Abstract
Synthetic derivatives of the microtubule-targeted agent maytansine, commonly known as drug maytansinoids or DMs, are emerging as potential cancer therapeutics. DM1 is an antibody-conjugatable maytansinoid that was developed to overcome systemic toxicity associated with maytansine and to enhance tumor-specific delivery. Antibody-DM1 conjugates showed promising results in preclinical and clinical evaluations. However, the molecular mechanism of the drug component DM1 was largely unknown. Recently, researchers have examined the mechanism of DM1 at molecular and cellular levels. According to their findings, DM1 binds at the tips of microtubules and suppresses the dynamicity of microtubules. The antibody-DM1 conjugate cleaves inside cells and releases the active drug in a time-dependent manner. The suppression of microtubule dynamics by DM1 induces mitotic arrest and cell death.
1. Introduction
1.1 Microtubules as drug targets
Microtubules are dynamic, polar polymers composed of αβ tubulin heterodimers arranged parallel to a cylindrical axis [1]. Several vital cellular processes depend directly or indirectly on the structural integrity and optimal functioning of microtubules [2]. For example, normal cell division requires formation of an intact mitotic spindle apparatus of the mitotic spindle apparatus and regulated dynamics of the component microtubules. Dynamic instability of microtubules, in other words the random length changes of microtubules, aids the accurate segregation of chromosomes during cell division and is fundamental to the optimal progression of the cell cycle [2]. The dynamic instability is regulated in cells by a variety of microtubule-interacting proteins such as the microtubule plus end tracking proteins (+TIPs; [1]) and G proteins [3]. Perturbations in the innate dynamic instability of microtubules deregulate the cell cycle and arrest cells at mitosis [2]. Therefore, drugs that suppress microtubule dynamics and thereby inhibit cancer cell proliferation are currently used in the clinic as effective anticancer agents for a wide variety of tumors [4]. By binding to microtubule tips or on the surface of the microtubules, these drugs suppress the normal dynamicity of microtubules and thereby induce cell-cycle arrest, inhibiting cell proliferation. Microtubule-targeted agents suppress the dynamic instability of microtubules at concentrations well below the concentration required to modify the polymer mass of microtubules [5].
1.2 Maytansine as a microtubule-targeted anticancer agent
Maytansine (Fig. 1) is an ansa macrolide first isolated from the plant maytenus ovatus by Kupchan et al. [6, 7]. It interacts with tubulin and microtubules and inhibits tubulin assembly into microtubules [8]. Maytansine has been reported to share its binding site with vinca alkaloids on tubulin [9]. Because it has the potential to target microtubules and arrest cell cycle progression, maytansine was evaluated for its clinical efficacy as a potential anticancer agent. In the late 1970s, the US National Cancer Institute evaluated the clinical efficacy of maytansine [10–14]. Patients with different types of cancers, including lymphoma and breast cancer, showed partial or complete responses. However, elevated toxic side effects, such as peripheral neuropathy, hampered maytansine’s progression as an anticancer drug [15]. In subsequent clinical trials also, researchers failed to obtain a clinically relevant outcome [16, 17]. The final clinical trial with maytansine was conducted to test its efficacy to regress advanced or recurrent adenocarcinoma of the cervix [18]. None of the patients treated with maytansine experienced promising results. Moreover, the patients suffered side effects such as myelosuppression [18]. Given these findings, researchers halted the clinical trials with maytansine.
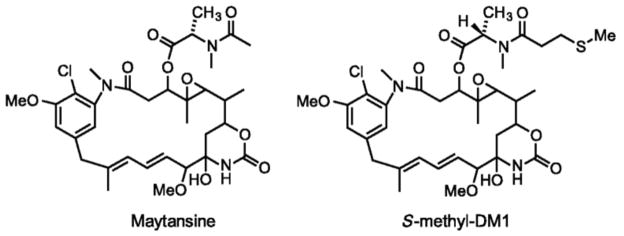
Fig. 1.
Structures of maytansine and the DM1 (S-methyl-DM1; [39]).
2. Development of novel, antibody-linkable maytansine analogs
For nearly a decade after the last clinical trial, no investigators considered using maytansine as an anticancer drug until a group at ImmunoGen Inc. developed synthetic derivatives of maytansine that can be conjugated to antibodies that target tumor-specific antigens [19]. The group synthesized derivatives of maytansine that possess 100- to 1000-fold higher cytotoxicity than the current anticancer drugs that are called drug maytansinoids or DMs [19]. By conjugating the maytansinoids with antibodies through disulfide-containing linkers that can be cleaved inside the cell to release the active drug, they revived interest in maytansine-derivative-based treatment.
2.1 Antibody-drug conjugates (ADCs)
An antibody-drug conjugates contains three distinct components, namely, the antibody, the linker that bonds the antibody with the drug, and the drug. In order to be effective, the ADC needs to be non-toxic until it reaches its target tumor cells. Once the ADC finds its target, it has to be activated.
2.1.1 The Antibody
Monoclonal antibodies that target tumor cell antigens are used in the treatment of a variety of tumors. In fact, there are treatment strategies based solely on antibodies, as these antibodies by themselves can be effective as anticancer agents. For example, trastuzumab, a monoclonal antibody that targets HER2 receptors, is used in the treatment of HER2+ breast cancers [20]. However, when patients developed resistance to these antibodies [21], researchers began looking for more effective therapeutic strategies. Although by themselves the antibodies are often ineffective for cancer treatment, their characteristic features, such as target specificity and high avidity binding to cancer cells, render them efficient carriers of drugs that kill cancer cells [22]. In addition, by “humanizing” the antibodies, host immune response can be circumvented [23].
2.1.2 The linker
A linker facilitates efficient conjugation of the drug to the antibody [24]. However, in order to be effective, the linker needs to be stable in the circulation, and it must be cleavable inside cancer cells [25]. Early studies evaluated acid-labile linkers that utilize the acidic environment of endosomes for cleavage [26]. However, subsequent studies found disulfide-based linkers to be a better choice because of their stability at physiological pH [25]. Moreover, the disulfide linkers utilize the concentration difference of glutathione inside cells and in circulation. The concentration of the tripeptide glutathione that cleaves disulfide bonds is a thousand-fold lower in the bloodstream than inside cancer cells [22]. Therefore, the linker is safe in the circulation and gets cleaved inside the cells.
2.1.3. The drug
In order to be an efficient component of ADC, the drug needs to be linkable to the antibody, it should be able to kill cancer cells with high potency, it should be sufficiently water soluble, and, like the linker, it should be stable in storage [25]. As maytansine alone does not possess many of these properties, derivatives of maytansines (drug maytansinoids or DMs) were developed, which are several times more potent than the parent compound and possess the other requirements mentioned above [27]. Among them, DM1 [N2’-deacetyl-N2’-(3-mercapto-1-oxopropyl)-maytansine] has been one of the most widely used drug candidates.
2.2 Antibody-DM1 conjugates
Antibody-DM1 conjugates are the most extensively studied of the antibody-maytansinoid conjugates. The first pre-clinical study of the antibody-DM1-conjugate was performed on colorectal cancer cells and on mice bearing human colon tumor xenografts [28]. The antibody-DM1 complex was prepared by conjugating DM1 to a colon-cancer specific monoclonal antibody C242, which recognizes CanAg, a mucin-type glycoprotein expressed on the surface of human colorectal cancer cells [28]. C242-DM1 was found to be effective against tumors in mice xenograft models, with complete tumor regression being observed. C242-DM1 progressed to clinical trials as cantuzumab-DM1 (huC242-DM1, cantuzumab mertansine), in which the C242 antibody was humanized [29]. Cantuzumab-DM1 was tested in patients with pancreatic and colorectal cancers [29]. According to the study, the ADC showed considerable tumor localization without inducing severe hematologic toxicity [29]. Anti-CD22-MCC-DM1 is an ADC in which DM1 is linked to an antibody that targets CD22, a siglec (which stands for sialic acid binding Ig-like lectins) family lectin expressed on mature B-cells. The ADC was found to be a promising agent for the treatment of non-Hodgkin's lymphoma [30]. Specifically, anti-CD22-MCC-DM1 demonstrated the ability to effectively inhibit the proliferation of several NHL B-cell lines in vitro. It also induced complete regression of tumors in xenograft mouse models [30]. In BB-10901, an ADC developed for the treatment of lung carcinoma, DM1 is linked to an antibody (huN901) that targets CD56, a homophilic glycoprotein expressed on a variety of tumors [31]. Another antibody-DM1 conjugate, MLN2704, contains MLN591, an antibody against the prostate-specific membrane antigen (PSMA), and was found to regress tumors in patients with progressive metastatic castration-resistant prostate cancer [32]. In addition, researchers are evaluating the maytansinoid-immunoconjugate IMGN901 that targets CD56, a neural cell adhesion molecule present on myeloma cells [33]. Trastuzumab is a humanized monoclonal antibody directed against domain IV of the extracellular domains of HER2 receptors [34]. Trastuzumab-DM1 is also undergoing clinical trials with promising results [35].
2.3 Other Antibody-maytansinoid conjugates
DM4 [N2’-deacetyl-N2’-(4-mercapto-4-methyl-1-oxopentyl) maytansine] is another antibody-conjugatable maytansinoid that showed promising results in vitro and in clinical settings. For example, IMGN388 is an antibody-DM4 conjugate in which DM4 linked to an antibody targeting αv integrin, an antigen expressed in a variety of solid tumors and on endothelial cells [36]. Thus, IMGN388 is a potential antitumor as well as antiangiogenic agent. Another antibody-DM4 conjugate, huMy9-6-DM4, targets CD33, a siglec family antigen expressed mostly on myeloid cells. huMy9-6-DM4 is undergoing clinical evaluation for the treatment of acute myeloid leukemia [37]. Like DM1, DM4 is also studied as the drug component of CanAg-targeted antibody huC242. huC242-DM4 was found to be effective in eradicating tumors in xenograft mouse models [37]. In BIIB015, DM4 is conjugated to an antibody that targets Cripto, an antigen that belongs to the EGF-CFC family of growth factor-like molecules, and that is expressed on several types of cancer cells, but is absent on normal cells [38].
3. Molecular Mechanism of Action of DM1
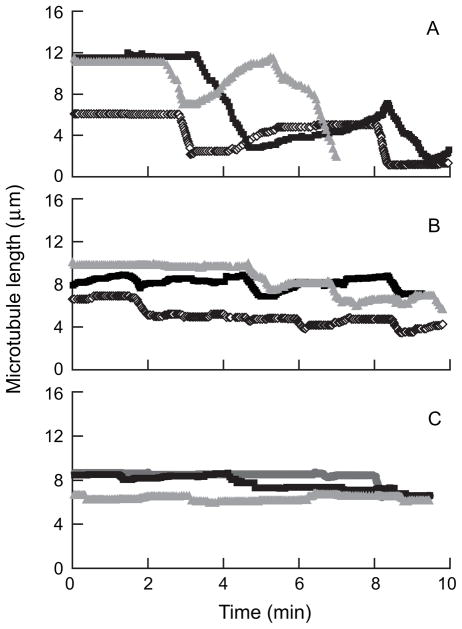
As indicated earlier, DM1 is a synthetic derivative of the tubulin-binding agent maytansine. Although maytansine is known to interact with microtubules, how maytansine and its synthetic derivatives inhibit cancer cell proliferation at sub-nanomolar concentrations remains unclear. Using a combination of biochemical and microscopy techniques, this author and colleagues [39] showed that maytansine and the DM1 (S-methyl-DM1; ((N2’-deacetyl-N2’-(3-thiomethyl-1-oxopropyl)-maytansine; Fig. 1) suppress microtubule dynamics at very low drug concentrations. Specifically, using video-enhanced differential interference contrast microscopy [40], they showed that maytansine as well as the DM1 derivative strongly suppress the dynamic instability parameters of microtubules assembled from MAP-free tubulin in vitro (Fig. 2). Maytansine and S-methyl-DM1 suppressed almost all dynamic instability parameters, including the growth rate, the shortening rate, the catastrophe frequency, and the rescue frequency, with the DM1 derivative showing stronger suppression of dynamics than the parent macrolide. The molecular mechanism of action of S-methyl-DM1 was found to be microtubule end poisoning. That is, S-methyl DM1 binds to the tips of microtubules and thereby inhibits the growth and the shortening of microtubules, leading to suppression of microtubule dynamics. Specifically, the maytansinoid showed high-affinity binding (KD, 0.1 μmol/L ) to approximately 37 sites per microtubule. The researchers also suggested that S-methyl-DM1 binds to high-affinity sites on microtubules 20 times more strongly than vinblastine and that the high-affinity binding of the maytansinoid at the tips might have suppressed the dynamics [39]. S-methyl DM1 is also reported to bind to soluble tubulin [39]. Although its affinity for soluble tubulin is approximately ten times less than that for microtubules, the possibility of tubulin-S-methyl-DM1 complex binding at the tips and thereby contributing to the suppression of microtubule dynamics cannot be ruled out.
Fig. 2.
Suppression of microtubule dynamics in vitro by maytansine and S-methyl-DM1 at 100 nM. Life history plots of microtubules assembled from purified bovine brain tubulin in the absence (A) and presence of 100 nM of maytansine (B) or S-methyl-DM1 (C) showing suppression of microtubule dynamics [39].
4. Cellular Mechanism of Action of the Antibody-DM1 conjugate
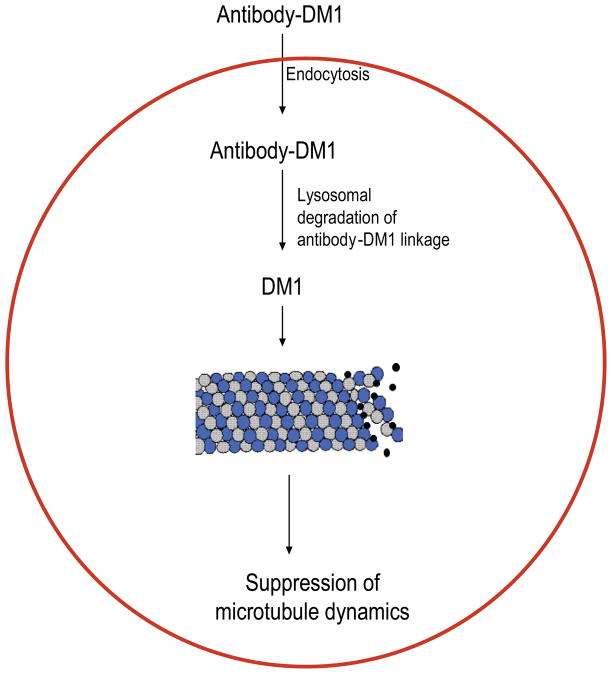
In an accompanying study, Oroudjev et al. [41] investigated the mechanism of action of antibody conjugated DM1 on cells and their effect on cellular microtubules. Maytansine and S-methyl-DM1 inhibited MCF7 cell proliferation [41]. The half-maximal inhibitory concentration for mitotic arrest (IC50) by maytansine was found to be 710 pM, and that of S-methyl DM1 was 330 pM. Both maytansine and S-methyl DM1 arrested cells at G2-M transition, with comparable IC50s (310 pM for maytansine and 340 pM for S-methyl DM1, [41]). In addition, both maytansine and S-methyl DM1 suppressed microtubule dynamics in cells as well (Fig. 3, [41]). To study the concentration and time-dependent effects of the antibody-DM1 conjugate on cancer cells, the authors used a cleavable anti-EpCAM-SPP-DM1 and an uncleavable anti-EpCAM-SMCC-DM1. The antibody anti-EpCAM was directed against the 40 KDa antigen called human epithelial adhesion molecule (EpCAM), a transmembrane glycoprotein expressed extensively in various carcinomas [42]. The study revealed that the antibody-DM1 conjugate enters the cells, undergoes lysosomal degradation, and with anti-EpCAM -SPP-DM1, the sulfhydryl bond that links the antibody with the drug, is cleaved and liberates the bound DM1. DM1 and its metabolites then suppress microtubule dynamics (Fig. 4). The authors found that lysine-linker- maytansinoid adduct (DM1-SPP-lysine) as the primary accumulated metabolites of the two conjugates [41]. Inside the cells, the DM1 strongly suppresses microtubule dynamics. For example, after 24 h of incubation with EpCAM-SPP-DM1, the overall dynamicity of microtubules was suppressed by 86% [41]. The researchers also noted that the suppression of microtubule dynamics by the maytansinoid showed qualitative and quantitative similarity in cells and in vitro with purified microtubules.
Fig. 3.
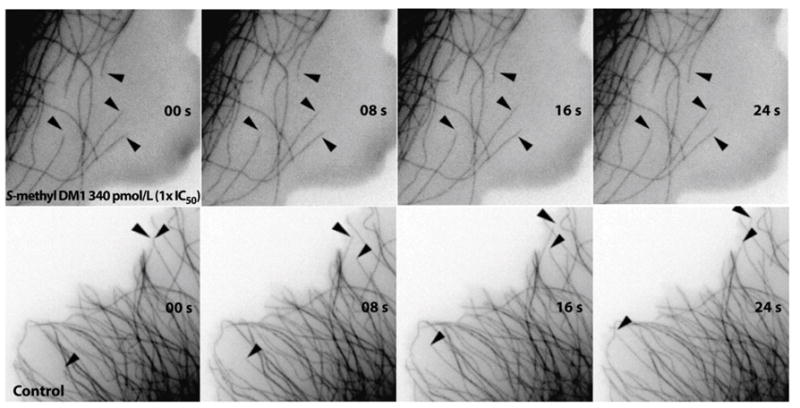
Suppression of microtubule dynamics in MCF7 cells by S-methyl-DM1. Time-lapse images of microtubules showing suppressed dynamics in the presence of S-methyl-DM1 (340 pM) [41].
Fig. 4.
Mechanism of action of Antbody-DM1 conjugates. The antibody-DM1 conjugate enters inside the cell by endocytosis. Inside the cells, the linker that bonds the antibody to DM1 is cleaved [22]. Free DM1 then binds at the tips of microtubules and suppress microtubule dynamics [39, 41].
5. Therapeutic Perspective
Antibody-DM1 conjugates are emerging as potential anticancer drugs and are under clinical evaluation. The stability of the conjugates in circulation, their tumor specificity, their high affinity for binding to target cells, their ability to become activated inside target cells, and the relatively high toxicity of the activated drug component make antibody-DM1 conjugates promising therapeutic agents. In addition to these qualities, Kovtun et al. found that antibody-DM1 conjugates linked via a reducible disulfide bond are effective against antigen positive and antigen negative cancer cells, when the antigen negative cells are within close proximity of the target tumor [43]. For instance, huC242-DM1 is known to induce such a “bystander effect” (that is, after intracellular processing of the antibody-DM1 conjugate, cells release free maytansinoids into the surrounding medium, which then kill cells that lie proximal to the target tumor cell) in several cancer cell lines in vitro and also in xenograft mouse models [43]. Moreover, efforts are underway to address the development of multidrug resistance to antibody-DM1 conjugates. On this front, novel linkers are being tested for their abilities to evade multidrug resistance strategies, including overexpression employed by the cells of the multidrug transporter MDR-1. For example, a recent study found that linking DM1 with tumor specific antibodies via maleimidyl-based hydrophilic linker PEG(4)Mal was able to overcome MDR-1 mediated drug resistance [44]. Specifically, antibody- PEG(4)Mal–DM1 treatment was shown to effectively eradicate human MDR-1 expressing xenograft tumors [44]. Current clinical trials of antibody-DM1 conjugates by the National Institute of Health in the United States include evaluation of the ADC as a monotherapy or a combinatorial therapy with existing anticancer agents. For example, two current phase I clinical studies are evaluating the safety, tolerability, pharmacokinetics, and efficacy of BB-10901 in patients with multiple myeloma [45] or solid tumors [46]. Several clinical trails using trastuzumab- DM1 are also underway [47–50]. For example, one study is evaluating the efficacy of trastuzumab-MCC-DM1 in combination with docetaxel in patients with HER2-positive metastatic breast cancer [47]. Successful outcomes of the clinical trials and enhanced understanding of the molecular mechanisms of the antibody-DM conjugates would facilitate approval of the antibody-DM1 conjugates as anticancer drugs.
Acknowledgments
Supported by a gift from ImmunoGen Inc. to Mary Ann Jordan and Leslie Wilson, and by USPHS grant NS13560 to Leslie Wilson. The author thanks Leslie Wilson, Mary Ann Jordan, and Silja Joseph for helpful suggestions.
Footnotes
Conflicts of interest Statement
None Declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lopus M, Yenjerla M, Wilson L. Microtubule Dynamics. In: Begley TP, editor. Wiley Encyclopedia of Chemical Biology. Vol. 3. Wiley; NJ: 2009. pp. 153–160. [Google Scholar]
- 2.Fojo AT. In The Role of Microtubules in Cell Biology, Neurobiology, and Oncology (Cancer Drug Discovery and Development) Humana Press; New York: 2009. [Google Scholar]
- 3.Dave RH, Saengsawang W, Lopus M, Dave S, Wilson L, Rasenick MM. A molecular and structural mechanism for G-protein mediated microtubule destabilization. J Biol Chem. 2011;286:4319–4328. doi: 10.1074/jbc.M110.196436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9:790–803. doi: 10.1038/nrd3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 6.Kupchan SM, Komoda Y, Court WA, Thomas GJ, Smith RM, Karim A, Gilmore CJ, Haltiwanger RC, Bryan RF. Maytansine, a novel antileukemic ansa macrolide from Maytenus ovatus. J Am Chem Soc. 1972;94:1354–1356. doi: 10.1021/ja00759a054. [DOI] [PubMed] [Google Scholar]
- 7.Kupchan SM, Komoda Y, Branfman AR, Sneden AT, Court WA, Thomas GJ, Hintz HP, Smith RM, Karim A, Howie GA, Verma AK, Nagao Y, Dailey RG, Jr, Zimmerly VA, Sumner WC., Jr The maytansinoids. Isolation, structural elucidation, and chemical interrelation of novel ansa macrolides. J Org Chem. 1977;42:2349–2357. doi: 10.1021/jo00434a001. [DOI] [PubMed] [Google Scholar]
- 8.Mandelbaum-Shavit F, Wolpert-DeFilippes MK, Johns DG. Binding of maytansine to rat brain tubulin. Biochem Biophys Res Commun. 1976;72:47–54. doi: 10.1016/0006-291x(76)90958-x. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya B, Wolff J. Maytansine binding to the vinblastine sites of tubulin. FEBS Lett. 1977;75:159–162. doi: 10.1016/0014-5793(77)80075-6. [DOI] [PubMed] [Google Scholar]
- 10.Blum RH, Kahlert T. Maytansine: a phase I study of an ansa macrolide with antitumor activity. Cancer Treat Rep. 1978;62:435–438. [PubMed] [Google Scholar]
- 11.Issell BF, Crooke ST. Maytansine. Cancer Treat Rev. 1978;5:199–207. doi: 10.1016/s0305-7372(78)80014-0. [DOI] [PubMed] [Google Scholar]
- 12.Chabner BA, Levine AS, Johnson BL, Young RC. Initial clinical trials of maytansine, an antitumor plant alkaloid. Cancer Treat Rep. 1978;62:429–433. [PubMed] [Google Scholar]
- 13.Eagan RT, Creagan ET, Ingle JN, Frytak S, Rubin J. Phase II evaluation of maytansine in patients with metastatic lung cancer. Cancer Treat Rep. 1978;62:1577–1579. [PubMed] [Google Scholar]
- 14.Cabanillas F, Rodriguez V, Hall SW, Burgess MA, Bodey GP, Freireich EJ. Phase I study of maytansine using a 3-day schedule. Cancer Treat Rep. 1978;62:425–428. [PubMed] [Google Scholar]
- 15.Cassady JM, Chan KK, Floss HG, Leistner E. Recent developments in the maytansinoid antitumor agents. Chem Pharm Bull (Tokyo) 2004;52:1–26. doi: 10.1248/cpb.52.1. [DOI] [PubMed] [Google Scholar]
- 16.Borden EC, Ash A, Enterline HT, Rosenbaum C, Laucius JF, Paul AR, Falkson G, Lerner H. Phase II evaluation of dibromodulcitol, ICRF-159, and maytansine for sarcomas. Am J Clin Oncol. 1982;5:417–420. doi: 10.1097/00000421-198208000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Ratanatharathorn V, Gad-el-Mawla N, Wilson HE, Bonnet JD, Rivkin SE, Mass R. Phase II evaluation of maytansine in refractory non-Hodgkin's lymphoma: a Southwest Oncology Group study. Cancer Treat Rep. 1982;66:1687–1688. [PubMed] [Google Scholar]
- 18.Thigpen JT, Ehrlich CE, Conroy J, Blessing JA. Phase II study of maytansine in the treatment of advanced or recurrent squamous cell carcinoma of the cervix. A Gynecologic Oncology Group study. Am J Clin Oncol. 1983;6:427–430. doi: 10.1097/00000421-198308000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Chari RV, Martell BA, Gross JL, Cook SB, Shah SA, Blättler WA, McKenzie SJ, Goldmacher VS. Immunoconjugates containing novel maytansinoids: promising anticancer drugs. Cancer Res. 1992;52:127–131. [PubMed] [Google Scholar]
- 20.Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 21.Valabrega G, Capellero S, Cavalloni G, Zaccarello G, Petrelli A, Migliardi G, Milani A, Peraldo-Neia C, Gammaitoni L, Sapino A, Pecchioni C, Moggio A, Giordano S, Aglietta M, Montemurro F. HER2-positive breast cancer cells resistant to trastuzumab and lapatinib lose reliance upon HER2 and are sensitive to the multitargeted kinase inhibitor sorafenib. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-1281-5. [DOI] [PubMed] [Google Scholar]
- 22.Chari RV. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc Chem Res. 2008;41:98–107. doi: 10.1021/ar700108g. [DOI] [PubMed] [Google Scholar]
- 23.Roguska MA, Pedersen JT, Keddy CA, Henry AH, Searle SJ, Lambert JM, Goldmacher VS, Blättler WA, Rees AR, Guild BC. Humanization of murine monoclonal antibodies through variable domain resurfacing. Proc Natl Acad Sci USA. 1994;91:969–973. doi: 10.1073/pnas.91.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietersz GA. The linkage of cytotoxic drugs to monoclonal antibodies for the treatment of cancer. Bioconjug Chem. 1990;1:89–95. doi: 10.1021/bc00002a001. [DOI] [PubMed] [Google Scholar]
- 25.Chari RV. Targeted delivery of chemotherapeutics: tumor-activated prodrug therapy. Adv Drug Deliv Rev. 1998;31:89–104. doi: 10.1016/s0169-409x(97)00095-1. [DOI] [PubMed] [Google Scholar]
- 26.Shen WC, Ryser HJP. cis-Aconityl spacer between daunomycin and macromolecular carriers: a model of pH-sensitive linkage releasing drug from a lysosomotropic conjugate. Biochem Biophys Res Commun. 1981;102:1048–1054. doi: 10.1016/0006-291x(81)91644-2. [DOI] [PubMed] [Google Scholar]
- 27.Widdison WC, Wilhelm SD, Cavanagh EE, Whiteman KR, Leece BA, Kovtun Y, Goldmacher VS, Xie H, Steeves RM, Lutz RJ, Zhao R, Wang L, Blättler WA, Chari RV. Semisynthetic maytansine analogues for the targeted treatment of cancer. J Med Chem. 2006;49:4392–4408. doi: 10.1021/jm060319f. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Tadayoni BM, Bourret LA, Mattocks KM, Derr SM, Widdison WC, Kedersha NL, Ariniello PD, Goldmacher VS, Lambert JM, Blättler WA, Chari RV. Eradication of large colon tumor xenografts by targeted delivery of maytansinoids. Proc Natl Acad Sci U S A. 1996;93:8618–8623. doi: 10.1073/pnas.93.16.8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolcher AW, Ochoa L, Hammond LA, Patnaik A, Edwards T, Takimoto C, Smith L, de Bono J, Schwartz G, Mays T, Jonak ZL, Johnson R, DeWitte M, Martino H, Audette C, Maes K, Chari RV, Lambert JM, Rowinsky EK. Cantuzumab mertansine, a maytansinoid immunoconjugate directed to the CanAg antigen: a phase I, pharmacokinetic, and biologic correlative study. J Clin Oncol. 2003;21:211–222. doi: 10.1200/JCO.2003.05.137. [DOI] [PubMed] [Google Scholar]
- 30.Polson AG, Williams M, Gray AM, Fuji RN, Poon KA, McBride J, Raab H, Januario T, Go M, Lau J, Yu SF, Du C, Fuh F, Tan C, Wu Y, Liang WC, Prabhu S, Stephan JP, Hongo JA, Dere RC, Deng R, Cullen M, de Tute R, Bennett F, Rawstron A, Jack A, Ebens A. Anti-CD22-MCC-DM1: an antibody-drug conjugate with a stable linker for the treatment of non-Hodgkin's lymphoma. Leukemia. 2010;24:1566–1573. doi: 10.1038/leu.2010.141. [DOI] [PubMed] [Google Scholar]
- 31.Fossella F, McCann J, Tolcher A, Xie H, Hwang L, Carr C, Berg K, Fram R. Phase II Trial of BB-10901 (huN901-DM1) given weekly for four consecutive weeks every 6 weeks in patients with relapsed SCLC and CD56-positive small cell carcinoma. JClin Oncology, ASCO Annual Meeting Proceedings. 2005 June 1;23(Supplement):7159. No. 16S, Part I of II. [Google Scholar]
- 32.Galsky MD, Eisenberger M, Moore-Cooper S, Kelly WK, Slovin SF, DeLaCruz A, Lee Y, Webb IJ, Scher HI. Phase I trial of the prostate-specific membrane antigen-directed immunoconjugate MLN2704 in patients with progressive metastatic castration-resistant prostate cancer. J Clin Oncol. 2008;26:2147–2154. doi: 10.1200/JCO.2007.15.0532. [DOI] [PubMed] [Google Scholar]
- 33.Lutz RJ, Whiteman KR. Antibody-maytansinoid conjugates for the treatment of myeloma. MAbs. 2009;1:548–551. doi: 10.4161/mabs.1.6.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldenberg MM. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther. 1999;21:309–318. doi: 10.1016/S0149-2918(00)88288-0. [DOI] [PubMed] [Google Scholar]
- 35.Isakoff SJ, Baselga J. Trastuzumab-DM1: Building a Chemotherapy-Free Road in the Treatment of Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer. J Clin Oncol. doi: 10.1200/JCO.2010.31.6679. [DOI] [PubMed] [Google Scholar]
- 36.Thompson D, Patnaik A, Bendell J, Papadopoulos K, Infante J, Carrigan C, Vyas V, Mastico RA, Johnson D, Qin A, O’Leary J, Tolcher AW. A phase I dose-escalation study of IMGN388 in patients with solid tumors. J Clin Oncol. 2010;28(suppl):15s. abstr 3058. [Google Scholar]
- 37.Lambert JM. Drug-conjugated monoclonal antibodies for the treatment of cancer. Curr Opin Pharmacol. 2005;5:543–549. doi: 10.1016/j.coph.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 38.http://www.cancer.gov/drugdictionary/?CdrID=596550
- 39.Lopus M, Oroudjev E, Wilson L, Wilhelm S, Widdison W, Chari R, Jordan MA. Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules. Mol Cancer Ther. 2010;9:2689–2699. doi: 10.1158/1535-7163.MCT-10-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yenjerla M, Lopus M, Wilson L. Analysis of dynamic instability of steady-state microtubules in vitro by video-enhanced differential interference contrast microscopy with an appendix by Emin Oroudjev. Methods Cell Biol. 2010;95:189–206. doi: 10.1016/S0091-679X(10)95011-5. [DOI] [PubMed] [Google Scholar]
- 41.Oroudjev E, Lopus M, Wilson L, Audette C, Provenzano C, Erickson H, Kovtun Y, Chari R, Jordan MA. Maytansinoid-antibody conjugates induce mitotic arrest by suppressing microtubule dynamic instability. Mol Cancer Ther. 2010;9:2700–2713. doi: 10.1158/1535-7163.MCT-10-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Litvinov SV, Velders MP, Bakker HA, Fleuren GJ, Warnaar SO. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol. 1994;125:437–446. doi: 10.1083/jcb.125.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, Leece BA, Chittenden T, Blättler WA, Goldmacher VS. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006;66:3214–3221. doi: 10.1158/0008-5472.CAN-05-3973. [DOI] [PubMed] [Google Scholar]
- 44.Kovtun YV, Audette CA, Mayo MF, Jones GE, Doherty H, Maloney EK, Erickson HK, Sun X, Wilhelm S, Ab O, Lai KC, Widdison WC, Kellogg B, Johnson H, Pinkas J, Lutz RJ, Singh R, Goldmacher VS, Chari RV. Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 2010;70:2528–2537. doi: 10.1158/0008-5472.CAN-09-3546. [DOI] [PubMed] [Google Scholar]
- 45.BB-10901 in Treating Patients With Relapsed and/or Refractory Multiple Myeloma. www.clinicaltrials.gov (ID: NCT00346255)
- 46.BB-10901 in Treating Patients With Relapsed or Refractory Solid Tumors. www.clinicaltrials.gov (ID: NCT00346385)
- 47.A Study of Trastuzumab-MCC-DM1 (T-DM1) in Combination With Docetaxel in Patients With Advanced Breast Cancer. www.clinicaltrials.gov (ID: NCT00934856)
- 48.A Study of Trastuzumab-DM1 Plus Pertuzumab Versus Trastuzumab [Herceptin] Plus a Taxane in Patients With Metastatic Breast Cancer (MARIANNE) www.clinicaltrials.gov (ID: NCT01120184)
- 49.A Study of Trastuzumab-DM1 (T-DM1) Sequentially With Anthracycline-Based Chemotherapy, As Adjuvant or Neoadjuvant Therapy for Patients With Early Stage HER2-Positive Breast Cancer. www.clinicaltrials.gov (ID: NCT01196052)
- 50.A Study of Trastuzumab-MCC-DM1, Paclitaxel, and Pertuzumab in Patients With HER2-Positive, Locally Advanced or Metastatic Breast Cancer. www.clinicaltrials.gov (ID: NCT00951665)