualized pain therapies.
Neuropathic pain, demonstrated by hypersensitivity to noxious and innocuous stimuli, is defined in broad terms as pain caused by damage or dysfunction in the peripheral nervous system (Merskey, 2002) leading to dysfunction of pain fibers of the CNS (Ossipov et al., 2000). This type of pain is generally diverse with unpredictable etiology; additionally, some patients present with genetic conditions, making treatment outcome even more difficult to predict (Ossipov and Porreca, 2005). Opiate therapy is one of the most commonly prescribed treatments for chronic neuropathic pain, however opioids do not address the mechanisms of neuropathic pain and often have limited efficacy against this type of pain. Opioids are plagued by analgesic tolerance, addiction, medication overuse hypersensitivities and other physical side effects (Jensen et al., 2001). Regardless of whether it is caused by a disease state or a nerve trauma, neuropathic pain generates unique challenges for the design of novel analgesic treatments due to the multiple mechanisms driving pain sequela.
Of particular importance are the descending facilitatory pathways of the brainstem, specifically CCK, which is co-localized in the CNS with endogenous opioids and opioid receptors (Ghilardi et al., 1992; Zhang et al., 2009). CCK release is increased in multiple models of injury in the descending pain facilitatory pathways (Kovelowski et al., 2000; Friedrich and Gebhart, 2003). Together, these data suggest that CCK plays a significant role in pain processing and endogenous opioid activity. In addition to activating nociceptive descending pain facilitatory neurons (Heinricher and Neubert, 2004), CCK administered into the RVM of normal rats produces time dependent behavioral signs of mechanical and thermal hypersensitivity of the viscera and hindpaw (Friedrich and Gebhart, 2003; Xie et al., 2005). Studies have demonstrated that exogenous opioid therapy also induces the release of CCK in the spinal cord, frontal cortex and brainstem (Becker et al., 2000; Gustafsson et al., 2001; Xie et al., 2005). Conversely, administration of a CCK antagonist into the RVM produces a time dependent attenuation of thermal and mechanical hypersensitivity in the rat L5/L6 SNL model (Kovelowski et al., 2000); the opioid-induced hyperalgesia model (Xie et al., 2005); as well as a model of visceral hypersensitivity (Friedrich and Gebhart, 2003). Lastly, systemic CCK antagonists significantly enhance the analgesic effects of opioids for the treatment of neuropathic pain and attenuate the development of opioid-induced antinociceptive tolerance in animals and humans (Dourish et al., 1990; McCleane, 1998; McCleane, 2002; McCleane, 2003; Nichols et al., 1996; Wiesenfeld-Hallin et al., 2002; Xie et al., 2005).
An emerging trend encompasses the use of hetero-bivalent compounds in the development of novel therapeutics. Licofelone, a pyrrolizine analogue, simultaneously inhibits both cyclooxygenases and 5-lipoxygenase; currently in clinical trials for osteoarthritis, it is the first NSAID to inhibit leukotriene and prostaglandin formation simultaneously (Raynauld et al., 2008). The only centrally acting analgesic approved in the United States since the 1970’s is itself a dual pharmacophore: tapentadol (Nucynta©), a μ opioid agonist and norepinephrine reuptake inhibitor in a single molecule (Frampton, 2010). Tapentadol sets itself apart from traditional opiate therapy with reduced side effects- most notably fewer patients experienced constipation, nausea, vomiting, pruritis and dizziness when compared to oxycodone in a long term safety and tolerability trial (Wild et al., 2010). Dual target non analgesics have also shown efficacy in chronic pain states: duloxetine (Cymbalta©), an SNRI, has been approved in the treatment of fibromyalgia and diabetic neuropathy in addition to major depressive disorder and generalized anxiety disorder in the United States (Wright et al., 2011). SNRIs alone have not resulted in the treatment for all types of chronic, neuropathic pain suggesting alternative underlying disease states such as increased levels of CCK. The increasing occurrence of bifunctional compounds in pain therapies is not accidental: it has been suggested that multivalent therapies offer a greater disease specificity and greater affinity than existing monovalent therapies (Lezoualc’h et al., 2009).
The bi-functional compounds examined here, RSA 504 (H-Tyr1-DNle2-Gly3-Trp4-Nle5-Asp6-Phe7-NH2) (Agnes et al., 2008) and RSA 601 (H-Tyr1-DPhe2-Gly3-DTrp4-NMeNle5-Asp6-Phe7-NH2) (Hruby et al., 2003) were based on the structure of SNF9007, a novel peptide analogue of CCK. SNF9007 was found to act simultaneously at the δ and μ opioid receptors to produce antinociception (Williams et al., 1994) but lacked efficacy due to some CCK agonist activity. The RSA compounds, designed to act as both an opioid agonists and CCK antagonists, have nanomolar binding affinity at both receptor classes with no CCK agonist activity and clear in vitro and in vivo antagonist activity at the CCK receptors. RSA 504 (913.02 g/mol) possesses Ki values of 23 nM and 27 nM at the δ and μ opioid receptors, respectively with Ki’s of 11.2 nM and 16 nM at the CCK-1 and CCK-2 receptors, respectively (Agnes et al., 2008). RSA 601 (m.w. 962.04 g/mol) displays 0.55 nM and 5.7 nM affinity at the δ and μ opioid receptors, respectively, as well as 1100 and 1.6 nM affinity for the CCK-1 and CCK-2 receptors, respectively (Hruby et al., 2003).
Overall, many compounds for neuropathic pain have been designed to act at a single selective target in a particular animal model of pain; however, they have not resulted in advancing clinical therapies. The RSA 504 and 601 compounds were designed towards the pathology of neuropathic pain in which two pharmacophores with different mechanisms of action result in better, longer lasting efficacy over the current monotherapy. These single entity compounds with agonist activity at the δ/μ opioid receptors in addition to CCK antagonist activity demonstrate reduced adverse side effects versus multiple compounds with pharmacokinetic and/or drug interactions. We advance the idea that these dual acting compounds address the pathology seen in many chronic pain syndromes, offering a multi-targeted approach to significantly improve antinociceptive efficacy.
2. Results
2.1 Biological Activities In Vitro
The RSA compounds were designed towards the pathology of neuropathic pain containing both CCK antagonist and opioid agonist pharmacophores. To expand upon the in vitro activities published by the Hruby and colleagues (Agnes et al., 2008; Hruby et al., 2003) demonstrating 0.45 or 23 nM in the MVD for delta opioid receptor activity and 63 or 210 nM in GPI for mu opioid receptor activity for RSA 601 or 504 respectively. The biological activity of RSA 601 was assessed using GTPγS binding assay for opioid receptors. At the hDOR in the GTP-γ-S binding assay RSA 601 displayed an EC50 of 20.9 nM and Emax of 33.8 nM; at MOR the EC50 was 520 nM and demonstrated an Emax of 52.7 nM. RSA 504 shows similar activity in the GTP-γ-S binding assay with an EC50 of 5.5 nM and an Emax of 81 nM at the hDOR and an EC50 of 0.47 nM and Emax of 110 nM at the MOR (Agnes et al., 2008).
2.2 RSA 504 and 601 Attenuate Tactile Allodynia When Administered Intrathecally
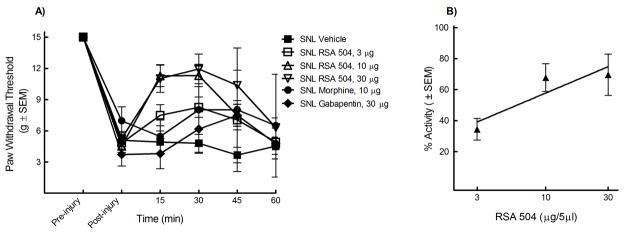
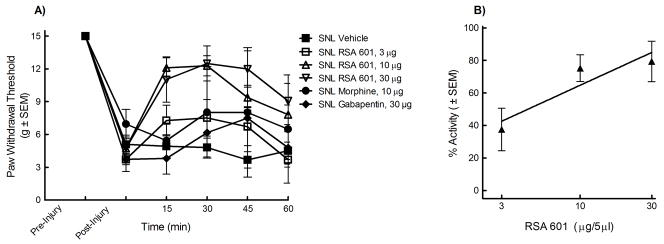
The L5/L6 SNL model was used to evaluate efficacy against mechanical hypersensitivity. Prior to and after injury, all animals were evaluated for mechanical response to non-noxious probing of the left hind paw with calibrated von Frey filaments. The mean paw withdrawal threshold before SNL was 15.0 ± 0.0 g. Seven days after nerve ligation, the mean withdrawal threshold was 4.7 ± 1.02 g, indicating the development of tactile allodynia (n = 74). The animals were separated at random into i.t. treatment groups: 33% EtOH vehicle, morphine (10 μg), gabapentin (30 μg), RSA 504 (3, 10, or 30 μg), and RSA 601 (3, 10, or 30 μg). Each group was given an acute bolus i.t. Following treatment administration, behavioral measurements of tactile allodynia were obtained every 15 min for the first hour. Responses were compared to control animal paw withdrawal thresholds. Significant attenuation of mechanical hypersensitivity using RSA 504 (15, 30, and 45 min post administration) was observed at doses of 3 μg/5mL, 10 μg/5μL, and 30 μg/5μL on day 7 after nerve injury when compared to post-injury baselines. RSA 504 induced significant attenuation of mechanical hypersensitivity (30 min post administration) at doses of 10 μg/5μL and 30 μg/5μL when compared to vehicle. No significance was seen at the 3 μg/5μL dose of RSA 504 when compared to vehicle. (Figure 1A). A dose response curve was generated at peak effect (30 min) (Figure 1B). The A50 at 30 min was 4.67 (2.81 – 9.88 μg/5μL; 95% CI). For additional comparative purposes, AUCs were calculated and compared to vehicle (AUC=22.07 ± 3.02). RSA 504 at doses of 3 μg/5μL (AUC= 32.53 ± 4.39), 10 μg/5μL (AUC = 39.38 ± 3.17), and 30 μg/5μL (AUC= 44.80 ± 4.93) differed significantly from vehicle. Similarly, RSA 601 when administered spinally displayed a time- and dose-related attenuation of SNL-induced mechanical hypersensitivity on day 7 after injury with significant attenuation of mechanical hypersensitivity at doses of 10μg/5μL and 30μg/5μL at all time points when compared to vehicle or post-injury baseline. RSA 601 at 3 μg/5 μL induced significant attenuation of mechanical hypersensitivity at the 15, 30, and 45 min time points when compared to post injury baseline, but not when compared to vehicle (Figure 2A). A dose response curve was generated for the time of peak effect (30 min) (Figure 2B). The A50 value at this time was 3.91 (2.75 – 6.34 μg/5μL; 95% CI). AUCs were calculated and compared to vehicle. RSA 601 at doses of 10 μg/5μL (AUC = 46.28 ± 2.43) and 30 μg/5μL (AUC= 49.34 ± 5.41) differed significantly from vehicle. No significant difference was observed with comparison of morphine or gabapentin to vehicle or post injury baseline at any time points tested or with total AUC (morphine AUC = 34.96 ± 7.99; gabapentin AUC = 25.97 ± 6.68).
Figure 1.
Mechanical hypersensitivity in L5/L6 spinal nerve ligated male Sprague Dawley rats (post-injury, day 7) was significantly reversed by RSA 504: (A) Dose- and time-related curves of RSA 504 (intrathecal) in nerve injury-induced mechanical hypersensitivity using calibrated von Frey filaments applied to the plantar aspect of the hind paw ± SEM. RSA 504 significantly reversed paw withdrawal thresholds at all three doses 3μg/5μl (p<0.05; n=9), 10μg/5μl (p<0.05; n=10), and 30μg/5μl (p<0.05; n=10)(p values calculated at the 30 min time point). No significant effect was seen in vehicle (n=10) (intrathecal) treated, morphine treated (n=6), or gabapentin treated animals (n=6). (B) Antiallodynic dose-response curve ± SEM for RSA 504 (intrathecal) was plotted at the time of peak effect (30 min) with an A50 of 4.67 (2.81 – 9.88 μg; 95% CI).
Figure 2.
Mechanical hypersensitivity in L5/L6 spinal nerve ligated male Sprague Dawley rats (post-injury, day 7) was significantly reversed by RSA 601: (A) Dose- and time-related curves of RSA 601 (intrathecal) in nerve injury-induced mechanical hypersensitivity using calibrated von Frey filaments applied to the plantar aspect of the hind paw ± SEM. RSA 601 significantly reversed paw withdrawal thresholds at all three doses 3μg/5μl (p<0.05; n=9), 10μg/5μl (p<0.05; n=12), and 30μg/5μl (p<0.05; n=9)(p values calculated at the 30 min time point). RSA 601 did not significantly change paw withdrawal thresholds in vehicle control (n=9) animals, morphine treated animals (n=6), or gabapentin treated animals (n=6) from baseline thresholds. (B) Antiallodynic dose-response curve ± SEM for RSA 601 (intrathecal) was plotted at the time of peak effect (30 min) with an A50 of 3.91 (2.75 – 6.34 μg; 95% CI).
2.3 RSA 504 and 601 Attenuate Thermal Hyperalgesia When Administered Intrathecally
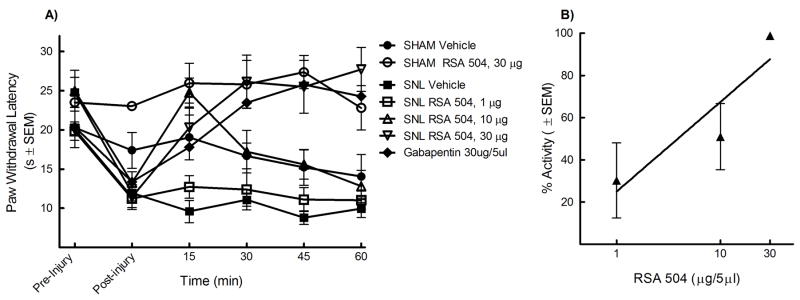
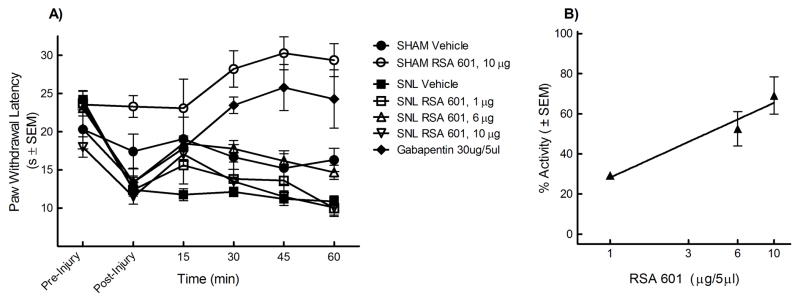
The L5/L6 SNL model was also used to evaluate efficacy against thermal hypersensitivity. Prior to and after injury, all animals were evaluated for hyperalgesic response to noxious probing of the left hind paw with an infrared cylinder. The mean paw withdrawal latency before SNL was 22.65 ± 1.6 seconds (n=80). Seven days after nerve ligation, the mean withdrawal latency was 12.21 ± 0.26 seconds (n=80), indicating the development of thermal hyperalgesia. The animals were separated at random into i.t. treatment groups: 33% EtOH vehicle, morphine (10 mg), gabapentin (30 mg), RSA 504 (1, 10, or 30 μg), and RSA 601 (1, 6, or 30 μg). An acute bolus was given i.t. to rats that had undergone L5/L6 SNL 7 days earlier. Additionally, sham control groups were included in the studies in which the animals received the same surgery as SNL animals minus the ligation of the L5 and L6 spinal nerves. No change in paw withdrawal latency was observed in sham-operated animals from pre-surgical baseline (22.09 ± 0.76 seconds, n=18) to 7 day post-surgical baseline (20.61 ± 0.98 seconds, n=18). Sham animals were separated into three groups: 33% EtOH vehicle, RSA 504 (30 μg/5 μL) and RSA 601 (10 μg/5 μL). Following treatment administration, behavioral measurements of thermal hyperalgesia were obtained every 15 min for the first hour. Responses were compared to pre-injury and post- injury paw withdrawal thresholds, and those of vehicle-treated animals. Administration of RSA 504 at doses of 10 μg/5μL and 30 μg/5μL in SNL animals significantly attenuated thermal hyperalgesia at the 30 min time point as compared to vehicle and post-injury baseline, whereas the 1μg/5μL dose did not produce a significant effect compared to vehicle or post injury baseline (Figure 3A). Peak effect of RSA 504 in the SNL animals occurred 30 minutes after intrathecal administration. A dose response curve was generated at the 30 min time point with an A50 value of 6.09 (3.45 – 13.96 μg/5μL; 95% CI) (Figure 3B). AUCs were calculated and compared to vehicle (AUC=55.52 ± 2.74). RSA 504 at doses of 10 μg/5μL (AUC = 83.61 ± 6.40) and 30 μg/5μL (AUC= 111.10 ± 7.97) differed significantly from vehicle. RSA 504 in sham operated animals and vehicle in the SNL animals had no effect on thermal hypersensitivity. RSA 601 administered intrathecally also significantly attenuated thermal hyperalgesia. Doses of 6 μg/5μL and 10 μg/5μL induced significant antihyperalgesic response as compared with vehicle treated animals or post-injury baselines (Figure 4A), however the lowest dose tested did not produce a significant effect 1 μg/5μL when compared to vehicle treated animals. A dose response curve for thermal latency in the SNL animals was generated for peak effect (15 min) with an A50 value of 5.17 (3.66 – 7.83 μg/5μL; 95% CI) (Figure 4B). RSA 601 (10 μg/5μL) demonstrated significant antinociception in sham animals. AUCs were calculated and compared to vehicle. RSA 601 at the 6 μg/5μL (AUC = 80.45 ± 4.13) differed significantly from vehicle. As compared to vehicle or post injury baseline, both gabapentin (figure 3A) and morphine (data not shown) displayed significant efficacy at all time-points as well as total AUC (morphine AUC = 77.33 ± 6.71; gabapentin AUC = 104.72 ± 11.01). In both SNL and sham-operated animals, intrathecal injection with control vehicle had no significant effect.
Figure 3.
Thermal hypersensitivity in L5/L6 spinal nerve ligated male Sprague Dawley rats (post-injury, day 7) was significantly reversed by RSA 504: (A) Dose- and time-related curves of RSA 504 (intrathecal) in nerve injury-induced thermal hypersensitivity using infrared radiant heat applied to the plantar aspect of the hind paw ± SEM. RSA 504 significantly reversed paw withdrawal latencies at doses of 10μg/5μl (p<0.05; n=9) and 30μg/5μl (p<0.05; n=10) but not at 1μg/5μl (n=8) (p values calculated at the 30 min time point). RSA 504 significantly increased paw withdrawal latencies in sham animals (p<0.05; n=10) at the 45 min time point. Gabapentin (n=6) and morphine (n=6) significantly increased paw withdrawal latencies at all timepoints (p<0.05). No significant effect was seen in vehicle (intrathecal) treated SNL (n=8) or Sham animals (n=6). (B) Antihyperalgesic dose-response curve ± SEM for RSA 504 (intrathecal) was plotted at the time of peak effect (30 min) with an A50 value of 6.09 (3.45 – 13.96 μg; 95% CI).
Figure 4.
Thermal hypersensitivity in L5/L6 spinal nerve ligated male Sprague Dawley rats (post-injury, day 7) was significantly reversed by RSA 601: (A) Dose- and time-related curves of RSA 601 (intrathecal) in nerve injury-induced thermal hypersensitivity using infrared radiant heat applied to the plantar aspect of the hind paw ± SEM. RSA 601 significantly reversed paw withdrawal latencies at the doses of 6μg/5μl (p<0.05; n=9) and 10μg/5μl (p<0.05; n=9) (p values calculated at the 15 min time point). RSA 601 significantly increased paw withdrawal latencies in sham animals (p<0.01; n=6) at the 45 min time point. Gabapentin (n=6) and morphine (n=6) significantly increased paw withdrawal latencies at all timepoints (p<0.05). No significant effect was seen in vehicle (intrathecal) treated SNL animals (n=10). (B) Antihyperalgesic dose-response curve ± SEM for RSA 601 (intrathecal) was plotted at the time of peak effect (15 min) with an A50 value of 5.17 (3.66 – 7.83 μg; 95% CI).
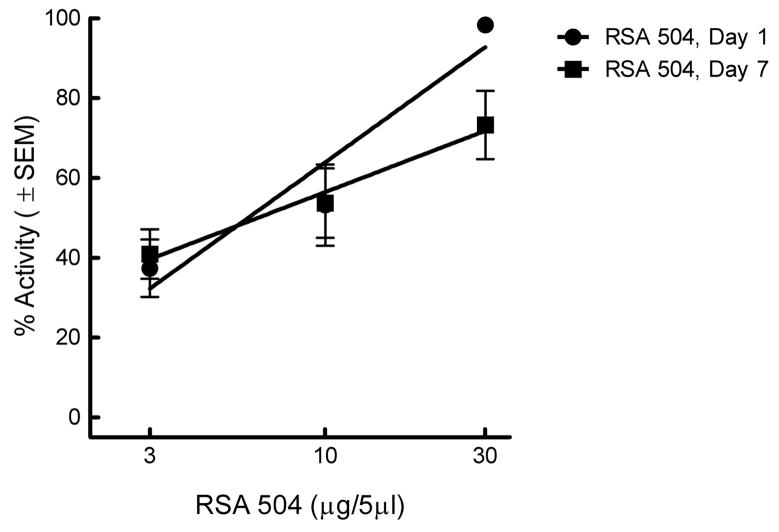
2.4 Chronic Spinal Administration of RSA 504 Does Not Induce Opioid Antinociceptive Tolerance
The L5/L6 SNL model was used to evaluate efficacy against thermal hyperalgesia over a period of chronic RSA 504 administration. Animals were tested for thermal paw withdrawal latencies on treatment day one (i.e., 7 days after SNL injury). An antihyperalgesic dose response curve was generated on the first treatment day using RSA 504, 3 μg/5μL, 10 μg/5μL and 30 μg/5μL (Figure 5). In order to test whether RSA 504 induces tolerance to its antihyperalgesic effects, these same animals were administered RSA 504 30 μg/5μL for 6 days and re-evaluated for thermal paw withdrawal latencies on the seventh treatment day. Reconstruction of the dose response curve in these chronically treated animals yielded significant attenuation of thermal hyperalgesia at doses of 3 μg/5μL (p<0.01), 10 μg/5μL, and 30 μg/5μL as compared with baseline (Figure 5). The A50 of RSA 504 on day one was 5.89 (4.11 – 8.45 μg/5μL; 95% CI) and was not significantly different when compared to the reconstructed RSA 504 dose response curve on day 7 after chronic treatment with an A50 of 6.45 (3.16 – 13.7 μg/5μL; 95% CI) (Figure 5). Chronic treatment with vehicle had no effect (data not shown).
Figure 5.
Antihyperalgesic dose-response curve ± SEM for intrathecal injection of RSA 504 in SNL animals does not result in tolerance: DRCs were generated at the time of peak effect (30 min) on day 1 (i.e., 7 days after SNL) and day 7 (i.e., 14 days after SNL) in the same animals. After testing on day one animals received sustained injections of RSA 504 (30 μg/5μl/day for 6 days; n=10). The DRC was re-constructed on day 7. The A50 value on day 1 was 5.89 (4.11 – 8.45 μg; 95% CI); the A50 value on day 7 was 6.45 (3.16 – 13.74 μg; 95% CI).
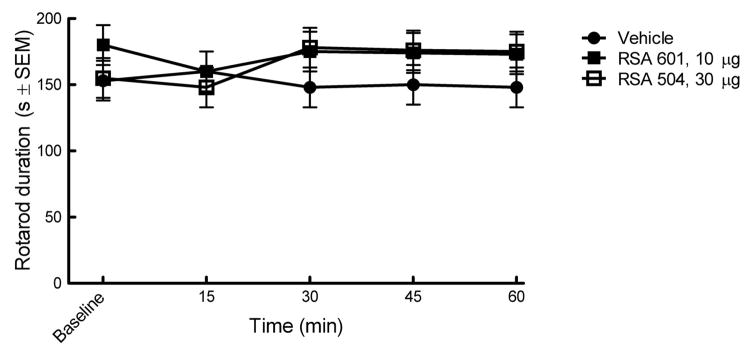
2.5 Intrathecal administration of RSA 504 and 601 do not induce motor impairment or sedation
Intrathecal administration of RSA 601 and 504 did not result in motor impairment as tested using the rotarod device (Figure 6) thus demonstrating that RSA 504 and 601 do not impair motor function or produce sedation.
Figure 6.
RSA 504, 601 or vehicle administered intrathecally did not result in motor impairment or sedation using the rotarod test: Animals were measured for their ability to stay on a rotating rod for 180 seconds ± SEM. No significant differences were seen between RSA 504 treated animals, RSA 601 animals or vehicle treated animals (n=6 per tested group).
2.6 In Vivo Demonstration of RSA 504 and RSA 601 CCK Antagonist Activity
Thermal hindpaw latencies were tested along with the RVM administration of RSA 504 and RSA 601 in with the presence of systemic naloxone (subcutaneous) to inhibit their opioid actions. Studies were performed in order to evaluate whether in vivo compound activity was being produced as a result of their CCK antagonist activity (Table 1). Thermal hypersensitivity was determined by paw withdrawal latencies to infrared radiant heat applied to the plantar aspect of the hind paw. Morphine produced significant (* p<0.01) analgesia as compared with baseline. Naloxone fully reversed this analgesia. CCK-8 administration alone produced thermal hypersensitivity as compared with baseline. RSA 504 and RSA 601 reversed the CCK-8 hypersensitivity and this effect was not significantly attenuated by naloxone as compared to CCK alone. These data suggest that RSA 504 and RSA 601, in the presence of naloxone in order to block the opioid effects, demonstrated an in vivo inhibition of the CCK-induced thermal hypersensitivity.
Table 1.
Thermal behavioral response in animals treated with RSA 504, RSA 601 and naloxone Thermal hypersensitivity was determined by paw withdrawal latencies to infrared radiant heat applied to the plantar aspect of the hind paw of male Sprague Dawley rats. The analgesic properties of RSA 504 (RVM; 60 ng/0.5μl) and RSA 601 (RVM; 60 ng/0.5μl) were compared with that of morphine sulfate (RVM; 60 ng/0.5μl) both with and without pretreatment (−20min) of naloxone (subcutaneous; 10mg/kg) and with pre-administration (−10min) of CCK-8sulf (RVM; 30 ng/0.5 μL). (*, **, ***, ****, or *****) denote significance when compared to baseline. (†or ††) denote significance when compared to administration of RSA 504 alone. (††† or ††††) denote significance when compared to administration of RSA 601 alone.
| Compound | Paw Withdrawal Latency (Sec ± SEM) | SEM | n |
|---|---|---|---|
| Baseline | 20.74 | 0.29 | 24 |
| Morphine Sulfate (MS) | 30.54* | 2.83 | 7 |
| Naloxone (NX) | 19.16 | 1.10 | 7 |
| MS + NX | 18.70** | 3.72 | 6 |
| Cholecystokinin (CCK) | 12.32*** | 1.49 | 6 |
| RSA 504 | 26.52**** | 1.79 | 6 |
| RSA 504 + CCK | 22.35† | 0.30 | 6 |
| RSA 504 + CCK + NX | 20.15†† | 0.55 | 6 |
| RSA 601 | 24.21***** | 2.52 | 8 |
| RSA 601 + CCK | 21.76††† | 1.27 | 6 |
| RSA 601 + CCK + NX | 20.81†††† | 1.30 | 6 |
3. Discussion
Widespread systemic changes and often prolonged treatment periods make many of the classic analgesics less viable for neuropathic pain. This may be due to issues of tolerance, adverse side effects, medication overuse hypersensitivities or simply a difference in the underlying pain mechanisms. While there are several mechanisms that are likely to play a role in this decreased efficacy, one such mechanism may be the increased activity of endogenous CCK at both spinal and supraspinal sites.
The PAG and RVM have been shown to mediate descending modulatory pathways that play a role in neuropathic pain (Behbehani, 1995; Mason, 2001; Ossipov et al., 2000; Urban and Gebhart, 1999). Several studies have shown that descending facilitation from the RVM is driven endogenously by CCK (Kovelowski et al., 2000; (Friedrich and Gebhart, 2003) and that inhibition is regulated by GABAergic interneurons (Cho and Basbaum, 1991; Gilbert and Franklin, 2001; Heinricher et al., 1991; Kovelowski et al., 2000; Moreau and Fields, 1986). CCK and opioid receptors as well as the associated endogenous peptides are not only co-localized in many of the pain modulating areas of the CNS (Ghilardi et al., 1992; Zhang et al., 2009), but also share some structural and pharmacological similarities (Hruby et al., 1994; Slaninova et al., 1991). Here we demonstrate, in rats with SNL, that morphine given spinally lacks efficacy for mechanical hypersensitivity after nerve ligation. However, when administered spinally, both RSA 504 and RSA 601 effectively inhibited tactile and thermal hypersensitivity caused by L5/L6 SNL at doses that do not produce sedation. By having dual pharmacophores, opioid agonist/CCK antagonist activity, the RSA compounds are more likely to reverse nerve injury-induced hypersensitivities.
Previous studies have demonstrated that a single injection of a CCK-2 antagonist into the RVM reversed L5/L6 SNL-induced mechanical and thermal hypersensitivity in a time dependent manner with peak effect occurring ten minutes post injection (Kovelowski et al., 2000). Similarly, mechanical hypersensitivity from nerve injury was reversed by spinal co-administration of a CCK-2 antagonist and morphine (Dourish et al., 1990) or spinal CCK-2 antagonist and inhibitors of endogenous enkephalin (Nichols et al., 1996). Studies by Friedrich and Gebhart (Friedrich and Gebhart, 2003) demonstrated that a CCK-2 antagonist administered into the RVM dose- and time-dependently reversed a visceral hypersensitive response induced by chronic inflammation of the GI tract. These data suggest that peripheral nerve injury to the lumbar region or chronic inflammation of visceral afferents results in the increase in endogenous CCK in the spinal cord and RVM promoting behavioral signs of pain and, therefore, a compound that acts to inhibit CCK receptors and activate opioid receptors is more likely to inhibit neuropathic and inflammatory pain.
Although in rodent studies the CCK-2 receptor does seem to be the primary arbiter of CCK descending modulation of the endogenous opioid system, this may not be the case in humans. Human trials conducted by McCleane evaluated the effects of the CCK-2 specific antagonist L365,260 in conjunction with morphine and did not show any augmentation to the analgesic effects of morphine (McCleane, 2003). Conversely, proglumide, a non specific CCK inhibitor, did augment the analgesic efficacy of morphine in human trials (McCleane, 1998), suggesting that in humans both CCK-1 and CCK-2 may play a role in pain modulation. Therefore, a non-selective CCK antagonist may be the most appropriate for clinical development. The compounds tested here have binding affinity in the nanomolar range at both CCK receptors, and simultaneously agonize δ and μ opioid receptors; a characteristic that may further increase utility of these compounds. Along with increases in affinity and specificity, patient care is also improved by simultaneously counteracting multiple facets of one disease state, lowering required doses, and reducing the risk of side effects and drug-drug interactions.
Our studies demonstrate that RSA 601, and to a lesser extent RSA 504, resulted in a significant increase in thermal thresholds above baseline in sham animals. By measuring their ability to attenuate CCK-8sulf induced thermal hyperalgesia using naïve animals in the presence of naloxone, the RSA compounds were shown to act as CCK antagonists in vivo. Since these compounds have opioid agonist activity, we blocked their ability to act at opioid receptors using naloxone and found that the CCK antagonist pharmacophore was active against CCK-8-induced thermal hyperalgesia confirming their dual acting biological activity. Preclinical studies have shown that long-term opioids can elicit abnormal pain states manifested as paradoxical algesia and hyperesthesias (Mao et al., 1995; Trujillo and Akil, 1991; Woolf, 1981; Yaksh and Harty, 1988). The development of thermal hyperalgesia in response to either repeated injections or constant infusion of morphine has also been reported (Mao et al., 1994), 1995). Thus, opioids given over time may maintain their level of efficacy, but the concurrent development of hyperalgesia could serve to counteract the antinociceptive effect of opioids, producing an impression of tolerance (Colpaert, 1996; Laulin et al., 1998; Laulin et al., 1999). Systems that may be activated upon sustained morphine administration include the descending pain facilitatory pathways from the RVM up-regulating the endogenous pronociceptive transmitter CCK.
Using in vivo microdialysis, Afrah and colleagues (Afrah et al., 2001) demonstrated that peripheral nerve injury results in a 3 fold increase in spinal endogenous CCK levels 7 days after injury. Likewise, using in vivo microdialysis we have demonstrated a 2 to 3 fold increase in endogenous CCK in the RVM, 7 days after L5/L6 SNL (abstract SfN, Vanderah Lab, 2008). The exogenous administration of CCK into the RVM results in a time dependent mechanical and thermal hypersensitivity as well as visceral hyperalgesia in naïve animals (Friedrich and Gebhart, 2003; Xie et al., 2005). CCK is thought to promote pain by activating a pain facilitatory pathway in the RVM. This pathway has been described as neurons that fire when pain is present, termed “ON cells” and are directly activated by the administration of CCK (Heinricher and Neubert, 2004). Collectively, these data strongly suggest that nerve injury or chronic inflammation results in the increase of endogenous CCK in the spinal cord and RVM resulting in pain facilitation, strongly supporting the concept of having a compound such as RSA 504 and 601 that not only activates opioid receptors but also blocks CCK receptors to inhibit neuropathic pain and antinociceptive tolerance.
Here we demonstrate that chronic administration of RSA 504 does not induce the onset of opioid antinociceptive tolerance: a promising finding consistent with previous findings that a CCK antagonist can reverse morphine-induced antinociceptive tolerance (Dourish et al., 1990). Likewise, several studies in humans with chronic pain have demonstrated significant opioid sparing effects by co-administering a CCK antagonist with an opioid and/or pain relief (McCleane, 1998; McCleane, 2002; McCleane, 2003). Unfortunately, such human studies cannot proceed since CCK antagonists, approved for human use, are no longer being manufactured most likely due to expired patents.
Over the past twenty years companies have continued to design highly selective compounds for a single target in order to attenuate neuropathic pain, however, such strategies have not resulted in a plethora of highly efficacious compounds that lack unwanted side effects. By designing compounds that target μ and δ opioid receptors, as well as the CCK receptors, we have produced novel compounds that demonstrate better, longer lasting antinociceptive efficacy in a neuropathic pain model. Novel compounds are being designed to target multiple sites that may offer better long-term efficacy and/or lack side effects due to over stimulation of one molecular target. Here we report in vivo behavioral studies with a compound designed to have agonist and antagonist activity at two distinctly different receptors for neuropathic pain. CCK is upregulated under conditions of neuropathic pain and therefore a compound is needed to block its pro-algesic effects. Unlike morphine, RSA 504 with CCK antagonist activity and opioid agonist activity did not result in antihyperalgesic tolerance over a 7 day period in nerve injured animals suggesting that the sustained administration of such dual acting pharmacophores may be effective over a long period of time in chronic pain patients. The results of this study show that the design of single compounds with dual pharmacophores leads to promising therapeutic agents for the treatment of neuropathic pain lacking unwanted side effects such as tolerance, sedation and opioid-induced hyperalgesia.
4. Experimental Procedure
4.1 Peptides
The peptides reported here were synthesized by standard solid phase peptide synthesis methods (Agnes et al., 2008; Hruby et al., 2003) by the Hruby lab in the University of Arizona department of Chemistry.
4.2 In Vitro Assays
4.2.1 GTPγS Binding
GTPγS binding was done using membranes from cells that express the human δ or rat μ opioid receptors. The procedure for this analysis was based on that of Lorenzen et al. (Lorenzen et al., 1993). Reactions were initiated by the addition of an aliquot of membrane preparation (15 μg) to a final volume of 300 μL of incubation mix [50 mM HEPES, pH.7.4, 1 mM EDTA, 5 mM MgCl2, 30 μM GDP, 1 mM dithiothreitol, 100 mM NaCl, 100 μM phenylmethylsulfonyl fluoride, 0.1% bovine serum albumin, 0.1 nM [35S]GTPγS (1250 Ci/mmol)] and indicated concentration range of agonist and incubated for 60 min at 30°C. Basal level of [35S]GTPγS binding was defined as the amount bound in the absence of agonist. Nonspecific binding was determined in the presence of 10 μM unlabeled GTPγS. Reactions were performed in triplicate and terminated by rapid filtration through Whatman GF/B filters presoaked in water followed by four washes with ice-cold wash buffer (50 mM Tris, 5 mM MgCl2, and 100 mM NaCl, pH 7.4). The radioactivity was determined by liquid scintillation counting. Data were fitted by nonlinear least-squares analysis using GraphPad Prism. 4.3 In Vivo Assays 4.3.1 Animals The experiments contained herein were carried out using male Sprague Dawley rats (250–350g; Harlan; Indianapolis, IN). All animals were maintained on a 12/12 hr light/dark cycle (lights on at 07:00 am) and provided food and water ad libitum except as noted during the experimental procedures. All experiments were performed under an approved protocol by the Institutional Animal Care and Use Committee of the University of Arizona, and in accordance with policies and guidelines for the care and use of laboratory animals as adopted by International Association for the Study of Pain and the National Institutes of Health.
4.3.2 L5/L6 SNL surgery
SNL injury was induced in male Sprague Dawley rats as described by Chung and colleagues (Kim and Chung, 1992). Anesthesia was induced with 2% isoflurane in O2 at 5 L/min and maintained with 2.5% isoflurane in O2. The dorsal vertebral column from L4 to S2 was exposed; the L5 and L6 spinal nerves were tightly ligated distal to the dorsal root ganglion using 4-0 silk suture. The incision was closed and the animals were allowed to recover for 5–7 days. Rats that exhibited motor deficiency (such as paw dragging) or failure to exhibit subsequent tactile allodynia were excluded from further testing (< 5%). Sham control rats underwent the same operation and handling as the experimental animals, but without ligation of the L5/L6 spinal nerves.
4.3.3 Intrathecal Catheter Surgery
Male Sprague Dawley rats were prepared for intrathecal drug administration as described by Yaksh and Rudy (Yaksh and Rudy, 1976) by placing anesthetized (ketamine/xylazine 100 mg/kg, intraperitoneal) animals in a stereotaxic head holder. The cisterna magna was exposed, an incision was made and animals were implanted with a catheter (PE: 10, 8 mm) that terminated in the lumbar region of the spinal cord. The animals were allowed to recover 5–7 days post-surgery before any pharmacological manipulations were made. Any rats showing impaired motor skills (e.g. paralysis) or greater than 10% weight loss were excluded from experiments (< 5%).
4.3.4 RVM Cannulation
Male Sprague Dawley rats were cannulated as described by the Porreca group (Roberts et al., 2009). Rats were anesthetized (ketamine/xylazine 100 mg/kg, intraperitoneal) and placed into a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). A 2 cm incision was made in the scalp, and the underlying connective tissue was retracted with hemostats to expose the skull. Paired guide cannulae 1.2 mm apart (26GA, #C235G-1.2 mm; Plastics One Inc., Roanoke, VA, USA) were directed towards the RVM (10.8 mm caudal to bregma, 0.6 mm to each side of the sagittal suture, and 7.0 mm ventral to the dura mater surface). Bone wax was used to seal the hole around the cannula. The paired guide cannula was secured to the skull using stainless steel screws and dental acrylic. A dummy cannula (#C235DC; Plastics One Inc.) was inserted to prevent contaminants from entering the RVM guide cannula. Rats were given an injection of antibiotic (amikacin C, 5 mg/kg, intraperitoneal) and allowed to recover for 7 days.
4.3.5 Drug Administration
RSA 504 and RSA 601 were dissolved in 33% EtOH and brought to volume with MilliPore H2O. CCK-8sulf was dissolved in Millipore H2O. Morphine sulfate was dissolved in 0.9% saline. Deltorphin (Bachem) was dissolved in 30% DMSO and brought to volume with 0.9% saline. Naloxone (RBI) was dissolved in 0.9% saline. For intrathecal compound administration, all compounds were injected in a 5 μL volume followed by a 9 μL saline flush. Blinded testing took place 15, 30, 45 and 60 min after drug injection, and dose–response curves were generated from data gathered at the time of peak effect. RVM drug administrations were performed by slowly expelling 0.5 μL bilaterally through an injection cannula protruding 1 mm beyond the tip of the guide to prevent backflow of drug into the guide cannula. Naloxone (10 mg/kg), when used, was administered subcutaneously 20 minutes before behavioral testing.
4.3.6 Behavioral Assessment
4.3.6.1 Antinociceptive Testing
Antinociception was assessed using the 52°C warm water tail-flick test in male Sprague Dawley rats (Vanderah et al., 2008). The latency to the first sign of a rapid tail flick was taken as the behavioral endpoint. Each rat was first tested for baseline latency by immersing the distal third of the tail in the water and recording the time to response. Rats not responding within 10 s were excluded from further testing (average latency = 4.26 ± 0.2s). One hour after baseline latencies were measured, rats were administered RSA 504, RSA 601, or vehicle and tested for tail or hindpaw withdrawal latency at 15, 30, 45, and 60 min post injection in a blinded fashion.
%Antinociception Calculation
4.3.6.2 Thermal Hypersensitivity
Thermal hypersensitivity was assessed using the rat plantar test following a modified method of Hargreaves and colleagues (Hargreaves et al., 1988). Male Sprague Dawley rats were allowed to acclimate within Plexiglas enclosures on a clear glass plate. A mobile radiant heat source (Ugo Basile, Italy) was located under the glass plate and focused onto the hind paw. Paw withdrawal latencies were recorded in seconds at 15 minute intervals for 60 minutes. An automatic cut off point of 33.0 s was set to prevent tissue damage. The apparatus was calibrated to give a paw withdrawal latency of approximately 20 seconds on the uninjured paw. The radiant heat source was activated with a timer and focused onto the plantar surface of the hindpaw. A motion detector which halted both heat source and timer when the paw was withdrawn determined paw-withdrawal latencies. The ipsilateral paws of SNL and sham animals were tested using the radiant heat source. The contralateral paws were not tested so that the injured paw would not be forced to bear weight unnecessarily. No thermal hypersensitivity was seen in sham animals. Crossover studies were performed when possible to reduce the total number of animals used: SNL or sham animals receiving vehicle during acute phase testing received a dose of either RSA 504 or 601 a minimum of 5 hours later for additional acute testing after repeating post-injury baseline testing.
Injury % Activity Calculation (% Antihyperalgesia)
Sham operated percent activities (% analgesia)
4.3.6.3 Mechanical Hypersensitivity
The assessment of mechanical hypersensitivity consisted of measuring the withdrawal threshold of the paw ipsilateral to the site of nerve injury in response to probing with a series of calibrated von Frey filaments. Prior to the SNL surgery, male Sprague Dawley rats were tested for pre-injury baseline mechanical sensitivity. Each filament was applied perpendicularly to the plantar surface of the left hind paw of rats kept in suspended wire-mesh cages. 7 days post SNL surgery on the left hind limb, measurements were taken both before (post-injury baseline) and after administration of RSA 504, RSA 601, or vehicle at 15 minute intervals for 60 minutes. The withdrawal threshold was determined by sequentially increasing and decreasing the stimulus strength (`up–down’ method) analyzed using a Dixon non-parametric test (Chaplan et al., 1994) and expressed as the mean withdrawal threshold. SNL animals were tested with the von Frey filaments while sham animals were not since maximum threshold was set to 15 grams. Higher calibrated filaments (>15g) result in the actual lifting of the paw that may be misinterpreted as a mechanical withdrawal (the higher calibrated filaments support the weight of the paw). Crossover studies were performed when possible to reduce the total number of animals used: SNL or sham animals receiving vehicle during acute phase testing received a dose of either RSA 504 or 601 a minimum of 5 hours later for additional acute testing after repeating post-injury baseline testing.
Injury % Activity Calculation (% Antiallodynia)
4.3.6.4 Motor Function
Male Sprague Dawley rats were trained to ambulate on a rotarod device (Columbus Instruments International, Columbus, OH) as previously described (Vanderah et al., 2008) until all could remain on the device for a duration of 180 s at a speed of 10 revolutions per minute. The rats were tested again after administration of RSA 504, RSA 601 or vehicle, and the time the rats were able to remain on the device without falling was recorded at 15 minute intervals for 60 minutes. The κ opioid agonist U50,488 was used as a positive control (not shown). A maximum cutoff time of 180 s was used.
4.4 Statistical Analysis
[35S]GTPγS binding data were analyzed by non-linear regression analysis using GraphPad Inplot. For binding affinity, the Ki value(s) for each ligand was calculated from the IC50 value(s) based on the Cheng and Prusoff equation from at least three independent experiments. For GTPγS binding, potency was expressed as log EC50 ± S.E.M. Maximal effect was expressed as Emax ± S.E.M.
Thermal and tactile hypersensitivity data were analyzed as previously published using one-way analysis of variance followed by students Neuman-Kuels testing for multiple comparisons in FlashCalc (Vanderah et al., 2000; Xie et al., 2005; Zhang et al., 2009). Differences were considered to be significant if p≤0.05. When possible, potencies (or A50) were determined by regression analysis of dose–response curve (log dose [x] vs response [y]) using a 95% confidence interval according to the method of analysis of the Graded Dose–Response (Tallarida and Murray, 1987). For the calculations of A50’s, the minimal possible response was set to 0%.
Acknowledgments
1. These studies were supported by NIDA grant 2PO1DA006284.
2. All peptides in this study were generously provided by the Hruby lab in the Department of Chemistry at the University of Arizona, Tucson, AZ.
Abbreviations
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- AUC
area under curve
- CCK
cholecystokinin
- CGRP
calcitonin gene related peptide
- CNS
central nervous system
- hDOR
human δ opioid receptor
- GABA
γ-aminobutyric acid
- HEK
human embryonic kidney cells
- IMDM
Iscove’s modified Dulbecco’s medium
- MOR
μ opioid receptor
- MS
morphine sulfate
- NOS
nitric oxide synthase
- NRI
norepinephrine reuptake inhibitor
- PAG
periaqueductal gray
- PKC
protein kinase C
- RVM
rostral ventromedial medulla
- SNL
spinal nerve ligation
- SNRI
serotonin-norepinephrine reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afrah AW, Gustafsson H, Olgart L, Brodin E, Stiller CO. Changes in spinal cholecystokinin release after peripheral axotomy. Neuroreport. 2001;12:49–52. doi: 10.1097/00001756-200101220-00018. [DOI] [PubMed] [Google Scholar]
- Agnes RS, Ying J, Kover KE, Lee YS, Davis P, Ma SW, Badghisi H, Porreca F, Lai J, Hruby VJ. Structure-activity relationships of bifunctional cyclic disulfide peptides based on overlapping pharmacophores at opioid and cholecystokinin receptors. Peptides. 2008;29:1413–23. doi: 10.1016/j.peptides.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Pohl M, Thiebot M-H, Collin E, Hamon M, Cesslin F, Benoliel J-J. δ-opioid receptor-mediated increase in cortical extracellular levels of cholecystokinin-like material by shbchronic morphine in rats. Neuropharmacology. 2000;39:161–171. doi: 10.1016/s0028-3908(99)00161-6. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Basbaum AI. GABAergic circuitry in the rostral ventral medulla of the rat and its relationship to descending antinociceptive controls. Journal of Comparative Neurology. 1991;303:316–28. doi: 10.1002/cne.903030212. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. System theory of pain and of opiate analgesia: no tolerance to opiates. Pharmacol Rev. 1996;48:355–402. [PubMed] [Google Scholar]
- Dourish CT, O’Neill MF, Coughlan J, Kitchener SJ, Hawley D, Iversen SD. The selective CCK-B receptor antagonist L-365,260 enhances morphine analgesia and prevents morphine tolerance in the rat. Eur J Pharmacol. 1990;176:35–44. doi: 10.1016/0014-2999(90)90129-t. [DOI] [PubMed] [Google Scholar]
- Frampton JE. Tapentadol immediate release: a review of its use in the treatment of moderate to severe acute pain. Drugs. 2010;70:1719–43. doi: 10.2165/11204470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Friedrich AE, Gebhart GF. Modulation of visceral hyperalgesia by morphine and cholecystokinin from the rat rostroventral medial medulla. Pain. 2003;104:93–101. doi: 10.1016/s0304-3959(02)00469-4. [DOI] [PubMed] [Google Scholar]
- Ghilardi JR, Allen CJ, Vigna SR, McVey DC, Mantyh PW. Trigeminal and dorsal root ganglion neurons express CCK receptor binding sites in the rat, rabbit, and monkey: possible site of opiate-CCK analgesic interactions. J Neurosci. 1992;12:4854–66. doi: 10.1523/JNEUROSCI.12-12-04854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert AK, Franklin KB. GABAergic modulation of descending inhibitory systems from the rostral ventromedial medulla (RVM). Dose-response analysis of nociception and neurological deficits. Pain. 2001;90:25–36. doi: 10.1016/s0304-3959(00)00383-3. [DOI] [PubMed] [Google Scholar]
- Gustafsson H, Afrah AW, Stiller CO. Morphine-induced in vivo release of spinal cholecystokinin is mediated by delta-opioid receptors--effect of peripheral axotomy. J Neurochem. 2001;78:55–63. doi: 10.1046/j.1471-4159.2001.00393.x. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Haws CM, Fields HL. Evidence for GABA-mediated control of putative nociceptive modulating neurons in the rostral ventromedial medulla: iontophoresis of bicuculline eliminates the off-cell pause. Somatosens Mot Res. 1991;8:215–25. doi: 10.3109/08990229109144745. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol. 2004;92:1982–9. doi: 10.1152/jn.00411.2004. [DOI] [PubMed] [Google Scholar]
- Hruby V, Fang S-N, Kramer T, Davis P, Parkhurst D, Nikiforovish G, Boteju L, Slaminova J, Yamamura H, Burks T. Analogues of cholecystokinin26–33 selective for B-type CCK receptors possess delta opioid receptor agonist activity in vitro and in vivo: evidence for similarieis in CCK-B and delta opioid receptor requirements. In: Hodges R, Smith J, editors. In peptides: Chemistry, Structure and Biology. ESCOM Publishers; Leiden: 1994. pp. 669–671. [Google Scholar]
- Hruby VJ, Agnes RS, Davis P, Ma SW, Lee YS, Vanderah TW, Lai J, Porreca F. Design of novel peptide ligands which have opioid agonist activity and CCK antagonist activity for the treatment of pain. Life Sci. 2003;73:699–704. doi: 10.1016/s0024-3205(03)00390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TS, Gottrup H, Sindrup SH, Bach FW. The clinical picture of neuropathic pain. Eur J Pharmacol. 2001;429:1–11. doi: 10.1016/s0014-2999(01)01302-4. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–63. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kovelowski CJ, Ossipov MH, Sun H, Lai J, Malan TP, Porreca F. Supraspinal cholecystokinin may drive tonic descending facilitation mechanisms to maintain neuropathic pain in the rat. Pain. 2000;87:265–73. doi: 10.1016/S0304-3959(00)00290-6. [DOI] [PubMed] [Google Scholar]
- Laulin JP, Larcher A, Celerier E, Le Moal M, Simonnet G. Long-lasting increased pain sensitivity in rat following exposure to heroin for the first time. Eur J Neurosci. 1998;10:782–5. doi: 10.1046/j.1460-9568.1998.00083.x. [DOI] [PubMed] [Google Scholar]
- Laulin JP, Celerier E, Larcher A, Le Moal M, Simonnet G. Opiate tolerance to daily heroin administration: an apparent phenomenon associated with enhanced pain sensitivity. Neuroscience. 1999;89:631–6. doi: 10.1016/s0306-4522(98)00652-6. [DOI] [PubMed] [Google Scholar]
- Lezoualc’h F, Jockers R, Berque-Bestel I. Multivalent-based drug design applied to serotonin. 5-HT(4) receptor oligomers. Curr Pharm Des. 2009;15:719–29. doi: 10.2174/138161209787315602. [DOI] [PubMed] [Google Scholar]
- Loeser JD, Bonica JJ. Bonica’s management of pain. Lippincott Williams & Wilkins; Philadelphia, Penn: 2001. [Google Scholar]
- Lorenzen A, Fuss M, Vogt H, Schwabe U. Measurement of guanine nucleotide-binding protein activation by A1 adenosine receptor agonists in bovine brain membranes: stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding. Mol Pharmacol. 1993;44:115–23. [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. Journal of Neuroscience. 1994;14:2301–12. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–74. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci. 2001;24:737–77. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- McCleane GJ. The cholecystokinin antagonist proglumide enhances the analgesic efficacy of morphine in humans with chronic benign pain. Anesth Analg. 1998;87:1117–20. [PubMed] [Google Scholar]
- McCleane GJ. A phase 1 study of the cholecystokinin (CCK) B antagonist L-365,260 in human subjects taking morphine for intractable non-cancer pain. Neurosci Lett. 2002;332:210–2. doi: 10.1016/s0304-3940(02)00934-5. [DOI] [PubMed] [Google Scholar]
- McCleane GJ. A randomised, double blind, placebo controlled crossover study of the cholecystokinin 2 antagonist L-365,260 as an adjunct to strong opioids in chronic human neuropathic pain. Neurosci Lett. 2003;338:151–4. doi: 10.1016/s0304-3940(02)01388-5. [DOI] [PubMed] [Google Scholar]
- Merskey H. Clarifying definition of neuropathic pain. Pain. 2002;96:408–9. doi: 10.1016/S0304-3959(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Fields HL. Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Research. 1986;397:37–46. doi: 10.1016/0006-8993(86)91367-3. [DOI] [PubMed] [Google Scholar]
- Nichols ML, Bian D, Ossipov MH, Malan TP, Jr, Porreca F. Antiallodynic effects of a CCKB antagonist in rats with nerve ligation injury: role of endogenous enkephalins. Neurosci Lett. 1996;215:161–4. doi: 10.1016/0304-3940(96)12964-5. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Malan TPJ, Porreca F. Spinal and Supraspinal Mechanisms of Neuropathic Pain. Annals of the New York Academy of Sciences. 2000;909:12–24. doi: 10.1111/j.1749-6632.2000.tb06673.x. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Porreca F. Challenges in the development of novel treatment strategies for neuropathic pain. NeuroRx. 2005;2:650–61. doi: 10.1602/neurorx.2.4.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynauld JP, Martel-Pelletier J, Abram F, Dorais M, Haraoui B, Choquette D, Bias P, Emmert KH, Laufer S, Pelletier JP. Analysis of the precision and sensitivity to change of different approaches to assess cartilage loss by quantitative MRI in a longitudinal multicentre clinical trial in patients with knee osteoarthritis. Arthritis Res Ther. 2008;10:R129. doi: 10.1186/ar2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J, Ossipov MH, Porreca F. Glial activation in the rostroventromedial medulla promotes descending facilitation to mediate inflammatory hypersensitivity. Eur J Neurosci. 2009;30:229–41. doi: 10.1111/j.1460-9568.2009.06813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaninova J, Knapp RJ, Wu JJ, Fang SN, Kramer T, Burks TF, Hruby VJ, Yamamura HI. Opioid receptor binding properties of analgesic analogues of cholecystokinin octapeptide. Eur J Pharmacol. 1991;200:195–8. doi: 10.1016/0014-2999(91)90688-m. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of Pharmacological Calculations with Computer Programs. Springer-Verlag Publishers; New York, NY: 1987. [Google Scholar]
- Trujillo KA, Akil H. Opiate tolerance and dependence: recent findings and synthesis. New Biologist. 1991;3:915–23. [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci U S A. 1999;96:7687–92. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM, Zhang ET, Malan TP, Jr, Ossipov MH, Lai J, Porreca F. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci. 2000;20:7074–9. doi: 10.1523/JNEUROSCI.20-18-07074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW, Largent-Milnes T, Lai J, Porreca F, Houghten RA, Menzaghi F, Wisniewski K, Stalewski J, Sueiras-Diaz J, Galyean R, Schteingart C, Junien JL, Trojnar J, Riviere PJ. Novel D-amino acid tetrapeptides produce potent antinociception by selectively acting at peripheral kappa-opioid receptors. Eur J Pharmacol. 2008;583:62–72. doi: 10.1016/j.ejphar.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Xu XJ, Hokfelt T. The role of spinal cholecystokinin in chronic pain states. Pharmacol Toxicol. 2002;91:398–403. doi: 10.1034/j.1600-0773.2002.910619.x. [DOI] [PubMed] [Google Scholar]
- Wild JE, Grond S, Kuperwasser B, Gilbert J, McCann B, Lange B, Steup A, Haufel T, Etropolski MS, Rauschkolb C, Lange R. Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract. 2010;10:416–27. doi: 10.1111/j.1533-2500.2010.00397.x. [DOI] [PubMed] [Google Scholar]
- Williams C, Rosenfeld G, Dafney N, Fang S-N, Hruby V, Bowden G, Cullinan C, Burks T. SNF9007: A novel analgesic that acts simultaneously at delta-1, delta-2, and mu opioid receptors. Journal of Pharmacology And Experimental Therapeutics. 1994;269:750–755. [PubMed] [Google Scholar]
- Woodcock J, Witter J, Dionne RA. Stimulating the development of mechanism-based, individualized pain therapies. Nat Rev Drug Discov. 2007;6:703–10. doi: 10.1038/nrd2335. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Intrathecal high dose morphine produces hyperalgesia in the rat. Brain Research. 1981;209:491–5. doi: 10.1016/0006-8993(81)90176-1. [DOI] [PubMed] [Google Scholar]
- Wright A, Luedtke KE, Vandenberg C. Duloxetine in the treatment of chronic pain due to fibromyalgia and diabetic neuropathy. J Pain Res. 2011;4:1–10. doi: 10.2147/JPR.S12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JY, Herman DS, Stiller CO, Gardell LR, Ossipov MH, Lai J, Porreca F, Vanderah TW. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J Neurosci. 2005;25:409–16. doi: 10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–6. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Harty GJ. Pharmacology of the allodynia in rats evoked by high dose intrathecal morphine. Journal of Pharmacology & Experimental Therapeutics. 1988;244:501–7. [PubMed] [Google Scholar]
- Zhang W, Gardell S, Zhang D, Xie JY, Agnes RS, Badghisi H, Hruby VJ, Rance N, Ossipov MH, Vanderah TW, Porreca F, Lai J. Neuropathic pain is maintained by brainstem neurons co-expressing opioid and cholecystokinin receptors. Brain. 2009;132:778–87. doi: 10.1093/brain/awn330. [DOI] [PMC free article] [PubMed] [Google Scholar]