Abstract
Total cellular RNA level is stable usually, although it may increase gradually during growth or decrease gradually under certain stressors. However, we found that mammal cell RNAs could be doubled within 24 h in response to free heme accumulation (ischemia reperfusion and malaria infection) or a high level of glucose treatment (diabetes). Clinical investigations in rats showed that pretreatment with heme (24 h for doubling total RNAs) alleviated oxidative damages caused by diabetes, and pretreatment with glucose (24 h for trebling total RNAs) alleviated oxidative damages caused by ischemia reperfusion or malaria infection. Therefore, this rapid RNA amplification may play an important role in mammal adaptation to diabetes, ischemia reperfusion and malaria infection–derived oxidative stress. This rapid RNA amplification is derived from glucose and heme, but not from their accompanying reactive oxygen species. Hexokinases endure glucose-derived reactive oxygen species accumulation but are not related glucose-derived RNA amplification. In contrast, the TATA box-binding protein (TBP) mediates all glucose- and heme-induced RNA amplification in mammal cells.
INTRODUCTION
Glucose is an essential molecule for maintaining life. It is a major metabolic fuel that (through its degradation via glycolysis and subsequent oxidative phosphorylation) generates high-energy phosphate compounds responsible for driving many cellular processes. Normal levels of glucose and growth factors may protect cells from apoptotic events (1). However, despite its clear beneficial roles, glycemia must be tightly regulated in mammals, because excessive glucose, such as in diabetes, may be harmful to tissues (1–3). Although the mechanism of glucose toxicity is not completely understood, many studies have shown that high levels of glucose increase the formation of advanced glycation end products, glucose flux through the aldose reductase pathway and production of reactive oxygen species (ROS) in different cell lines (1–3). It has been proposed that the production of ROS by mitochondria via the respiratory chain is a causal link between high glucose and the main pathways responsible for hyperglycemic damage (1).
Heme is another essential molecule to living aerobic organisms and plays a role in various biological reactions, such as oxygen transport, respiration, drug detoxification and signal transduction (4). Heme interacts with various inactive apoproteins giving rise to functional heme proteins, such as hemopexin, albumin, α1-microglobulin and heme-binding protein 23 (HBP23). The function of the heme molecule is ultimately determined by the properties of the polypeptide bound to it (5). In hemoglobin and myoglobin, it is used for oxygen transport and storage, respectively, whereas in cytochromes, it is involved in electron transport, energy generation and chemical transformation (5). In catalases, heme functions in H2O2 degradation; in peroxidases, it serves different biological functions in the presence of H2O2 (5). Furthermore, heme is indispensable for other important enzyme systems, such as cyclooxygenase and nitric oxide synthase. In erythroid cells, heme serves as a positive feedback regulator for heme synthesis and inhibits its degradation. Heme is also important, in expression control of numerous proteins, such as globin, heme biosynthetic enzymes, cytochromes, myeloperoxidase, heme oxygenase-1 and the transferrin receptor. Heme also regulates differentiation and proliferation of various cell types (5). However, during the past three decades, reports have accumulated regarding the release of free heme from hemoproteins. This occurs during several pathological states and the toxic products lead to undesirable side effects (4,5).
Stress-induced hyperglycemia and diabetes increase blood glucose substantially (6,7). Severe hemolysis or myolysis occurring during pathological states, such as sickle cell disease, ischemia reperfusion (IR), icterohepatitis and malaria, results in high levels of free heme (5,8,9). High levels of both glucose and free heme cause undesirable oxidative stress to mammal cells (1,5,8).
Levels of total cellular RNA are usually stable within a cell cycle, although they may increase gradually during growth or decrease gradually as a result of stressors (10). Therefore, Northern blots are usually carried out on the basis of the total RNA level (10). However, several years ago, we found an interesting phenomenon when we extracted total RNAs from diabetic or IR tissues (11,12). High glucose– or high heme-stressed cells contained much higher levels of total cellular RNAs than unstressed cells. We repeated the experiments several times and found that those results were not due to experimental errors or different extraction efficiencies. Here we report rapid “RNA amplification” in mammalian cells. We reproduced this regulation by in vitro glucose or heme feeding and found this rapid RNA amplification plays an important role in mammal cells’ adaptation to diabetes, IR or malaria infection–derived oxidative stress. Possible factors in mammal cells mediating this rapid RNA amplification have been studied, including some transcription factors. One putative factor, the TATA box-binding protein (TBP), has been defined. The molecular signaling pathways are proposed here.
MATERIALS AND METHODS
Materials
Adult Sprague-Dawley rats (average body weight 350 ± 50 g) were purchased from Li-Nuo Biotechnology (Chengdu, China). Adult chickens were purchased from a local market.
DNA Constructs and Human Embryonic Kidney 293 Gene–Silenced Cell Lines
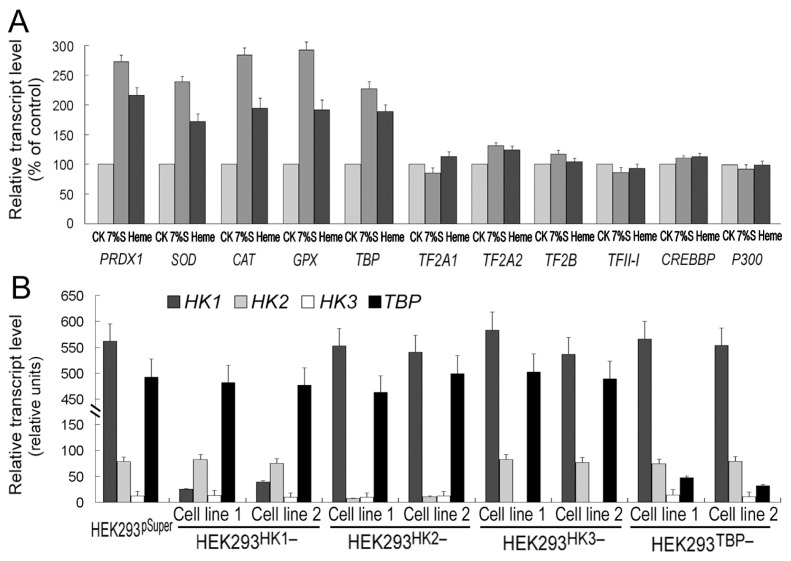
Human embryonic kidney (HEK)-293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) as described previously (13). Human Chang liver cells were cultured in minimum essential medium Eagle supplemented with 2 mmol/L l-glutamine, 1% nonessential amino acids (NEAA), 1 mmol/L sodium pyruvate and 10% fetal bovine serum (14). Human HK1, HK2, HK3 and TBP gene fragments and their inverted-repeat fragments were amplified by using the primer-pairs HK1 and HK1-Anti, HK2 and HK2-Anti, HK3 and HK3-Anti and TBP and TBP-Anti, respectively (Table Supplementary Material [SM]-1). Then the gene fragments and their inverted- repeat fragment were cloned in a pSuper vector expression system (Oligoengine, Seattle, WA, USA) for gene silencing by RNA interference (RNAi) (10). The recombinant vector was cotransfected (ratio 1:10) with the G418 antibiotic- resistance pCDNA3 vector (Invitrogen, Carlsbad, CA, USA) in HEK293 cells. After 3 wks of selection, stable HEK293HK1−, HEK293HK2−, HEK293HK3− and HEK293TBP− cell clones and HEK293pSuper control clones were isolated. HK1, HK2, HK3 or TBP gene expression was detected by reverse transcriptase–polymerase chain reaction (RT-PCR). We designed two independent siRNAs against each target gene. The efficiencies of RNAi suppression achieved about 90% for both gene-silenced cell lines. Therefore, we randomly chose one cell line for further experiments.
Chemical Treatment and Heme Feeding
For HEK293 cells, sterile glucose (7%), heme (50 μmol/L), TNF-α (10 ng/mL) or 1 mmol/L H2O2 were applied to the DMEM directly.
Quantitative Real-Time PCR
RNA was extracted by the TRIzol DNA/RNA kit (Invitrogen). The purification of RNA samples was detected by measuring the absorbance ratios of A260/A280, all of which were about 1.9. All RNA samples were treated with DNase I before RT-PCR. Minus RT (with minus RT–specific primers) was used as the control for the possible DNA contamination (15) (data not shown).
We used stationary phase HEK293 cells (anchorage-dependent rate achieved over 90%). The treatment time was only 1 d. Thus, the effect of the cell cycle should be negligible, and the cells were synchronized.
It may be difficult to normalize the total RNAs in the measurements. The number of cells per unit weight of tissue may change after the treatments. And for the long-term treatments (over 48 h), the cell cycles could not be neglected. Regardless of whether the cell cycles could be affected by the treatments, DNA is the template of RNA. Therefore, DNA (template) content is the most reliable reference parameter, and the RNA levels were presented as the ratio of total RNAs to total DNAs. The ratios in control animal cells without any treatment are normalized to 100%. We used a TRIzol DNA/RNA kit (Invitrogen) to isolate DNAs and RNAs simultaneously to guarantee the uniform extraction efficiency.
The quantitative real-time PCR analysis was performed with the primers shown in Table SM-1. Relative quantitation of the target gene expression level was performed by using the comparative Ct (threshold cycle) method (16). Three technical replicates were performed for each experiment. Amplification of the human Actin1 gene was used as an internal control for HEK293 cells. The expression levels of control animal cells without any treatment are normalized to 100%.
mRNA, tRNA, snRNA and snoRNA Isolation and Polyribosome Population Estimation
mRNAs, tRNAs, snRNAs and snoR-NAs were isolated from total RNAs as described previously (17–20). Polyribosomes were isolated by precipitation. For accurate polyribosome analysis, polyribosomes were applied to glucose density gradient centrifugation as described previously (21). The ratio of polyribosomes to total ribosomes was calculated as follows: (area of polyribosomes)/(area of polyribosomes + ribosomal subunits + mono-ribosomes). Ribosomal protein content and total cellular protein content were determined as described previously (22).
Transcription Rate Determination
Cells were incorporated with 32P-dCTP and then subjected to 50 μmol/L heme or 5% sucrose treatment for 48 h. rRNA, snRNA, snoRNA, mRNA and tRNA were extracted, respectively, and separated in agarose gel and visualized by using an autoradiography instrumentation (23).
Western Blots to Hexokinases
Cells were lysed in 1 × sodium dodecyl sulfate (SDS) sample buffer and resolved by electrophoresis using SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes (equal amounts of total cellular proteins were loaded). The membranes were probed with primary antibodies (against human HK1, HK2 and HK3 respectively; Abcam, Cambridge, MA) overnight and then incubated with appropriate horseradish peroxide–conjugated secondary antibodies for 3 h, followed by detection with a SuperSignal Enhanced Chemiluminescence kit (Pierce, Rockford, IL, USA) (24).
Superoxide and H2O2 Detection
The formation of H2O2 in HEK293 cells and kidneys was followed by measuring the oxidation of acetylated ferrocytochrome C catalyzed by cytochrome C peroxidase at room temperature (25), whereas the formation of superoxide in HEK293 cells and kidneys was monitored by the reduction of acetylated ferri-cytochrome C (26).
H2O2 levels in HEK293 cells were also visually detected with a solution of 5-(and 6-) chloromethyl-2′,7′-dichlorodihy-drofluorescein diacetate (CM-DCFH DA) (Molecular Probes, TM Invitrogen, Eugene, OR, USA), which was prepared daily in absolute ethanol and kept at 4°C. The HEK293 cells were then washed with DMEM for fluorescence microscopy. The imaging system consisted of a Zeiss Axiovert 200M epifluorescence microscope equipped with ×10 and ×20 objectives and filter sets: a 450–490 nm excitation/515–565 nm emission filter set that was used for CM-DCF fluorescence. CM-DCF fluorescence from fibers was recorded at 15-min intervals over 45 min at 25°C (27,28).
Renal Ischemia and Reperfusion Surgery, Diabetes Experiments and Malaria Infection
Renal ischemia (IR) was induced by nontraumatic vascular clamps over the pedicles for 20 min (29). After clamps were released, the incision was closed in two layers with 2-0 sutures. Twenty-four h after renal ischemia reperfusion to normal animals, kidney samples and kidney blood samples were collected. For alleviating IR injury, renal arterial infusion (30) with 1 mL 30% glucose solution was performed a single time 24 h before IR.
Diabetic rats were acquired by streptozotocin treatment (31). The diabetic state was assessed by daily monitoring of blood glucose levels. Oral glucose feeding to fasted (24 h) diabetic rats (30% glucose solution, every 8 h, three times total) and then blood glucose level was determined as described previously (31). Twenty-four h after the first glucose feeding, kidney samples were collected. For alleviating glucose-derived oxidative stress, renal arterial infusion (32) with 1 mmol/L heme was performed a single time upon fasting.
Malaria infection was applied with 106 Plasmodium berghei (ANKA) to normal rats (32). The number of erythrocytes/μL renal blood (×104) and the number of parasitized erythrocytes/μL renal blood (×104) were examined by a microscopy (32). For alleviating malaria infection, rat kidneys were treated with 30% glucose solution (renal arterial infusion, every 2 d, 4x total) paralleling with Plasmodium berghei infection for 8 d.
At the end of the experiment, the right kidney was removed and quickly frozen for molecular studies and the left kidney was perfused through the femoral catheter with a phosphate buffer, thereby preserving the mean arterial pressure of each animal. After blanching of the kidney, the perfusate was replaced by a freshly prepared 10% formalin buffer, and perfusion was continued until fixation was completed. After appropriate dehydration, kidney slices were embedded in paraffin, sectioned at 4 μm and stained via the periodic acid–Schiff technique. Ten subcortical and juxtamedullary fields were recorded from each kidney slide by using a digital camera incorporated in a microscope. Tubular damage was characterized by a loss of brush border, lumen dilatation or collapse and detachment from basement membrane. The damaged tubular area was expressed as a proportion of the affected tubular area to total tubular area (29).
At the end of the experiment, individual 24-h urine samples were collected. Urinary protein excretion from treated tissues was measured by a trichloroacetic acid (TCA) turbidimetric method (33) and N-acetyl-β glucosaminidase was measured spectrophotometrically (34).
The experimental procedures were approved by the local Animal Welfare Committee in accordance with the guidelines issued by the China Council on Animal Care.
Nitric Oxide Assay
Nitric oxide (NO) production from control and IR kidneys was evaluated by measuring nitrite using a chemiluminescence NO analyzer (Siever Instruments, Boulder, CO, USA) as described previously (35). The protein levels in each tube were quantified by bicinchoninic acid protein assay (Pierce) and were used as a basis to normalize the NO production.
Blood Free Heme Detection
Hemolysates were prepared by the method of Garrick et al. (36). The lysates were run through a 0.5 × 4 cm column (0.8 mL) of Dowex 1-X8 resin equilibrated in 2 mol/L NaCl and 5 mmol/L NaPO4, pH 7.4. Hemoglobin does not bind to the column under these conditions, but “free” heme does (36). The column was rinsed with 5× the bed volume of 2 mol/L NaCl and 5 mmol/L NaPO4, pH 7.4, to assure that all the hemoglobin had passed through, and then with 10× the bed volume of 5 mmol/L NaPO4, pH 7.4. The latter low salt rinse was done to avoid precipitation of SDS by high salt. “Free” heme was then eluted from the column with 10% SDS. Fractions of 900 μL were collected; 90 μL 100 mmol/L KCN was added to each fraction. A405 and A540 were read for each fraction. Elution of the column was continued until the A540 returned to background. The amount of “free” heme was calculated from the A540 by using an extinction coefficient of 11.1 or A405 = A540 × 8.15, providing greater sensitivity at the expense of a modest loss in precision and reproducibility (37).
Statistical Analysis
All quantitative real-time PCR and other determinations were repeated 3–5×, and typical results are presented and the SDs are shown. An independent (unpaired) Student t test (two-tailed) was chosen to test the significance of differences (P < 0.05) among means of small n sample sets.
All supplementary materials are available online at www.molmed.org.
RESULTS
Heme and Glucose Double Mammal Cell Total RNAs within 24 h
The 7% glucose or 50 μmol/L heme doubled HEK293 cell total RNAs within 24 h (Figure 1A). Considering that both glucose and heme induce ROS (Figure 1B), this increase may be caused by the side effect of ROS. However, 1 mmol/L H2O2 had no such effect, although H2O2 may cause the total RNA level to fluctuate (the error bars were a little large, see Figure 1A). Tumor necrosis factor (TNF)-α also induces great ROS accumulation (see Figure 1B) (38). However, with long-term treatment (over 24 h) with 10 ng/mL TNF-α, total cellular RNA content decreased (see Figure 1A), which may be due to the apoptotic death induced by TNF-α (38). Thus, glucose and heme may function as signaling molecules to induce an increase in RNA, independent of ROS.
Figure 1.
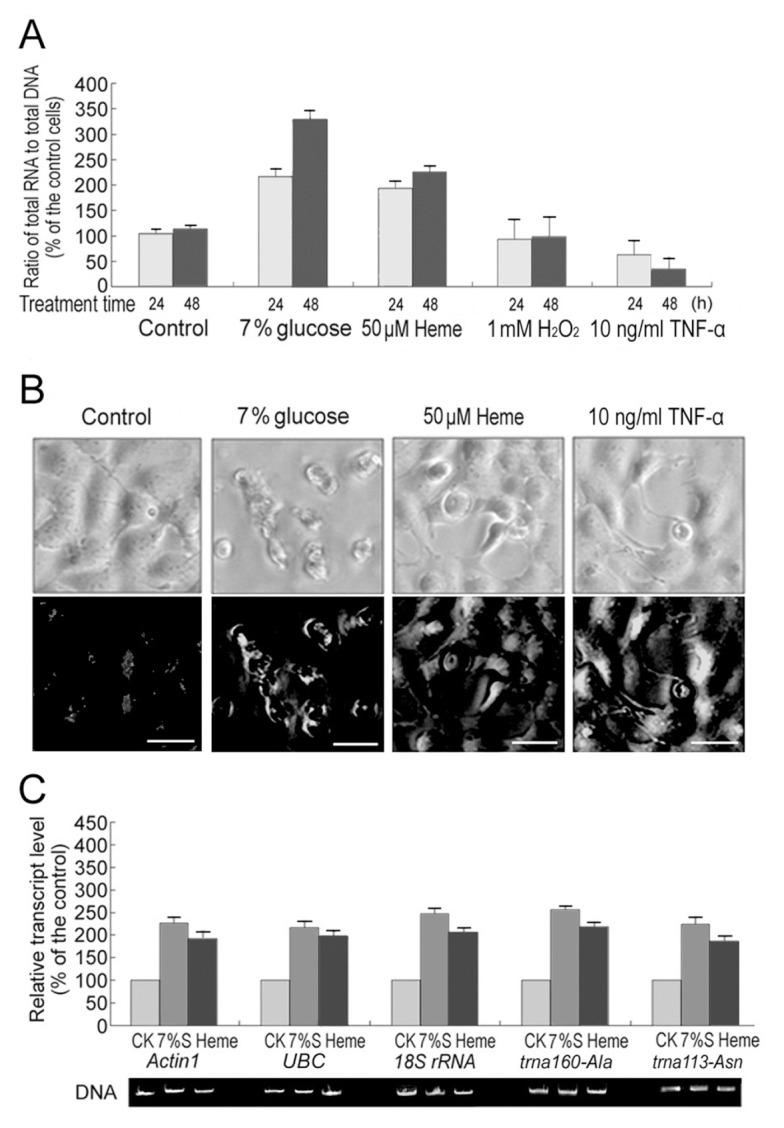
Glucose and heme induce RNA amplification in HEK293 cells. (A) The 7% glucose or 50 μmol/L heme but not 1 mmol/L H2O2 or 10 ng/mL TNF-α doubles HEK293 cell total RNAs within 24 h. (B) Cell forms (upper panel) and H2O2 levels (lower panel) of heme, glucose or TNF-α–treated cells (24 h). H2O2 was visualized by CM-DCFH-DA stain and observed with a fluorescence microscopy. (C) Effects of 7% glucose (7%S) and 50 μmol/L heme treatments (24 h) on Actin1 and UBC (two representative mRNAs), trna160-Ala and trna113-Asn (two representative tRNAs) and 18S rRNA expression. Gene expressing detection was derived on an equal DNA basis. Error bars show standard deviations (n = 3).
mRNAs, tRNA, rRNAs, snRNAs and snoRNAs were extracted from HEK293 cells separately. They were equally promoted by heme or glucose (Table 1). Their transcription rates were equally enhanced by the treatments (Table 2). Increases of mRNAs, tRNAs and rRNAs have been further confirmed by quantitative real-time PCR for two constitutively expressed mRNAs Actin1 and UBC, four representative tRNAs and 5S, 5.8S, 18S and 28S rRNAs (Figure 1C and Figure SM-1). Their polyribosome distribution was also not changed after glucose or heme treatments, although the total ribosome content increased (Table 1 and Figure 2). Correspondingly, the ribosomal proteins and the total cellular proteins were significantly increased, although not as large as total cellular RNAs (Table 2). From the data on polyribosome distribution and protein levels, we infer that the translation rate may not be changed, but the protein changes lagged behind the RNA changes.
Table 1.
mRNA, tRNA and rRNA changes in 293 cells after heme or 5% sucrose treatment for 48 h.a
| mRNA (μg/μg DNA) | mRNA/total RNAs (%) | tRNA (μg/μg DNA) | tRNA/total RNAs (%) | rRNA (μg/μg DNA) | rRNA/total RNAs (%) | P/T | |
|---|---|---|---|---|---|---|---|
| Control | 0.022 ± 0.003 | 2.8 ± 0.4 | 0.11 ± 0.02 | 14.1 ± 1.3 | 0.64 ± 0.05 | 83.1 ± 7.3 | 0.38 |
| 50 μmol/L Heme 48 h | 0.039 ± 0.005b | 2.9 ± 0.6 | 0.20 ± 0.03b | 14.5 ± 2.1 | 1.12 ± 0.12b | 82.6 ± 6.4 | 0.40 |
| 5% sucrose 48 h | 0.062 ± 0.008b | 2.5 ± 0.5 | 0.32 ± 0.05b | 12.7 ± 3.2 | 2.11 ± 0.26b | 84.8 ± 6.8 | 0.37 |
The data represent the mean with SE of at least five independent experiments. P/T, the ratio of polyribosomes to total ribosomes.
P < 0.05, t test; statistically significant difference between control and heme or sucrose-treated cells.
Table 2.
Relative RNA synthesis rates and protein contents (% of control) of 293 cells treated with heme or 5% sucrose for 48 h.a
| rRNA | snRNA | snoRNA | Large mRNA | Small mRNA | tRNA | Ribosomal proteins | Total proteins | |
|---|---|---|---|---|---|---|---|---|
| 50 μmol/L Heme 48 h | 182 ± 21b | 188 ± 24b | 179 ± 25b | 187 ± 27b | 184 ± 27b | 185 ± 23b | 158 ± 17b | 152 ± 15b |
| 5% sucrose 48 h | 274 ± 32b | 294 ± 28b | 282 ± 36b | 288 ± 30b | 291 ± 37b | 277 ± 27b | 195 ± 22b | 189 ± 19b |
Cells were incorporated with 32P-dCTP and then subjected to 50 μmol/L Heme or 5% sucrose treatment for 48 h. rRNA, snRNA, snoRNA, mRNA and tRNA were extracted, respectively, and separated in agarose gel and visualized by using an autoradiography instrumentation. Ribosomal protein and cell total protein contents were also determined immediately after the treatments. “Large mRNA” equals mRNAs longer than 1 kb; “Small mRNA” equals mRNAs shorter than 1 kb. The data represent the mean with SE of at least three independent experiments.
P < 0.05, t test; statistically significant difference between control and heme or sucrose-treated cells.
Figure 2.
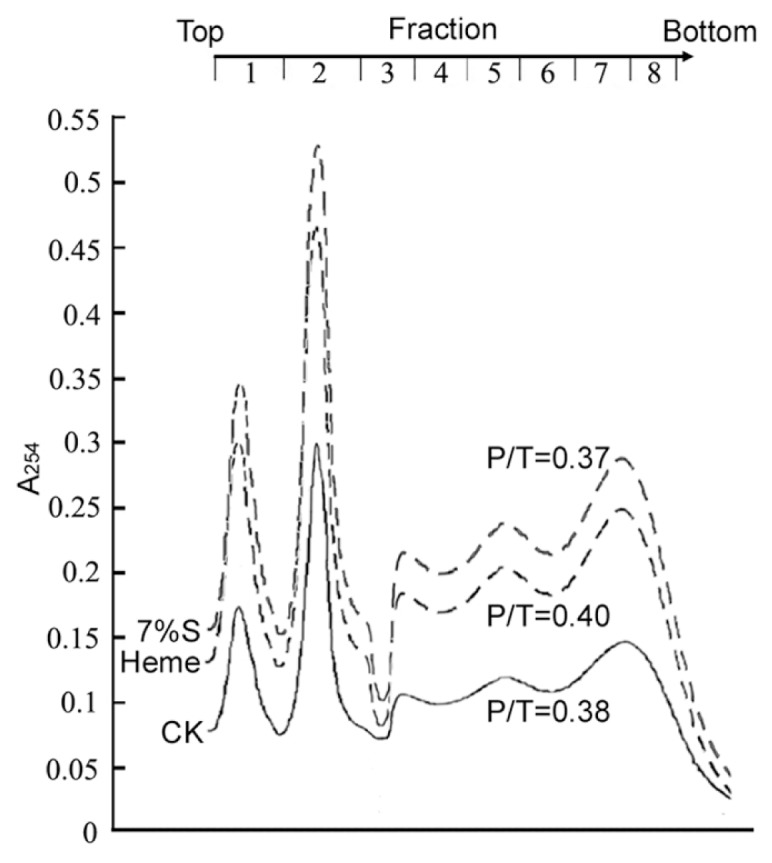
Effect of heme and glucose on polyribosome profiles. Ribonucleoprotein material was extracted from rats treated with or without 7% glucose (7%S) or 50 μmol/L heme for 24 h and was resolved on glucose gradients. Absorbance readings were at 254-nm polyribosomes (fractions 3–8) and the 40S and 60S ribosomal subunits (fractions 1 and 2). Dashed lines represent the sample after glucose or heme treatment. The top of the gradients is to the left. The ratios of polyribosomes to total ribosomes (P/T) are shown.
HEK293 epithelial cells were originally derived from HEK cell cultures. Renal epithelial cells live in a condition that glucose and heme may fluctuate violently and frequently (during nephritic filtration) (29). Thus, we chose HEK293 cells as a representative and valid model for our studies. As future evidence, rapid RNA amplification and ROS accumulation were reproduced in Chang liver cells (Figure SM-2).
TBP Mediates Rapid RNA Amplification in Mammal Cells
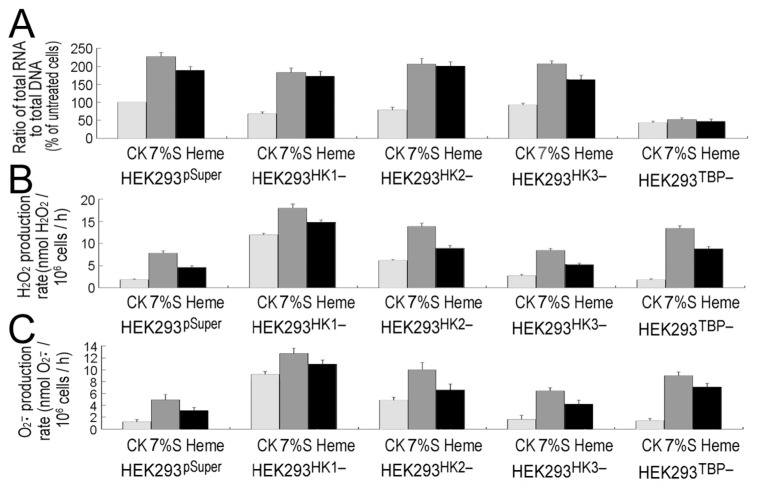
The factors mediating RNA amplification could be easily defined in mammalian cells. First, we wanted to identify possible glucose signaling–related factors. It is well known that transport and the first step of glucose utilization within the cells are catalyzed by hexokinase (EC 2.7.1.1), which participates in blood glucose homeostasis. In mammals, there are four isoforms of hexokinase (I, II and III are most important), differing in their affinities for glucose and inhibition by glucose-6-phosphate and inorganic phosphorus, as well as in their subcellular distribution (39,40). Therefore, the relationship between hexokinase and RNA amplification was investigated. Of the four isoforms of hexokinase (hexokinase-I to hexokinase-IV), three main hexokinase genes were examined: HK1, HK2 and HK3. Hexokinase-silenced HEK293 cell lines were generated by RNAi method, and testified by real-time PCR (Figure 3B) and Western blotting (Figure SM-3). HK1 mRNA was amplified with 26 cycles, HK2 with 29 cycles and HK3 with 32 cycles. Even by 32 cycles, the HK3 transcript still could not be detected apparently. Therefore, HK1 is the predominant isoform in HEK293 cells, and HK3 is the least isoform. Furthermore, among the hexokinase isoforms, only HK2 can be induced by glucose (Figure SM-3) (40). Cellular RNAs were similarly enhanced by glucose or heme in HEK293HK1−, HEK293HK2− or HEK293HK3− cells (Figure 3A), suggesting that mammal hexokinase is not involved in RNA amplification. A large part of glucose signals are sensed by hexokinase in plant cells (41); however, it has been suggested that hexokinase is unrelated to glucose signaling in mammal cells (40), which is consistent with our observations.
Figure 3.
Representative gene expression in control or silenced HEK293 cell lines. (A) The 7% glucose (7%S) and 50 μmol/L heme increase PRDX1, SOD, CAT, GPX and TBP expression but have no effect on the other six representative transcription factor genes in HEK293 cells. The treatment time was 24 h. Gene expression levels of the cells without any treatment (CK) are normalized to 100%. (B) HK1, HK2, HK3 and TBP expression levels in HEK293HK1−, HEK293HK2−, HEK293HK3− and HEK293TBP− gene-silenced cell lines and the HEK293pSuper control cell lines. Two independent gene-silenced cell lines for each gene were tested. Gene expression levels were detected by quantitative real-time PCR. Gene-expressing detection was performed with Actin1 transcript as a loading control. Error bars show standard deviations (n = 3).
Considering that all RNAs increased after heme/glucose treatment, one could speculate that RNA polymerase I, II and III and all polymerase activities are induced by the stress signals. Consequently, there should be some transcription factor response to all polymerases. Transcription factors belong to a large complex protein family; however, the number of transcription cofactors for all RNA polymerases is relatively small (42). TBP (42), cAMP response element–binding protein (CREB)-binding protein (CREBBP) and P300 are the only factors shared by all RNA polymerases (42). We selected TBP, CREBBP and P300, and some representative RNA polymerase I/II transcription factors, and tested their expression under heme/glucose treatment. The results showed that except for TBP, all other transcription factor transcripts could not be enhanced by heme or glucose (see Figure 3A). The role of TBP in total RNA amplification was further confirmed in HEK293TBP− cells. Total cellular RNAs were not increased by either 7% glucose or heme treatment in HEK293TBP− cells (Figure 4A), but the TBP transcript itself was enhanced by glucose or heme treatments (Figure SM-3).
Figure 4.
Effects of 7% glucose (7%S) and 50 μmol/L heme treatments (8 h) on total RNA levels (A), H2O2 (B) and superoxide (C) production rates of HEK293 HK1−, HEK293HK2−, HEK293HK3− and HEK293TBP− gene-silenced cell lines and the HEK293pSuper control cell lines. CK, untreated control cells. Error bars show standard deviations (n = 3).
Rapid RNA Amplification Helps Mammal Cells Protect against Heme/Glucose-Derived Oxidative Stress
What is the physiological significance of RNA amplification? Why do glucose and heme signals tightly correlate with cellular ROS levels? To answer these questions, we must pay attention to anti-oxidant genes. The increase in oxidative load can damage lipids, proteins and nucleic acids. A number of defensive mechanisms, including antioxidant vitamins, are available that allow scavenging of free radicals. The balance between ongoing oxidative stress and the antioxidant defense system determines the extent of oxidative damage in tissues (9,43,44). Expression of four representative anti-ROS genes in HEK293 cells was detected. Human PRDX1 (encoding peroxiredoxin I) (44), SOD (encoding a superoxide dismutase), CAT (encoding a catalase) and GPX (encoding a glutathione peroxidase) transcripts were increased two- to three-fold under the glucose or heme treatments (see Figure 3A). Considering that total cellular RNAs increased two- to three-fold, the SOD and CAT gene transcripts per cell increased four- to nine-fold.
The role of rapid RNA amplification in antioxidant adaptation of mammal cells has been further confirmed in HK-or TBP-silenced HEK293 cell lines. Although HEK293HK1− cells accumulated high levels of ROS under normal growth conditions (see “Discussion”), cellular ROS was similarly enhanced by glucose or heme in HEK293HK1−, HEK293HK2− or HEK293HK3− cells (Figure 4B, C). On the contrary, HEK293TBP− cells are more sensitive to high levels of glucose or free heme. Deficiency of rapid RNA amplification in HEK293TBP− cells would result in more severely oxidative stress (see Figure 4B, C).
Role of RNA Amplification in Diabetes/IR/Malaria-Induced Oxidative Stress Resistance
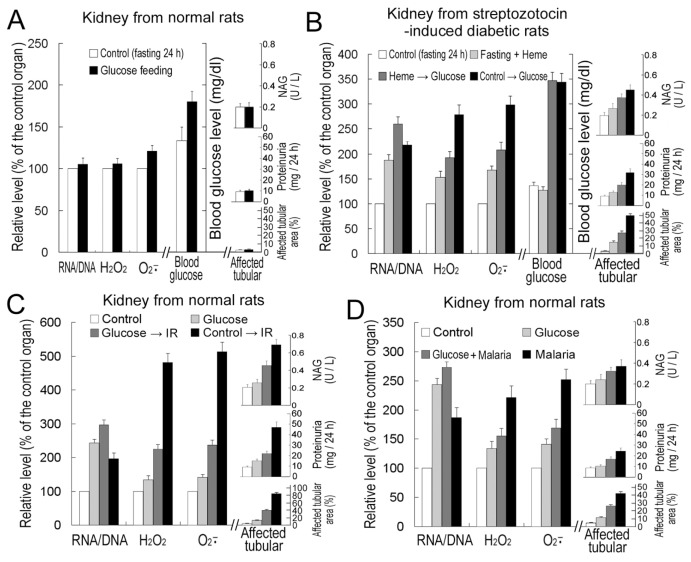
When diabetic rats (fasted 24 h before glucose feeding) were fed 30% glucose solution (every 8 h, 3× total), their blood glucose levels doubled (Figure 5B). Renal ischemia (20 min) and 24-h reperfusion caused similar damage to rat kidneys (indicated by greatly increased urinary protein excretion and N-acetyl-β glucosaminidase activity [Figure 5C], increased NO production [Figure SM-4] and large free-heme accumulation [Figure SM-5]). Eight-day Plasmodium infections resulted in hemolysis and erythrocyte parasitism (about 30% for rats, Figure SM-6), also causing great free-heme accumulation (Figure SM-5). All these stresses induced RNA amplification, ROS accumulation (Figure 5) and subsequently injuries to rat kidneys (tubular dilation, Figure SM-7).
Figure 5.
The role of RNA amplification in diabetes/IR/malaria-induced oxidative stress resistance of rat kidney cells. Kidney RNA/DNA ratio, H2O2 and superoxide levels and tubular damages (tubular dilation area [Figure SM-4], proteinuria level and N-acetyl-β glucosaminidase [NAG] level) and blood glucose levels in normal (A, C, D) and diabetic rats (B) were determined. The rat (fasted for 24 h for the “control” sample to diabetic experiments) kidneys were treated with or without 1 mmol/L heme or 30% glucose (renal arterial infusion), and then 30% glucose solution (every 8 h, 3× total) was administered orally or IR or malaria infection was performed. Samples were taken at 24 h after IR or glucose administration, or 8 d after malaria infection. The sample “glucose” means four times glucose infusion (every 2 d) without malaria infection. See “Materials and Methods” for details. Data in control samples are normalized to 100%. Error bars show standard deviations (n = 3).
The possible relationship between rapid RNA amplification and organs’ adaptation to diabetes, IR or malaria infection was further investigated. Pretreatment with heme in rat kidneys (renal arterial infusion 24 h before glucose feeding to double total cellular RNAs) alleviated subsequently oxidative damages caused by diabetes (Figure 5B). This result was not because heme infusion decreased the blood glucose level (see Figure 5B). Pretreatment with glucose in rat kidneys (to treble total cellular RNAs) alleviated subsequent oxidative damages caused by IR or malaria infection (Figure 5C, D); this result was not because glucose infusion inhibited heme releasing or erythrocyte parasitism (Figures SM-5 and SM-6).
DISCUSSION
Stress-induced hyperglycemia (such as head injury or acute stroke) and diabetes increased blood glucose substantially (6,7). Glucose-6-phosphate accumulation leads to great increases in mitochondrial membrane potential and ROS generation (1,45). If mitochondrial hexokinase is displaced from mitochondria, such as by RNAi in this report, then normal glucose levels would not be able to reduce membrane potential values and ROS production. Under this condition, mitochondria would rely only on other reactions to maintain ADP/ATP cycling. Thus, the endogenous antioxidant defenses present in these mitochondria could not be sufficient to scavenge all ROS generated upon the increase of membrane potential, establishing an oxidative stress situation (1,45,46). From Figure 3B, we know that hexokinase 1 (HK1) is the predominant isoform in HEK293 cells. Therefore, HEK293HK1− cells accumulated high levels of ROS under the normal growth condition. No matter what levels of ROS in hexokinase-silenced HEK293 cell lines, no isoform of hexokinases is involved in rapid RNA amplification or adaptation to oxidative stress induced by additional heme/glucose. The glucose signaling, especially the pathway to induce RNA amplification, needs further investigation.
Besides TBP, human CREBBP (also called CBP) and P300 are the other two transcription cofactors for all three RNA polymerases (47,48); however, they could not be enhanced by heme or glucose (see Figure 3A). Furthermore, no close homolog to human TBP, P300 or CREBBP could be found in the human genome. Thus, TBP is the only known candidate transcription cofactor responsible for rapid RNA amplification in mammal cells (Figure SM-8).
This rapid RNA amplification plays an important role in mammal cell’s resistance to diabetes/IR/malaria-induced oxidative stress. Heme or glucose pretreatment 24 hours before diabetes/IR/ malaria could double total cellular RNAs and subsequently alleviate oxidative damages significantly. Similar roles of hepatocellular glycogen in alleviation of liver IR injury (49) and heme in alleviation of heart IR injury (50) have been reported before, although they did not consider the role of cellular RNA amplification. When a cell encounters oxidative stress (such as high glucose or free heme to mammal cells), heme and glucose generate multiple signals to enhance all RNAs (coarse regulation) and prompt anti-stress gene expression simultaneously (fine regulation, Figure SM-8). Under the background that all RNAs were prompted, anti-ROS gene expression could be enhanced exponentially and quickly. Most of the stress-associated gene promoters should have the TATA box, and therefore they should be under the control of TBP and be enhanced by glucose/heme signals commonly. Considering that total cellular RNAs increased two- to three-fold, the antioxidant enzyme gene transcripts per cell were increased four- to nine-fold. On the other hand, this rapid RNA amplification may disperse the oxidative injuries to nucleic acids (oxidative damage per gram of RNA decrease), therefore providing a natural protective screen against oxidative stress. Deficiency of the RNA amplification causes a more severely oxidative situation. Such manipulation of RNAs may offer new direction for diabetic treatment, prevention of ischemia-reperfusion injury and curing malaria.
Supplemental Data
ACKNOWLEDGMENTS
We thank Dr. Xiao-Chao Xu (Sichuan University, China) for technical assistance with RNAi to HEK293 cells. This work was supported by the National Key Basic Research “973” Program of China (2009CB118500), National Nature Science Foundation of China (30970214 and 30800071).
Footnotes
Online address: http://www.molmed.org
Current affiliation: College of Biology and Science, Sichuan Agriculture University, Ya’an 625014, China.
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.da-Silva WS, et al. Mitochondrial bound hexokinase activity as a preventive antioxidant defense: steady-state ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J Biol Chem. 2004;279:39846–55. doi: 10.1074/jbc.M403835200. [DOI] [PubMed] [Google Scholar]
- 2.Negre-Salvayre A, et al. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal. 2009;11:3071–09. doi: 10.1089/ars.2009.2484. [DOI] [PubMed] [Google Scholar]
- 3.Nishikawa T, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 4.Abraham NG. Molecular regulation: biological role of heme in hematopoiesis. Blood Rev. 1991;5:19–28. doi: 10.1016/0268-960x(91)90004-v. [DOI] [PubMed] [Google Scholar]
- 5.Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157:175–88. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 6.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17:107–24. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2007;9:343–53. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- 8.Haga S, et al. Hepatic ischemia induced immediate oxidative stress after reperfusion and determined the severity of the reperfusion-induced damage. Antioxid Redox Signal. 2009;11:2563–72. doi: 10.1089/ars.2009.2681. [DOI] [PubMed] [Google Scholar]
- 9.Reddy MK, Labhasetwar V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia-reperfusion injury. FASEB J. 2009;23:1384–95. doi: 10.1096/fj.08-116947. [DOI] [PubMed] [Google Scholar]
- 10.Yuan S, Lin HH. Transcription, translation, degradation, and circadian clock. Biochem Biophys Res Commun. 2004;321:1–6. doi: 10.1016/j.bbrc.2004.06.093. [DOI] [PubMed] [Google Scholar]
- 11.Li JZ, et al. Cideb regulates diet-induced obesity, liver steatosis, and insulin sensitivity by controlling lipogenesis and fatty acid oxidation. Diabetes. 2007;56:2523–32. doi: 10.2337/db07-0040. [DOI] [PubMed] [Google Scholar]
- 12.Wang N, et al. Effects of ischemic postconditioning on reperfusion injury in rat liver grafts after orthotopic liver transplantation. Hepatol Res. 2009;39:382–90. doi: 10.1111/j.1872-034X.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- 13.Pelucchi B, et al. Nonspecific cation current associated with native polycystin-2 in HEK-293 cells. J Am Soc Nephrol. 2006;17:388–97. doi: 10.1681/ASN.2004121146. [DOI] [PubMed] [Google Scholar]
- 14.Chae S, et al. Centrosome amplification and multinuclear phenotypes are induced by hydrogen peroxide. Exp Mol Med. 2005;37:482–7. doi: 10.1038/emm.2005.59. [DOI] [PubMed] [Google Scholar]
- 15.Chappell DB, Zaks TZ, Rosenberg SA, Restifo NP. Human melanoma cells do not express Fas (Apo-1/CD95) ligand. Cancer Res. 1999;59:59–62. [PMC free article] [PubMed] [Google Scholar]
- 16.Backliwal G, Hildinger M, Hasija V, Wurm FM. High-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEI. Biotechnol Bioengin. 2007;99:721–7. doi: 10.1002/bit.21596. [DOI] [PubMed] [Google Scholar]
- 17.Riccio A, et al. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res. 2002;109:95–104. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- 18.Bawnik N, Beckmann JS, Sarid S, Daniel V. Isolation and nucleotide sequence of a plant tRNA gene: petunia asparagine tRNA. Nucleic Acids Res. 1983;25:1117–22. doi: 10.1093/nar/11.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zöphel D, Jansen HW, Reichel R, Benecke BJ. Isolation and characterization of snRNA and scRNA gene candidates from a human genomic library. Mol Biol Rep. 1983;9:59–64. doi: 10.1007/BF00777474. [DOI] [PubMed] [Google Scholar]
- 20.Lübben B, Marshallsay C, Rottmann N, Lührmann R. Isolation of U3 snoRNP from CHO cells: a novel 55 kDa protein binds to the central part of U3 snoRNA. Nucl. Acids Res. 1993;21:5377–85. doi: 10.1093/nar/21.23.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortimer SE, et al. IMP dehydrogenase type 1 associates with polyribosomes translating rhodopsin mRNA. J Biol Chem. 2008;283:36354–60. doi: 10.1074/jbc.M806143200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warner JR. Distribution of newly formed ribosomal proteins in HeLa cell fractions. J Cell Biol. 1979;80:767–72. doi: 10.1083/jcb.80.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson TR, et al. Newly synthesized RNA: simultaneous measurement in intact cells of transcription rates and RNA stability of insulin-like growth factor I, actin, and albumin in growth hormone-stimulated hepatocytes. Proc Natl Acad Sci U S A. 1991;88:5287–91. doi: 10.1073/pnas.88.12.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Zhang H, Lu W, Huang P. Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim Biophys Acta. 2009;1787:553–60. doi: 10.1016/j.bbabio.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holland MK, Storey BT. Oxygen metabolism of mammalian spermatozoa: generation of hydrogen peroxide by rabbit epididymal spermatozoa. Biochem J. 1981;198:273–80. doi: 10.1042/bj1980273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland MK, Alvarez JG, Storey BT. Production of superoxide and activity of superoxide dismutase in rabbit epididymal spermatozoa. Biol Reprod. 1982;27:1109–18. doi: 10.1095/biolreprod27.5.1109. [DOI] [PubMed] [Google Scholar]
- 27.Palomero J, et al. In situ detection and measurement of intracellular reactive oxygen species in single isolated mature skeletal muscle fibers by real time fluorescence microscopy. Antioxid Redox Signal. 2008;10:1463–74. doi: 10.1089/ars.2007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhee SG, Chang TS, Jeong W, Kang D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol. Cells. 2010;29:539–49. doi: 10.1007/s10059-010-0082-3. [DOI] [PubMed] [Google Scholar]
- 29.Mejía-Vilet JM, et al. Renal ischemia-reperfusion injury is prevented by the mineralo-corticoid receptor blocker spironolactone. Am J Physiol Renal Physiol. 2007;293:F78–86. doi: 10.1152/ajprenal.00077.2007. [DOI] [PubMed] [Google Scholar]
- 30.Hamza SM, Kaufman S. A vibrator prevents streaming during close-arterial infusion into the kidney. Am J Physiol Renal Physiol. 2004;286:F643–6. doi: 10.1152/ajprenal.00290.2003. [DOI] [PubMed] [Google Scholar]
- 31.Elias D, et al. Autoimmune diabetes induced by the beta-cell toxin STZ: immunity to the 60-kDa heat shock protein and to insulin. Diabetes. 1994;43:992–8. doi: 10.2337/diab.43.8.992. [DOI] [PubMed] [Google Scholar]
- 32.Favila-Castillo L, et al. Protection of rats against malaria by a transplanted immune spleen. Parasite Immunol. 1996;18:325–31. doi: 10.1046/j.1365-3024.1996.d01-117.x. [DOI] [PubMed] [Google Scholar]
- 33.Henry RJ, Sobel C, Segalove M. Turbidimetric determination of proteins with sulfosalicylic and trichloroacetic acid. Proc Soc Exp Biol Med. 1956;92:48–751. doi: 10.3181/00379727-92-22601. [DOI] [PubMed] [Google Scholar]
- 34.Wellwood JM, Price RG, Ellis BG, Thompson AE. A note on the practical aspects of the assay of N-acetyl-beta-glucosaminidase in human urine. Clin Chim Acta. 1976;69:85–91. doi: 10.1016/0009-8981(76)90475-7. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C, et al. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB J. 2001;15:1264–6. doi: 10.1096/fj.00-0681fje. [DOI] [PubMed] [Google Scholar]
- 36.Garrick MD, et al. Evidence for and consequences of chronic heme deficiency in Belgrade rat reticulocytes. Biochim Biophys Acta. 1999;1449:125–36. doi: 10.1016/s0167-4889(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 37.Liu SC, Zhai S, Palek J. Detection of hemin release during hemoglobin S denaturation. Blood. 1988;71:1755–8. [PubMed] [Google Scholar]
- 38.Lee JY, et al. Protective role of cytosolic 2-Cys peroxiredoxin in the TNF-α-induced apoptotic death of human cancer cells. Free Rad Biol Med. 2009;47:1162–71. doi: 10.1016/j.freeradbiomed.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 39.Russell JW, et al. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002;16:1738–48. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- 40.Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–57. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 41.Zhang ZW, et al. The plastid hexokinase pHXK: a node of convergence for sugar and plastid signals in Arabidopsis. FEBS Lett. 2010;584:3573–9. doi: 10.1016/j.febslet.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 42.Vaquerizas JM, Kummerfeld SK, Teichmann SA, Luscombe NM. A census of human transcription factors: function, expression and evolution. Nat Rev Genet. 2009;10:252–63. doi: 10.1038/nrg2538. [DOI] [PubMed] [Google Scholar]
- 43.Vento M, et al. Antenatal steroids and antioxidant enzyme activity in preterm infants: influence of gender and timing. Antioxid Redox Signal. 2009;11:2945–55. doi: 10.1089/ars.2009.2671. [DOI] [PubMed] [Google Scholar]
- 44.Woo HA, et al. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell. 2010;140:517–28. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Xu Y, et al. Glucose-6-phosphate dehydrogenase-deficient mice have increased renal oxidative stress and increased albuminuria. FASEB J. 2010;24:609–16. doi: 10.1096/fj.09-135731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan S, et al. Voltage-dependent anion channel 1 is involved in endostatin-induced endothelial cell apoptosis. FASEB J. 2008;22:2809–20. doi: 10.1096/fj.08-107417. [DOI] [PubMed] [Google Scholar]
- 47.Klejman MP, et al. Mutational analysis of BTAF1-TBP interaction: BTAF1 can rescue DNA-binding defective TBP mutants. Nucleic Acids Res. 2005;33:5426–36. doi: 10.1093/nar/gki850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi D, et al. CBP and p300 are cytoplasmic E4 polyubiquitin ligases for p53. Proc Natl Acad Sci U S A. 2009;106:16275–80. doi: 10.1073/pnas.0904305106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang LJ, Tian FZ, Tao W, Cui JF. Hepatocellular glycogen in alleviation of liver ischemia-reperfusion injury during partial hepatectomy. World J Surg. 2007;31:2039–43. doi: 10.1007/s00268-007-9186-0. [DOI] [PubMed] [Google Scholar]
- 50.Masini E, et al. Heme oxygenase-1 and the ischemia-reperfusion injury in the rat heart. Exp Biol Med. 2003;228:546–9. doi: 10.1177/15353702-0322805-25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.