Abstract
Cardiovascular insults such as myocardial infarction and chronic hypertension can trigger the heart to undergo a remodeling process characterized by myocyte hypertrophy, myocyte death and fibrosis, often resulting in impaired cardiac function and heart failure. Pathological cardiac remodeling is associated with inflammation, and therapeutic approaches targeting inflammatory cascades have shown promise in patients with heart failure. Small molecule histone deacetylase (HDAC) inhibitors block adverse cardiac remodeling in animal models, suggesting unforeseen potential for this class of compounds for the treatment of heart failure. In addition to their beneficial effects on myocardial cells, HDAC inhibitors have potent antiinflammatory actions. This review highlights the roles of HDACs in the heart and the potential for using HDAC inhibitors as broad-based immunomodulators for the treatment of human heart failure.
INTRODUCTION
Heart failure is a major health problem and a growing economic burden worldwide. There are more than five million heart failure patients in the United States alone, and treatment of this condition is estimated to cost the American health care system over $37 billion annually. Furthermore, the 5-year mortality rate following first admission for heart failure is over 40%, highlighting an urgent need for new therapeutic approaches (1).
Heart failure typically is classified as either systolic, in which there is reduced pump function, or diastolic, which is characterized by impaired cardiac relaxation and abnormal ventricular filling. At the cellular level, systolic heart failure is associated with myocyte hypertrophy and myocyte death, which often lead to development of interstitial fibrosis, chamber dilation and ventricular wall thinning. Diastolic heart failure is typified by myocyte hypertrophy and fibrosis without chamber dilation. It is estimated currently that close to 50% of the heart failure population in the U.S. has diastolic heart failure, which also is known as heart failure with preserved ejection fraction (HFpEF) (1).
First-line therapy for heart failure includes drugs aimed at inhibiting signaling pathways elicited by cell surface receptors, such as the angiotensin receptor (angiotensin-converting enzyme inhibitors [ACEi] and angiotensin receptor II blockers [ARBs]) and the β-adrenergic receptors (β-blockers). Despite efficacy of these drugs, the high mortality rate for patients with heart failure underscores the need to target alternative pathogenic mechanisms. In this regard, it has long been recognized that acute and chronic heart failure is associated with inflammatory cell activation (2–4), raising the possibility for synergy between antiinflammatory drugs and heart failure standards-of-care.
An exhaustive review of cytokine and cytokine receptor expression in human heart failure was published recently (5). Multiple studies have revealed that circulating levels of interleukin-6 (IL-6) and TNFα are increased in patients with heart failure, and expression of these cytokines appears to correlate with disease severity and prognosis; ACEi treatment is associated with reduced expression of TNFα as well as IL-1 (6). Several other inflammatory mediators, including IL-18 and monocyte chemoattractant protein (MCP-1), also have been implicated in human heart failure, and antiinflammatory approaches have been shown to be efficacious in animal models of heart failure and in small scale clinical trials in humans. For example, intraperitoneal (i.p.) administration of anakinra, a recombinant form of a naturally occurring IL-1 receptor antagonist, reduces cardiac apoptosis and improves cardiac function in rodent models of myocardial infarction (MI) (7). Consistent with this, mice in which the gene for the IL-1 receptor has been knocked out exhibit attenuated post-MI cardiac remodeling (8). In a trial of patients with ST-segment elevation acute MI (STEMI), subcutaneous administration of anakinra once daily for two weeks led to reduced left ventricular (LV) wall remodeling, as measured by echocardiography and magnetic resonance imaging after three months post-MI (9).
Studies in animal models also suggested a pathological role for TNFα in the heart, and in small-scale phase I/II trials of the injectable, soluble tumor necrosis factor (TNF) antagonist, etanercept, blockade of TNF receptor signaling led to improved LV ejection fraction in heart failure patients (10,11). However, etanercept failed to reduce death or hospitalization in phase III trials of ~1,500 heart failure patients (12). Infliximab, a chimeric monoclonal antibody against TNFα, also failed to improve cardiac function in patients with moderate-to-severe heart failure, and actually worsened clinical symptoms (13), suggesting a protective role for TNFα in the heart (14).
It is possible that agents that target multiple proinflammatory pathways will provide enhanced efficacy in the setting of heart failure. In this regard, histone deacetylase (HDAC) inhibitors represent a promising new class of compounds with broad-based antiinflammatory activities. This review highlights preclinical evaluations of HDAC inhibitors in animal models of heart failure, and discusses the potential for translating these findings to human clinical trials.
HDACs
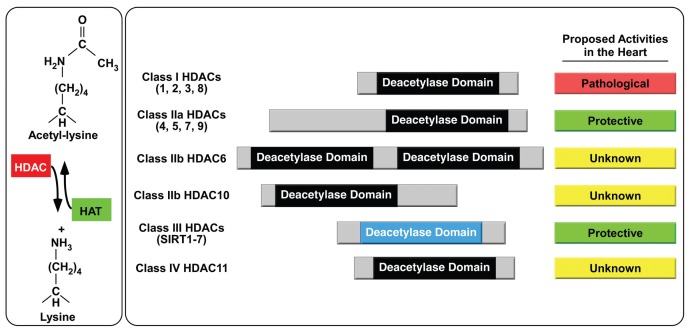
HDACs catalyze removal of acetyl groups from ɛ-amino groups of lysine residues in a variety of proteins (Figure 1). HDACs have been studied mainly in the context of chromatin, where they serve an epigenetic function by deacetylating nucleosomal histones and altering the electrostatic properties of chromatin in a manner that leads to gene repression. However, it is now clear that HDACs deacetylate many nonhistone proteins, and thus the enzymes also are called lysine deacetylases (KDACs) (15).
Figure 1.
Regulation of lysine acetylation by histone deacetylases. Histone acetyltransferases (HATs) transfer acetyl groups from acetyl-CoA to ɛ-amines of lysine residues on a variety of proteins, and HDACs catalyze removal of these groups. HDACs are categorized into four distinct classes. Class II HDACs are further divided into two subclasses, IIa and IIb. Class III HDACs also are known as sirtuins. Current data based on genetic and pharmacological investigations suggests that class I HDACs promote pathological cardiac remodeling while class IIa and class III HDACs are protective. There is nothing known about the functions of class IIb and class IV HDACs in the heart.
The 18 HDACs are encoded by distinct genes and are grouped into four classes on the basis of similarity to yeast transcriptional repressors (see Figure 1). Class I HDACs (HDACs 1, 2, 3 and 8) are related to yeast RPD3, class II HDACs (HDACs 4, 5, 6, 7, 9 and 10) to yeast HDA1, and class III HDACs (SirT1 – 7) to yeast Sir2. Class II HDACs are further divided into two subclasses, IIa (HDACs 4, 5, 7 and 9) and IIb (HDACs 6 and 10). HDAC11 falls into a fourth class (16).
HDACs IN THE HEART
The first connection between HDACs and regulation of pathological cardiac remodeling was provided by the discovery that class IIa HDACs interact with members of the myocyte enhancer factor-2 (MEF2) transcription factor family (17), which are key regulators of cardiac hypertrophy. Cardiac hypertrophy has long been viewed as a compensatory mechanism that normalizes wall stress and enhances cardiac performance. However, long-term suppression of cardiac hypertrophy is associated with reduced morbidity and mortality in patients with hypertension, and thus chronic cardiac hypertrophy is considered maladaptive (18,19).
All class IIa HDACs were found to associate with MEF2 on DNA (20,21), resulting in repression of downstream target genes. Ectopic overexpression of either HDAC4 (22), HDAC5 (23–25) or HDAC9 (25) in cultured rat cardiomyocytes coordinately suppresses MEF2-dependent transcription and agonist-dependent cardiac hypertrophy. In contrast, disruption of the gene encoding HDAC9 in mice leads to superactivation of cardiac MEF2 activity (25), and mouse knockouts for HDAC5 (26) or HDAC9 (25) develop exaggerated cardiac hypertrophy in response to pressure overload and spontaneous, pathologic hypertrophy with advancing age. These results support a general role for class IIa HDACs as endogenous suppressors of pathological cardiac hypertrophy (see Figure 1).
Since class IIa HDACs block cardiac hypertrophy, we hypothesized that HDAC inhibitors would promote cardiomyocyte growth. However, experiments with cultured cardiac myocytes revealed a striking ability of HDAC inhibitors to suppress myocyte hypertrophy (27). Two subsequent discoveries explain these seemingly paradoxical findings. First, recently described class IIa HDAC enzymatic assays revealed that these HDACs are relatively insensitive to standard HDAC inhibitors, including those used in the initial hypertrophy studies (28,29). Second, it was determined that class IIa HDACs do not require catalytic activity to suppress hypertrophic signaling in cardiomyocytes (25).
EFFICACY OF HDAC INHIBITORS IN PRECLINICAL MODELS OF HEART FAILURE
The fortuitous discovery of the antihypertrophic action of HDAC inhibitors suggested a novel application for these compounds for the treatment of human heart failure. Since dysregulation of HDACs is associated with a variety of pathophysiological processes, most notably cancer, there is intense focus in the pharmaceutical industry and in academic labs on development of novel small molecule HDAC inhibitors with enhanced potency, selectivity and pharmacokinetic properties. These efforts were further justified when SAHA/vorinostat (Zolinza) reached the market in 2006 with United States Food and Drug Administration (FDA) approval for the treatment of cutaneous T-cell lymphoma (30).
Most HDAC inhibitors possess a zinc-binding “warhead” group that docks in the active site, a linker and a surface recognition domain that interacts with residues near the entrance to the active site. This generic HDAC inhibitor pharmacophore is represented in at least four chemical classes: hydroxamic acids (for example, SAHA), short chain fatty acids (for example, valproic acid), benzamides (for example, MS-275) and cyclic peptides (for example, depsipeptide). Relative potencies and selectivity profiles differ between and within these classes (28). The strong zinc-chelating properties of the hydroxamic acid warhead produce potent (low nanomolar) pan-HDAC inhibitors. In contrast, the short chain fatty acids are weak (millimolar) HDAC inhibitors, with perhaps modest selectivity toward class I HDACs. Benzamide HDAC inhibitors are generally highly selective for HDACs 1, 2 and 3, as are the cyclic peptides.
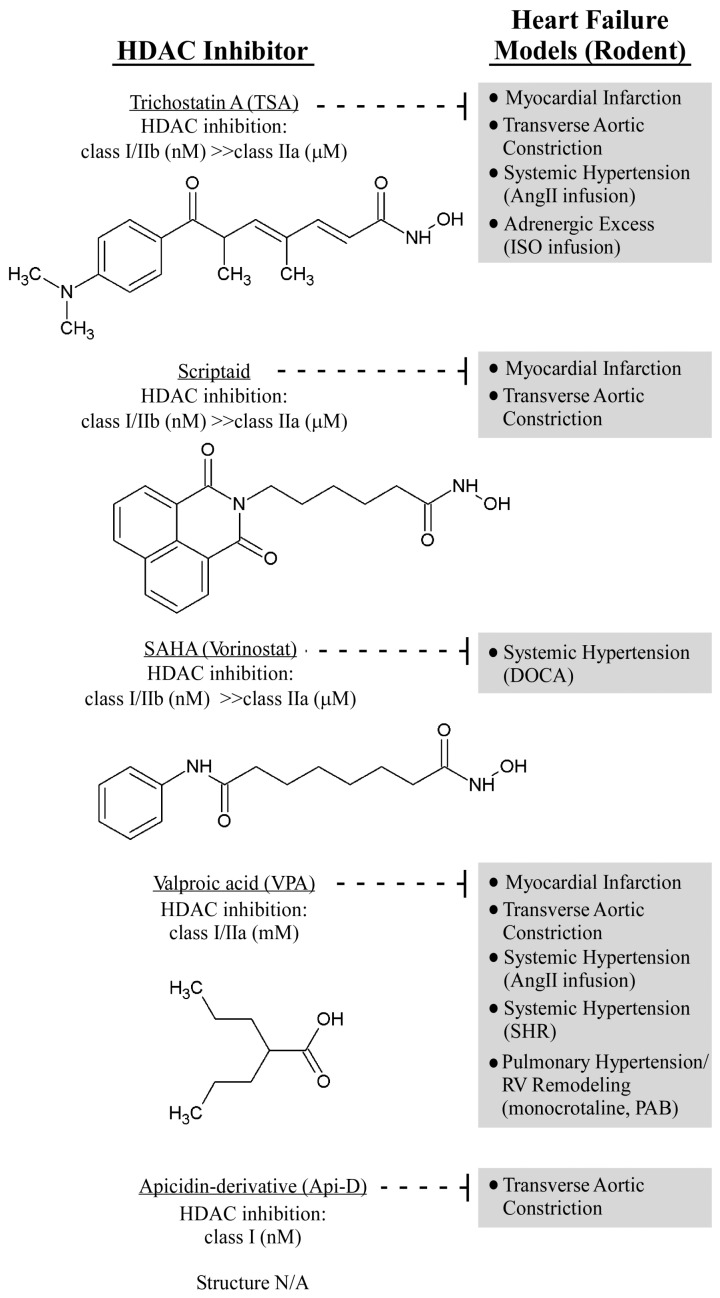
In vivo studies demonstrated that non-selective, pan-HDAC inhibitors can effectively halt, and even reverse, pathological cardiac hypertrophy (Figure 2). Treatment with the hydroxamic acid, pan-HDAC inhibitor, trichostatin A (TSA), or valproic acid for 2 weeks blocked the development of cardiac hypertrophy in transgenic mice that overexpress an HDAC2-dependent serum response factor (SRF) inhibitor, Hop, selectively in the heart (31). Similarly, pan-HDAC inhibitor treatment effectively suppressed cardiac hypertrophy induced by continuous infusion of the β-adrenergic receptor agonist, isoproterenol (31), or infusion of angiotensin II (32), as well as pressure overload imposed by transverse aortic constriction (32). Importantly, TSA treatment also was shown to regress established cardiac hypertrophy in mice subjected to aortic constriction (32), and also reversed established atrial fibrosis in Hop-transgenic mice (33), suggesting potential for HDAC inhibitors for the treatment of preexisting heart failure. Of note, data obtained with valproic acid should be interpreted with caution since this compound is a weak HDAC inhibitor that has many other pharmacological activities (34,35), including regulation of ion channels, glycogen synthase kinase-3β and mitogen-activated protein kinases (MAPK) (36).
Figure 2.
In vivo activities of HDAC inhibitors in rodent heart failure models. The indicated compounds have been tested in rodent models of heart failure. Models in which the compounds have demonstrated efficacy are shown, as are the relative potencies of compounds for different HDAC classes. The structure of apicidin-derivative (Api-D) is not available (N/A). AngII, angiotensin II; ISO, isoproterenol; PAB, pulmonary artery banding; RV, right ventricle.
Additional studies confirmed that 3 weeks of treatment with TSA and another pan-HDAC inhibitor, scriptaid, blunted cardiac hypertrophy in a pressure-overload mouse model, reducing cardiomyocyte size and improving ventricular performance significantly (37). The reduction in cardiac hypertrophy and functional improvements were maintained in a 9-week study, and TSA appeared to be well tolerated, since chronic administration over the course of the investigation did not impact survival negatively. Pan-HDAC inhibitors also have been shown to reduce cell death and prevent mal-adaptive ventricular remodeling in rodent models of MI (38–41), and in the setting of chronic hypertension in rats (42,43). Valproic acid recently was shown to block right ventricular (RV) cardiac hypertrophy in response to pulmonary artery banding, as well as in the setting of pulmonary hypertension caused by monocrotaline-induced lung injury (44). However, since valproic acid has many pharmacological activities, it is difficult to know whether the efficacy observed in these models was related directly to HDAC inhibition. Additional investigation of the role(s) of HDACs in RV remodeling is warranted, especially since maintenance of RV function in patients with pulmonary hypertension confers a survival advantage (45).
It will be important to determine which HDAC isoforms promote pathological remodeling of the heart. Studies in genetically engineered mice and cultured cardiomyocytes have suggested a role for HDAC2 in heart failure (46,47), although these findings remain contentious (48,49). More definitive answers likely will come from the use of small molecule inhibitors of select HDAC isoforms. An apicidin derivative, which is selective predominantly for class I HDACs 1, 2 and 3, was shown to suppress hypertrophy effectively and to improve cardiac performance in the setting of pressure overload (50). However, this compound appeared to exhibit activity, albeit modest, against HDAC6 in vitro. A critical next step is to extend these findings by testing additional class I HDAC inhibitors and newer generations of HDAC1/2-, HDAC3-, HDAC6- and HDAC8-selective compounds in animal models of heart failure (51).
Cardiac Inflammation, HDAC Inhibitors and Tregs
The impact of HDAC inhibition on inflammation in heart failure models has only been addressed recently. In spontaneously hypertensive rats (SHRs), treatment with valproic acid for 20 weeks led to reduced LV expression of IL-1β and TNFα, which correlated with inhibition of cardiac hypertrophy and fibrosis and improved cardiac function (42). Iyer and colleagues performed an exhaustive analysis of the effect of SAHA on plasma cytokine levels in the rat deoxycorticosterone acetate (DOCA)-salt model of hypertensive cardiomyopathy (43). After 4 weeks of treatment, SAHA reduced circulating levels of multiple proinflammatory cytokines significantly, including IL-1β, IL-6 and TNFα, and these decreases correlated with reduced cardiac hypertrophy and suppression of interstitial fibrosis in the LV. Of note, HDAC inhibition lowered mean systemic blood pressure in both SHR and DOCA rats, suggesting possible effects of HDACs on vascular remodeling.
The mechanism(s) for the general anti-inflammatory effects of HDAC inhibitors in the setting of heart failure is unknown. As discussed elsewhere in this review series, HDAC inhibitors have shown remarkable efficacy in models of organ rejection, reducing proinflammatory cytokine expression and increasing survival in a mouse bone marrow transplant model of graft versus host disease (GVHD) (52,53). Additionally, coadministration of TSA with a subtherapeutic dose of rapamycin induces allograft tolerance and improves survival in mouse cardiac and pancreatic islet allograft models dramatically (52). The protective effects of HDAC inhibitors in these models appears to be due to induction of regulatory T cells (Tregs), which possess potent anti-inflammatory properties (54). HDAC inhibitors stimulate Treg production by promoting acetylation of the FoxP3 transcription factor, which is a master regulator of Treg differentiation (53). Efficacy of HDAC inhibitors in mouse models of collagen-induced arthritis (55) and colitis (56) also were shown to correlate with induction of Tregs.
A recent report showed that Treg numbers and function were reduced significantly in patients with chronic heart failure, and the degree of Treg impairment correlated with severity of the heart failure phenotype (57). Consistent with these clinical findings, adoptive transfer of Tregs reduced angiotensin II–mediated cardiac remodeling in a mouse model (58). Improved cardiac function in this model was associated with reduced expression of proinflammatory cytokines and blunted inflammatory cell infiltration in the heart. It will be interesting to determine if beneficial effects of HDAC inhibitors in heart failure models correlate with enhanced production and activity of Tregs.
ADDITIONAL MECHANISMS: REGULATION OF HYPERTROPHY, CONTRACTILITY AND EndoMT
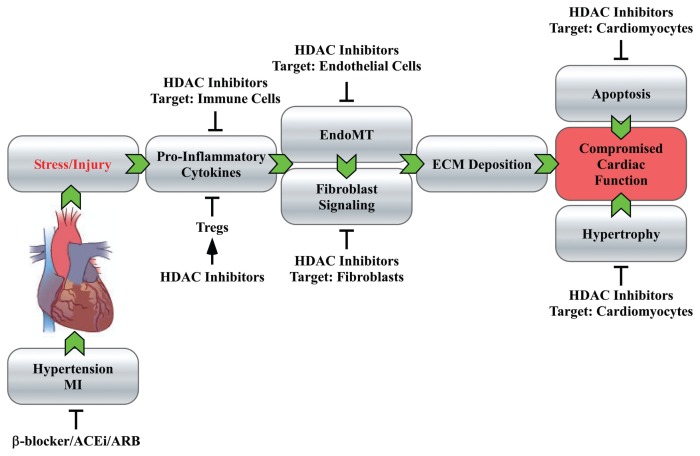
The remarkable efficacy of HDAC inhibitors in heart failure models is likely due to the ability of the compounds to affect multiple cells types (for example, myocytes, fibroblasts and immune cells) and diverse pathological mechanisms (for example, myocyte hypertrophy, fibrosis and inflammation) that culminate in organ damage (Figure 3). Thus, although HDAC inhibitors have one biochemical target (HDACs), they have multiple disease modifying mechanisms-of-action.
Figure 3.
HDAC inhibitors target multiple cell types and mechanisms controlling heart failure. Stresses such as hypertension and MI can trigger remodeling of the heart, resulting in impaired systolic and diastolic function and, ultimately, heart failure. Inflammation contributes to cardiac remodeling, in part, by stimulating fibroblasts to produce excess extra-cellular matrix (ECM). HDAC inhibitors appear to block several pathogenic mechanisms controlling heart failure, including inflammation, EndoMT and fibroblast signaling, as well as myocyte hypertrophy and death. The antiinflammatory effects of HDAC inhibitors could be governed by induction of Tregs, although this hypothesis has not been tested in heart failure models. Thus, HDAC inhibitors intervene with heart failure progression at multiple steps that are downstream of the cell surface receptors targeted by standards-of-care, such as β-blockers, ACEi and ARBs. The unique mechanisms-of-action of HDAC inhibitors provide intriguing possibilities for disease modulation and synergy with current standards-of-care for heart failure.
The means by which HDAC inhibitors suppress pathological cardiac hypertrophy are still being elucidated. Based on recent findings, it seems clear that a combination of histone and nonhistone targets will play key roles. One transcriptional mechanism for efficacy of HDAC inhibitors in the heart involves the anti-hypertrophic transcription factor, krüppel-like factor 4 (KLF4). KLF4 over-expression blocks cardiac hypertrophy in culture (59,60), and KLF4 knockout mice develop exaggerated cardiac hypertrophy and fibrosis in response to pressure overload (60). Pan-HDAC inhibitors were shown to increase expression of KLF4 in cultured cardiomyocytes (59), most likely by increasing acetylation of histones near KLF4 gene regulatory elements. The resulting increase in KLF4 expression appeared to be sufficient to block agonist-dependent hypertrophy of the cells.
Nontranscriptional effects of HDAC inhibitors in the heart also have been described. HDAC4 was shown to associate with cardiac sarcomeres and to decrease myofilament calcium sensitivity by promoting deacetylation of muscle Lim protein (MLP); HDAC inhibitor treatment increased calcium sensitivity of myofilaments from skinned fibers (61). However, it should be noted that the HDAC inhibitor concentrations used in these contractility studies were insufficient to inhibit catalytic activity of HDAC4, which is a class IIa HDAC (28,61). Further studies are needed to address the involvement of HDAC4 and other HDACs in the control of cardiac contractility.
HDAC inhibitors have profound suppressive effects on pathological cardiac fibrosis. Given the fact that proinflammatory cytokines activate cardiac fibroblasts to produce extracellular matrix (62,63), at least part of the antifibrotic action of HDAC inhibitors is likely due to immunomodulation. HDAC inhibitors also appear to have direct effects on cardiac fibroblasts. Indeed, TSA blocks transforming growth factor-β (TGF-β)- mediated induction of collagen synthesis in cultured rat ventricular fibroblasts (37). HDAC inhibitors do not affect TGF-β-driven phosphorylation or nuclear translocation of SMAD transcription factors, which control collagen gene expression, but do appear to suppress other signaling mediators (for example, ERK, AKT and PI3K) that impact collagen synthesis (64,65). Furthermore, HDAC inhibitors are capable of blocking differentiation of fibroblasts into contractile myofibroblasts by inhibiting expression of smooth muscle α actin (66).
Endothelial-to-mesenchymal transition (EndoMT) has emerged recently as a mechanism for production of excessive numbers of cardiac fibroblasts in adult hearts in response to pressure overload (67). Endo-MT is stimulated by TGF-β and suppressed by bone morphogenic protein-7 (BMP-7) (67), which is known to block fibrosis (68). Endothelin-1, a potent vasoconstrictor with promitogenic properties, also was shown to stimulate cardiac fibrosis by promoting EndoMT (69). Given that part of the antioncogenic action of HDAC inhibitors is through blockade of epithelial-to-mesenchymal transition (EMT) (70), future studies should address whether HDAC inhibition alters EndoMT in the heart.
TRANSLATION TO THE CLINIC
The preclinical results described above justify evaluation of HDAC inhibitors in patients with heart failure. Given the novelty and inherent high risk of this approach, phase IIa proof-of-concept trials with small numbers of patients would be particularly enlightening. HFpEF is an attractive indication for HDAC inhibitors since it is characterized by myocyte hypertrophy and interstitial fibrosis, two processes that are highly sensitive to HDAC inhibition. Furthermore, there are no FDA-approved drugs for HFpEF, and current standards-of-care for systolic heart failure provide little benefit to patients with this condition (71,72). Interestingly, patients with rheumatoid arthritis were recently shown to have increased prevalence of diastolic cardiac dysfunction, providing an additional link between inflammation and HFpEF (73). Post-MI remodeling is another indication for which efficacy of HDAC inhibitors could be addressed easily, and these trials could be patterned after the recent study of anakinra in patients with STEMI (9).
In the context of cancer, HDAC inhibitors are regarded currently as effective and generally well-tolerated chemotherapeutics (74). In addition to nausea and fatigue, HDAC inhibitors can produce transient thrombocytopenia and, in some instances, myelosuppression (75–78). The thrombocytopenia appears to be mechanism based, involving suppression of GATA-1 expression (79), but the specific HDAC isoform(s) responsible for this effect remains to be determined. It is hypothesized that isoform-selective HDAC inhibitors will be safer than pan-HDAC inhibitors, although clinical experience with isoform-selective HDAC inhibitors is quite limited (51).
Cancer therapy frequently is based on maximum tolerated doses of compounds. However, in patients with systemic onset juvenile idiopathic arthritis, the pan-HDAC inhibitor, ITF2357, was shown to be safe and efficacious at relatively low concentrations (1.5 mg/kg/day) (80). As such, with regard to targeting inflammation in the setting of heart failure, it is likely that efficacious doses of HDAC inhibitors will be significantly lower than those required for cancer therapy, and thus may be well tolerated.
CONCLUSION
HDAC inhibition continues to hold promise as an innovative approach for treating heart failure. Several questions remain as we move toward clinical testing of HDAC inhibitors for this indication. For example, it is unknown which HDAC isoform(s) is pathological in the heart, or whether selective inhibition of this HDAC sufficient for the treatment of heart failure. Preclinical safety and efficacy studies with emerging classes of HDAC1/2-, HDAC3-, HDAC6- and HDAC8-selective compounds are needed to determine whether isoform-selective HDAC inhibition will provide a more favorable therapeutic index than pan-HDAC inhibitors for the treatment of a chronic, nononcology indications such as heart failure. It is unclear why HDAC inhibitors are efficacious in models of heart failure. As described above (see Figure 3), HDAC inhibitors appear to block multiple pathogenic mechanisms that control heart failure. However, the mechanistic details of these effects are still lacking. For example, it is not known if the antiinflammatory effects of HDAC inhibitors in the heart are due to induction of Tregs or if they are mediated by independent mechanisms. Finally, it will be important to determine whether HDAC inhibitors synergize with current standards-of-care for heart failure. Answers to these questions should be forthcoming rapidly as momentum builds for translating HDAC-related discoveries from the lab to the heart failure clinic.
ACKNOWLEDGMENTS
I thank MA Cavasin and DD Lemon for helpful discussions.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The author declares to have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Lloyd-Jones D, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Deswal A, et al. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–9. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 3.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 4.Rauchhaus M, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102:3060–7. doi: 10.1161/01.cir.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 5.Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Fail Rev. 2010;15:331–41. doi: 10.1007/s10741-009-9140-3. [DOI] [PubMed] [Google Scholar]
- 6.Schindler R, Dinarello CA, Koch KM. Angiotensin-converting-enzyme inhibitors suppress synthesis of tumour necrosis factor and inter-leukin 1 by human peripheral blood mononuclear cells. Cytokine. 1995;7:526–33. doi: 10.1006/cyto.1995.0071. [DOI] [PubMed] [Google Scholar]
- 7.Abbate A, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–83. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 8.Bujak M, et al. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbate A, et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study) Am J Cardiol. 2010;105:1371–7. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 10.Bozkurt B, et al. Results of targeted anti-tumor necrosis factor therapy with etanercept (ENBREL) in patients with advanced heart failure. Circulation. 2001;103:1044–7. doi: 10.1161/01.cir.103.8.1044. [DOI] [PubMed] [Google Scholar]
- 11.Deswal A, et al. Safety and efficacy of a soluble P75 tumor necrosis factor receptor (Enbrel, etanercept) in patients with advanced heart failure. Circulation. 1999;99:3224–6. doi: 10.1161/01.cir.99.25.3224. [DOI] [PubMed] [Google Scholar]
- 12.Mann DL, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 13.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–40. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 14.Burchfield JS, et al. The cytoprotective effects of tumor necrosis factor are conveyed through tumor necrosis factor receptor-associated factor 2 in the heart. Circ Heart Fail. 2010;3:157–64. doi: 10.1161/CIRCHEARTFAILURE.109.899732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–61. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 17.McKinsey TA, Zhang CL, Olson EN. MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem Sci. 2002;27:40–7. doi: 10.1016/s0968-0004(01)02031-x. [DOI] [PubMed] [Google Scholar]
- 18.Devereux RB, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–6. doi: 10.1001/jama.292.19.2350. [DOI] [PubMed] [Google Scholar]
- 19.Gardin JM, Lauer MS. Left ventricular hypertrophy: the next treatable, silent killer. JAMA. 2004;292:2396–8. doi: 10.1001/jama.292.19.2396. [DOI] [PubMed] [Google Scholar]
- 20.Han A, et al. Sequence-specific recruitment of transcriptional co-repressor Cabin1 by myocyte enhancer factor-2. Nature. 2003;422:730–4. doi: 10.1038/nature01555. [DOI] [PubMed] [Google Scholar]
- 21.Han A, He J, Wu Y, Liu JO, Chen L. Mechanism of recruitment of class II histone deacetylases by myocyte enhancer factor-2. J Mol Biol. 2005;345:91–102. doi: 10.1016/j.jmb.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;116:1853–64. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bush E, et al. A small molecular activator of cardiac hypertrophy uncovered in a chemical screen for modifiers of the calcineurin signaling pathway. Proc Natl Acad Sci U S A. 2004;101:2870–5. doi: 10.1073/pnas.0308723101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vega RB, et al. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24:8374–85. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang CL, et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–88. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang S, et al. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol Cell Biol. 2004;24:8467–76. doi: 10.1128/MCB.24.19.8467-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antos CL, et al. Dose-dependent blockade to cardiomyocyte hypertrophy by histone deacetylase inhibitors. J Biol Chem. 2003;278:28930–7. doi: 10.1074/jbc.M303113200. [DOI] [PubMed] [Google Scholar]
- 28.Bradner JE, et al. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–43. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heltweg B, et al. Subtype selective substrates for histone deacetylases. J Med Chem. 2004;47:5235–43. doi: 10.1021/jm0497592. [DOI] [PubMed] [Google Scholar]
- 30.Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- 31.Kook H, et al. Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein Hop. J Clin Invest. 2003;112:863–71. doi: 10.1172/JCI19137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kee HJ, et al. Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation. 2006;113:51–9. doi: 10.1161/CIRCULATIONAHA.105.559724. [DOI] [PubMed] [Google Scholar]
- 33.Liu F, et al. Histone-deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin. J Mol Cell Cardiol. 2008;45:715–23. doi: 10.1016/j.yjmcc.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurvich N, Tsygankova OM, Meinkoth JL, Klein PS. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 2004;64:1079–86. doi: 10.1158/0008-5472.can-03-0799. [DOI] [PubMed] [Google Scholar]
- 35.Matalon S, et al. The histone deacetylase inhibitor ITF2357 decreases surface CXCR4 and CCR5 expression on CD4(+) T-cells and monocytes and is superior to valproic acid for latent HIV-1 expression in vitro. J Acquir Immune Defic Syndr. 2010;54:1–9. doi: 10.1097/QAI.0b013e3181d3dca3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terbach N, Williams RS. Structure-function studies for the panacea, valproic acid. Biochem Soc Trans. 2009;37:1126–32. doi: 10.1042/BST0371126. [DOI] [PubMed] [Google Scholar]
- 37.Kong Y, et al. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation. 2006;113:2579–88. doi: 10.1161/CIRCULATIONAHA.106.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granger A, et al. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J. 2008;22:3549–60. doi: 10.1096/fj.08-108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee TM, Lin MS, Chang NC. Inhibition of histone deacetylase on ventricular remodeling in infarcted rats. Am J Physiol Heart Circ Physiol. 2007;293:H968–77. doi: 10.1152/ajpheart.00891.2006. [DOI] [PubMed] [Google Scholar]
- 40.Zhang LX, et al. Targeted deletion of NF-kappaB p50 diminishes the cardioprotection of histone deacetylase inhibition. Am J Physiol Heart Circ Physiol. 2010;298:H2154–63. doi: 10.1152/ajpheart.01015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao TC, Cheng G, Zhang LX, Tseng YT, Pad-bury JF. Inhibition of histone deacetylases triggers pharmacologic preconditioning effects against myocardial ischemic injury. Cardiovasc Res. 2007;76:473–81. doi: 10.1016/j.cardiores.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Cardinale JP, et al. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension. 2010;56:437–44. doi: 10.1161/HYPERTENSIONAHA.110.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iyer A, et al. Antifibrotic activity of an inhibitor of histone deacetylases in DOCA-salt hypertensive rats. Br J Pharmacol. 2010;159:1408–17. doi: 10.1111/j.1476-5381.2010.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho YK, et al. Sodium valproate, a histone deacetylase inhibitor, but not captopril, prevents right ventricular hypertrophy in rats. Circ J. 2010;74:760–70. doi: 10.1253/circj.cj-09-0580. [DOI] [PubMed] [Google Scholar]
- 45.Voelkel NF, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–91. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 46.Kee HJ, et al. Activation of histone deacetylase 2 by inducible heat shock protein 70 in cardiac hypertrophy. Circ Res. 2008;103:1259–69. doi: 10.1161/01.RES.0000338570.27156.84. [DOI] [PubMed] [Google Scholar]
- 47.Trivedi CM, et al. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3 beta activity. Nat Med. 2007;13:324–31. doi: 10.1038/nm1552. [DOI] [PubMed] [Google Scholar]
- 48.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montgomery RL, et al. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 2007;21:1790–802. doi: 10.1101/gad.1563807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallo P, et al. Inhibition of class I histone deacetylase with an apicidin derivative prevents cardiac hypertrophy and failure. Cardiovasc Res. 2008;80:416–24. doi: 10.1093/cvr/cvn215. [DOI] [PubMed] [Google Scholar]
- 51.Bush EW, McKinsey TA. Protein acetylation in the cardiorenal axis: the promise of histone deacetylase inhibitors. Circ Res. 2010;106:272–84. doi: 10.1161/CIRCRESAHA.109.209338. [DOI] [PubMed] [Google Scholar]
- 52.Tao R, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 2007;13:1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, de Zoeten EF, Greene MI, Hancock WW. Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells. Nat Rev Drug Discov. 2009;8:969–81. doi: 10.1038/nrd3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 55.Saouaf SJ, et al. Deacetylase inhibition increases regulatory T cell function and decreases incidence and severity of collagen-induced arthritis. Exp Mol Pathol. 2009;87:99–104. doi: 10.1016/j.yexmp.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Zoeten EF, Wang L, Sai H, Dillmann WH, Hancock WW. Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice. Gastroenterology. 2010;138:583–94. doi: 10.1053/j.gastro.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang TT, et al. Defective circulating CD4CD25+Foxp3+CD127(low) regulatory T-cells in patients with chronic heart failure. Cell Physiol Biochem. 2010;25:451–8. doi: 10.1159/000303050. [DOI] [PubMed] [Google Scholar]
- 58.Kvakan H, et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–12. doi: 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- 59.Kee HJ, Kook H. Kruppel-like factor 4 mediates histone deacetylase inhibitor-induced prevention of cardiac hypertrophy. J Mol Cell Cardiol. 2009;47:770–80. doi: 10.1016/j.yjmcc.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 60.Liao X, et al. Kruppel-like factor 4 regulates pressure-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010;49:334–8. doi: 10.1016/j.yjmcc.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta MP, Samant SA, Smith SH, Shroff SG. HDAC4 and PCAF bind to cardiac sarcomeres and play a role in regulating myofilament contractile activity. J Biol Chem. 2008;283:10135–46. doi: 10.1074/jbc.M710277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–87. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 63.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–78. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Barter MJ, et al. HDAC-mediated control of ERK- and PI3K-dependent TGF-beta-induced extracellular matrix-regulating genes. Matrix Biol. 2010;29:602–12. doi: 10.1016/j.matbio.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Yoshikawa M, Hishikawa K, Idei M, Fujita T. Trichostatin a prevents TGF-beta1-induced apoptosis by inhibiting ERK activation in human renal tubular epithelial cells. Eur J Pharmacol. 2010;642:28–36. doi: 10.1016/j.ejphar.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 66.Guo W, Shan B, Klingsberg RC, Qin X, Lasky JA. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol. 2009;297:L864–70. doi: 10.1152/ajplung.00128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeisberg EM, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–61. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 68.Weiskirchen R, et al. BMP-7 as antagonist of organ fibrosis. Front Biosci. 2009;14:4992–5012. doi: 10.2741/3583. [DOI] [PubMed] [Google Scholar]
- 69.Widyantoro B, et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121:2407–18. doi: 10.1161/CIRCULATIONAHA.110.938217. [DOI] [PubMed] [Google Scholar]
- 70.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–19. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borlaug BA, Kass DA. Mechanisms of diastolic dysfunction in heart failure. Trends Cardiovasc Med. 2006;16:273–9. doi: 10.1016/j.tcm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 72.Borlaug BA. Treatment of heart failure with preserved ejection fraction. Curr Treat Options Cardiovasc Med. 2009;11:79–87. doi: 10.1007/s11936-009-0009-5. [DOI] [PubMed] [Google Scholar]
- 73.Liang KP, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1665–70. doi: 10.1136/ard.2009.124362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marks PA. The clinical development of histone deacetylase inhibitors as targeted anti-cancer drugs. Expert Opin Investig Drugs. 2010;19:1049–66. doi: 10.1517/13543784.2010.510514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galli M, et al. A phase II multiple dose clinical trial of histone deacetylase inhibitor ITF2357 in patients with relapsed or progressive multiple myeloma. Ann Hematol. 2010;89:185–90. doi: 10.1007/s00277-009-0793-8. [DOI] [PubMed] [Google Scholar]
- 76.Giles F, et al. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006;12:4628–35. doi: 10.1158/1078-0432.CCR-06-0511. [DOI] [PubMed] [Google Scholar]
- 77.Klimek VM, et al. Tolerability, pharmaco-dynamics, and pharmacokinetics studies of depsipeptide (romidepsin) in patients with acute myelogenous leukemia or advanced myelodys-plastic syndromes. Clin Cancer Res. 2008;14:826–32. doi: 10.1158/1078-0432.CCR-07-0318. [DOI] [PubMed] [Google Scholar]
- 78.O’Connor OA, et al. Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoy-lanilide hydroxamic acid in patients with advanced hematologic malignancies. J Clin Oncol. 2006;24:166–73. doi: 10.1200/JCO.2005.01.9679. [DOI] [PubMed] [Google Scholar]
- 79.Matsuoka H, et al. Mechanisms of HDAC inhibitor-induced thrombocytopenia. Eur J Pharmacol. 2007;571:88–96. doi: 10.1016/j.ejphar.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 80.Vojinovic J, et al. Safety and efficacy of an oral histone deacetylase inhibitor in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2011;63:1452–8. doi: 10.1002/art.30238. [DOI] [PubMed] [Google Scholar]