Abstract
OBJECTIVES
To investigate a single institution experience with radical retropubic prostatectomy (RRP), laparoscopic radical prostatectomy (LRP) and robot-assisted radical prostatectomy (RARP) with respect to pathological and biochemical outcomes.
SUBJECTS AND METHODS
A group of 522 consecutive patients who underwent RARP between 2003 and 2008 were matched by propensity scoring on the basis of patient age, race, preoperative prostate-specific antigen (PSA), biopsy Gleason score and clinical stage with an equal number of patients who underwent LRP and RRP at our institution. Pathological and biochemical outcomes of the three cohorts were examined.
RESULTS
Overall positive surgical margin rates were lower among patients who underwent RRP (14.4%) and LRP (13.0%) compared to patients who underwent RARP (19.5%) (P = 0.010). There were no statistically significant differences in positive margin rates between the three surgical techniques for pT2 disease (P = 0.264). In multivariate logistic regression analysis, surgical technique (P = 0.016), biopsy Gleason score (P < 0.001) and preoperative PSA (P < 0.001) were predictors of positive surgical margins. Kaplan–Meier analysis did not show any statistically significant differences with respect to biochemical recurrence for the three surgical groups.
CONCLUSIONS
RRP, LRP and RARP represent effective surgical approaches for the treatment for clinically localized prostate cancer. A higher overall positive SM rate was observed for the RARP group compared to RRP and LRP; however, there was no difference with respect to biochemical recurrence-free survival between groups. Further prospective studies are warranted to determine whether any particular technique is superior with regard to long-term clinical outcomes.
Keywords: prostate cancer, biochemical recurrence, radical prostatectomy, robotic prostatectomy, laparoscopic prostatectomy
INTRODUCTION
Prostate cancer is the most frequently diagnosed cancer among men in the USA. In 2008, it is estimated that 186 320 new cases of prostate cancer will be diagnosed and 28 660 patients will die of the disease [1]. The widespread use of PSA screening and increased public awareness regarding the early detection of prostate cancer has led to a stage migration towards lower stage disease [2]. Patients with localized prostate cancer are candidates for surgery, radiation or active surveillance. Typically, younger men with localized prostate cancer and an extended life expectancy choose surgery for treatment. The gold standard for surgical treatment of prostate cancer is radical retropubic prostatectomy (RRP), with excellent success rates for postoperative continence and erectile function [3].
Over the past decade, minimally-invasive approaches for surgical management of prostate cancer have become increasingly utilized. In 2006, it was estimated that approximately 35% of all radical prostatectomies were performed using a minimally-invasive approach that was either laparoscopically or robotically assisted [4]. The evolution of surgical technique from pure laparoscopic to robotic prostatectomy has been attributed in part to the steep learning curve associated with laparoscopic radical prostatectomy (LRP). Robotic-assisted surgical techniques incorporate features such as three-dimensional viewing, improved ergonomics, hand tremor elimination and refined dexterity [5].
There is a limited amount information available with regard to oncological outcomes associated with robotic prostatectomy because this technique has been in use for less than a decade [6]. Furthermore, there is a limited body of literature comparing open RRP with minimally-invasive approaches and a lack of data directly comparing all three techniques (RRP vs LRP vs RALP). We investigated the impact of surgical technique (open vs laparoscopic vs robotic-assisted) at one institution on pathological and biochemical outcomes following radical prostatectomy utilizing propensity score matching to adjust for multiple preoperative variables.
SUBJECTS AND METHODS
Between 2000 and 2008, more than 9000 men underwent radical prostatectomy for clinically localized adenocarcinoma of the prostate at our institution. Because robot-assisted radical prostatectomy (RARP) was the newest technique with the most limited patient numbers, this group was chosen as the reference dataset for patient matching. Men treated with neoadjuvant hormonal therapy, clinical stage T1a or T1b disease or incomplete preoperative information, including preoperative PSA, clinical stage and biopsy Gleason score, were excluded from analysis. This resulted in 522 men who underwent RARP from 2003 to 2008 who were matched 1 : 1 to men who underwent LRP and RRP from 2000 to 2008 based on propensity scores. We only included patients who were operated on by surgeons who completed their urological surgical training within the past decade. This selection was carried out in an attempt to normalize surgeon experience because LRP and RARP surgeons were younger than most RRP surgeons and therefore had less experience. A total of 1566 men formed the overall study population. All data were collected under an Internal Review Board-approved protocol with Health Insurance Portability and Accountability Act compliance. All patients provided their informed consent when indicated by the Institutional Review Board.
A central pathology laboratory performed all biopsy grading used for propensity scoring between surgical treatment groups. Prostatectomy specimens were processed and reviewed per standard institutional protocol at the two campuses where the surgeries were performed: one for RRP and RARP (John Hopkins Hospital) and one for LRP (John Hopkins Bayview Medical Campus).
Propensity scores were used to match patients in each of the three surgical cohorts based on a range of previously described characteristics [7,8]. Propensity scores were calculated for each patient using multivariable logistic regression based upon the covariates: age, race, preoperative PSA level, biopsy Gleason score and clinical tumour stage. Patients were not matched for the year of surgery because they were exclusively operated on during the same treatment era.
The method of propensity score matching is an approach to control for imbalances in confounding factors among discrete study cohorts. Continuous and categorical factors are combined to yield a propensity score for each individual in the study population. Individuals in each of the different study cohorts are matched to patients in the reference cohort based upon their calculated propensity scores. The greatest advantage of implementing this method of matching is that variables are weighted by their relative importance, rather than being assigned equal weights. Furthermore, it has been shown that cohort means and standard deviations related to covariates used for matching are equivalent when composite propensity scores are matched. Matching was performed with a computer application implemented in SPSS (SPSS Inc., Chicago, IL, USA) to select for the most similar propensity scores across each of the different surgical strata in a 1 : 1 ratio with respect to the reference group of patients who underwent RARP.
We compared the clinical and pathological characteristics of the three surgical cohorts using chi-squared for categorical variables and one-way ANOVA for continuous variables. Patient age, preoperative PSA values and year of surgery were evaluated as continuous variables. Clinical stage, biopsy and prostatectomy Gleason score (≥6, 7, 8–10) and race (white or black) were considered categorical variables.
Univariate and multivariable logistic regression models were then performed to examine the relationship between clinical features and the likelihood of positive surgical margins in the radical prostatectomy specimens. We adjusted for surgeon experience by incorporating the number of cases performed by each surgeon at entry into this series stratified as: <50 cases, 51–200 cases and >200 cases. The actuarial risk of PSA recurrence was calculated using the Kaplan–Meier method and compared across the three surgical cohorts using the log-rank test. All statistical analyses were performed using SPSS, version 15.0.
RESULTS
Clinical and pathological characteristics of the three matched study cohorts separately listed for matched and unmatched variables are shown in Table 1. Mean ± SD follow-up was 2.5 ± 1.6, 1.4 ± 0.7 and 1.3 ± 0.6 years for the RRP, LRP and RARP cohorts, respectively. There were no statistically significant differences between the three surgical cohorts with respect to the variables used for propensity score matching, including patient age at the time of surgery, race, preoperative PSA level, biopsy Gleason score and clinical tumour stage.
TABLE 1.
Clinicopathological characteristics of matched radical retropubic prostatectomy (RRP), laparoscopic radical prostatectomy (LRP) and robot-assisted radical prostatectomy (RARP) patients
| Characteristic | Surgical technique |
P* | ||
|---|---|---|---|---|
| RRP | LRP | RARP | ||
| Patients (n) | 522 | 522 | 522 | |
| Mean follow-up (years) | 2.5 ± 1.6 | 1.4 ± 0.7 | 1.3 ± 0.6 | |
| Matched variables | ||||
| Age (years) | 0.496‡KW | |||
| Mean ± SD | 58.8 ± 6.1 | 58.4 ± 6.4 | 58.3 ± 6.3 | |
| Median | 59 | 59 | 59 | |
| Range | 40–73 | 40–77 | 35–77 | |
| Race | 0.135 | |||
| Caucasian | 454 (87.0) | 429 (82.2) | 435 (83.3) | |
| African-American | 46 (8.8) | 72 (13.8) | 62 (11.9) | |
| Other | 22 (4.2) | 21 (4.0) | 25 (4.8) | |
| PSA (ng/mL) | 0.929‡KW | |||
| Mean ± SD | 5.4 ± 3.2 | 5.4 ± 2.7 | 5.4 ± 3.2 | |
| Median | 4.9 | 4.8 | 4.8 | |
| Range | 0.2–35.6 | 0.3–26.0 | 0.2–37.9 | |
| Biopsy Gleason score (%) | 0.192‡ | |||
| ≥6 | 371 (71.1) | 390 (74.7) | 394 (75.5) | |
| 7 | 140 (26.8) | 114 (21.8) | 114 (21.8) | |
| 8–9 | 11 (2.1) | 18 (3.4) | 14 (2.7) | |
| Clinical stage (%) | 0.778‡ | |||
| cT1 | 423 (81.0) | 414 (79.3) | 417 (79.9) | |
| cT2 | 99 (19.0) | 108 (20.7) | 105 (20.1) | |
| Unmatched variables | ||||
| Pathology weight (g) | <0.0001KW | |||
| Mean ± SD | 54 ± 17 | 52 ± 18 | 49 ± 16 | |
| Median | 51 | 48 | 46 | |
| Range | 12–150 | 13–130 | 15–150 | |
| Prostatectomy Gleason score (%) | <0.001 | |||
| ≥6 | 316 (60.5) | 380 (72.8) | 306 (58.6) | |
| 7 | 177 (33.9) | 122 (23.4) | 188 (36.0) | |
| 8–9 | 29 (5.6) | 20 (3.8) | 28 (5.4) | |
| Extraprostatic extension | 158 (30.3) | 82 (15.7) | 136 (26.1) | <0.001 |
| Seminal vesicle invasion | 18 (3.4) | 10 (1.9) | 17 (3.3) | 0.271 |
| Lymph node invasion | 5 (1.0) | 1 (0.2) | 0 (0) | 0.030 |
| Positive surgical margin | 75 (14.4) | 68 (13.0) | 102 (19.5) | 0.010 |
Indicates test for comparison among the three age cohorts. All tests are chi-squared, unless stated otherwise.
Indicates matched variables. KW, Kruskal–Wallis test.
Examination of postoperative variables showed significant differences between the surgical cohorts. RRP patients exhibited higher mean pathology weight compared to LRP and RARP patients (P < 0.0001). LRP patients had significantly higher proportions of Gleason 6 and lower proportions of Gleason 8–9 on final pathology compared to RRP and RARP patients (P < 0.001). Patients who underwent RRP had higher rates of extraprostatic extension compared to LARP and RARP (P < 0.001). Additionally, patients who underwent RRP had higher rates of lymph node invasion compared to patients who underwent LRP and RARP (P = 0.030). There was a higher overall positive surgical margin rate for RARP compared to RRP and LRP (P = 0.010). There were no differences with respect to seminal vesicle invasion between the three surgical groups (P = 0.271).
Table 2 shows the positive surgical margin rate for each surgical cohort stratified by pathological stage (pT2 vs pT3). For pT2 disease, there were no statistically significant differences with respect positive surgical margin rates between the three groups (P = 0.264). However, patients with pT3 disease who underwent LRP and RARP had higher positive surgical margin rates compared to the RRP cohort (P = 0.013).
TABLE 2.
Positive surgical margin (SM) rates of radical retropubic prostatectomy (RRP), laparoscopic radical prostatectomy (LRP) and robot-assisted radical prostatectomy (RARP) patients stratified by pathological tumour stage
| Pathological stage | Surgical group |
P* | |||
|---|---|---|---|---|---|
| SM | RRP | LRP | RARP | ||
| pT2 n (%) | SM positive | 24 (6.6) | 29 (6.7) | 36 (9.3) | 0.264 |
| pT3 n (%) | SM positive | 51 (32.1) | 39 (43.8) | 66 (48.5) | 0.013 |
| All patients n (%) | SM positive | 75 (14.4) | 68 (13.0) | 102 (19.5) | 0.010 |
Indicates test for comparison among the three surgical groups. All tests are chi-squared.
Table 3 shows the association of preoperative variables in predicting positive surgical margins following radical prostatectomy. In univariate analysis, surgical technique (P = 0.010), biopsy Gleason score (P < 0.001) and preoperative PSA (P < 0.001) were predictors of positive surgical margins following radical prostatectomy. There was no association between age, race and clinical stage with surgical margin status in univariate analysis. There was a trend towards lower positive surgical margin rates with increased number of cases performed on entry into this study (P = 0.070). In multivariable analysis, surgical technique (P = 0.016), biopsy Gleason score (P < 0.001) and preoperative PSA (P < 0.001) remained independent predictors of a positive surgical margin.
TABLE 3.
Univariate and multivariable logistic regression models of preoperative clinical variables predicting positive surgical margins following radical prostatectomy
| Variable | Univariate analyses |
Multivariable analyses |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Surgical technique | 0.010 | 0.016 | ||||
| RRP | 1.00 (reference) | 1.00 (reference) | ||||
| LRP | 0.89 | 0.63–1.27 | 0.529 | 1.10 | 0.65–1.86 | 0.731 |
| RARP | 1.45 | 1.04–2.00 | 0.025 | 1.64 | 1.06–2.53 | 0.026 |
| Age | 1.02 | 0.99–1.04 | 0.137 | 1.01 | 0.99–1.03 | 0.462 |
| Race | 0.692 | 0.909 | ||||
| Caucasian | 1.00 (reference) | 1.00 (reference) | ||||
| African-American | 1.20 | 0.79–1.80 | 0.396 | 1.10 | 0.72–1.68 | 0.674 |
| Other | 1.07 | 0.55–2.07 | 0.850 | 0.97 | 0.50–1.91 | 0.937 |
| Biopsy Gleason score | <0.001 | <0.001 | ||||
| ≥6 | 1.00 (reference) | 1.00 (reference) | ||||
| 7 | 1.98 | 1.48–2.66 | <0.001 | 1.84 | 1.35–2.52 | <0.001 |
| 8–10 | 0.86 | 0.33–2.21 | 0.746 | 0.68 | 0.25–1.82 | 0.438 |
| Preoperative PSA | 1.10 | 1.05–1.14 | <0.001 | 1.09 | 1.04–1.14 | <0.001 |
| Clinical stage T2 relative to T1 | 1.07 | 0.76–1.50 | 0.703 | 1.01 | 0.71–1.44 | 0.959 |
| Number of cases performed | 0.070 | 0.440 | ||||
| ≥50 cases | 1.00 (reference) | 1.00 (reference) | ||||
| >50 cases | 1.37 | 1.00–1.87 | 0.048 | 1.29 | 0.86–1.93 | 0.220 |
| >200 cases | 0.87 | 0.57–1.32 | 0.509 | 1.11 | 0.61–2.03 | 0.729 |
LRP, laparoscopic radical prostatectomy; OR, odds ratio; RRP, retropubic prostatectomy; RARP, robot-assisted radical prostatectomy.
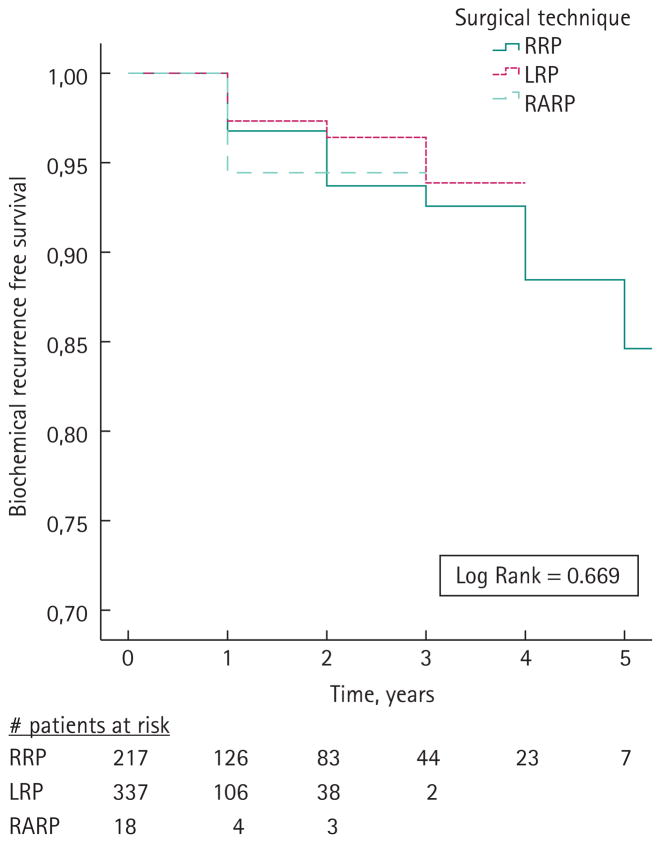
Kaplan–Meier analysis gave biochemical recurrence-free survival rates of 93%, 94%, and 94% for RRP, LRP and RARP patients, respectively (Fig. 1). There was no statistically significant difference in biochemical-free survival across the three surgical cohorts (log-rank P = 0.669). Univariate and multivariable proportional hazard analyses for the prediction of biochemical recurrence are shown in Table 4. Univariate analysis showed a significant association between preoperative PSA (P = 0.020), prostatectomy Gleason score (P < 0.001), pathological stage (P < 0.001) and positive surgical margin status (P = 0.001) with biochemical recurrence. In multivariable analysis adjusted for significant variables from univariate analyses and surgical technique, prostatectomy Gleason score (P = 0.001) and pathological stage (P = 0.004) remained independent predictors of biochemical recurrence.
FIG. 1.
Biochemical recurrence-free survival following radical prostatectomy. LRP, laparoscopic radical prostatectomy; RARP, robot-assisted radical prostatectomy; RRP, radical retropubic prostatectomy.
TABLE 4.
Univariate and multivariable proportional hazards models for risk of biochemical recurrence following radical prostatectomy
| Variable | Univariate analyses |
Multivariable analyses |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Surgical technique | 0.669 | 0.479 | ||||
| RRP | 1.00 (reference) | 1.00 (reference) | ||||
| LRP | 0.73 | 0.32–1.67 | 0.459 | 1.75 | 0.69–4.42 | 0.232 |
| RARP | 1.39 | 1.18–10.86 | 0.750 | 1.02 | 0.13–8.36 | 0.979 |
| Age | 1.02 | 0.96–1.08 | 0.537 | |||
| Race | 0.119 | |||||
| Caucasian | 1.00 (reference) | |||||
| African-American | 2.19 | 0.87–5.51 | 0.094 | |||
| Other | 2.93 | 0.68–12.66 | 0.149 | |||
| Prostatectomy Gleason score | <0.001 | 0.001 | ||||
| ≥6 | 1.00 (reference) | 1.00 (reference) | ||||
| 7 | 5.44 | 2.17–13.66 | <0.001 | 3.35 | 1.27–8.83 | 0.015 |
| 8–10 | 16.54 | 5.78–47.36 | <0.001 | 9.98 | 3.07–32.42 | <0.001 |
| Preoperative PSA | 1.10 | 1.01–1.17 | 0.020 | 1.05 | 0.96–1.15 | 0.254 |
| Pathological stage | <0.001 | 0.004 | ||||
| Organ-confined disease | 1.00 (reference) | 1.00 (reference) | ||||
| Extraprostatic extension | 6.26 | 2.62–14.94 | <0.001 | 3.70 | 1.45–9.44 | 0.006 |
| Seminal vessicle invasion | 19.86 | 5.94–66.37 | <0.001 | 7.87 | 2.03–30.48 | 0.003 |
| Lymph node metastasis | 20.58 | 2.56–165.61 | 0.004 | 14.40 | 1.62–128.23 | 0.017 |
| Positive surgical margin | 3.57 | 1.62–7.83 | 0.001 | 2.11 | 0.90–4.92 | 0.085 |
| Number of cases performed | 0.716 | |||||
| ≥50 cases | 1.00 (reference) | |||||
| >50 cases | 1.21 | 0.47–3.10 | 0.679 | |||
| >200 cases | 1.48 | 0.57–3.90 | 0.419 | |||
HR, hazard ratio; LRP, laparoscopic radical prostatectomy; RRP, retropubic prostatectomy; RARP, robot-assisted radical prostatectomy.
DISCUSSION
Open RRP remains the standard of care for surgical treatment of prostate cancer worldwide. Minimally-invasive approaches such as LRP and RARP are becoming increasingly popular as a result of extensive marketing and patient preference. Minimally-invasive techniques for treatment of prostate cancer have been associated with decreased blood loss, less postoperative pain and shorter hospitalization [9]. LRP is a challenging technique because of its lengthy learning curve, ergonomics associated with instrumentation, and the requirement for expertise in laparoscopic surgery [10]. RARP is more attractive to many surgeons compared to LRP as a result of the more rapid transfer of open-surgery skills to a minimally-invasive setting, three-dimensional vision, six degrees of freedom, downscaling of movements and elimination of physiological tremor [11–13].
To our knowledge, this is the first study to investigate the impact of all three surgical techniques (RRP vs LRP vs RARP) on pathological and clinical outcomes following radical prostatectomy. We found that surgical technique was an independent predictor of positive surgical margin rate, with RARP being associated with a higher overall positive surgical margin rate compared to RRP and LRP. However, this finding did not translate into a higher risk of biochemical recurrence at short-term follow-up. Laurila et al. [14]. compared pathological outcomes of patients who underwent RRP and RARP. They showed that pathological outcomes (i.e. specifically positive surgical margin rates) did not differ between the two groups. Because of the significantly higher rates of patients with high-risk disease in their RRP cohort, their margin analysis was limited to low- and intermediate-risk patients. They found a positive surgical margin rate of 14% after RRP and 13% after RALP [13]. In the present study, we observed overall positive surgical margin rates of 14.4%, 13.0% and 19.5% for RRP, LRP and RALP, respectively. Although the positive surgical margin rates in the present study were similar for RRP compared to the series of Laurila et al. [00], the proportion of patients with overall positive margins in the present study was higher for the RARP cohort compared to RRP and LRP (P = 0.010). This observation could be explained by differences in study design, patient selection, or surgeon experience for each technique.
In the present study, RARP was performed by multiple surgeons at various levels of experience and many of these cases were performed during the learning curve associated with RARP. Although there is no consensus regarding the number of cases required to overcome the learning curve for LRP or RARP, Herrell et al. [15] suggested that at least 150 RARP cases were required to obtain results that were comparable to those routinely obtained with RRP. Greater surgeon experience was associated with a lower risk of 5-year biochemical recurrence following surgery in a recent study examining the learning curve of LRP [16]. The LRP learning curve was found to be flatter than RRP and >250 cases may be required to achieve optimal outcomes with LRP.
The majority of surgeons at our institution who performed RRP had extensive surgical experience with open procedures, whereas minimally-invasive approaches were primarily performed by younger urologists earlier in their learning curve. We attempted to control for this variable by including only surgeons who completed their urology training within the past 10 years. Despite this, however, many of the minimally-invasive surgeons included in the present study had performed <150 RARP cases. Taken together, variances in surgical experience likely explain at least in part the difference found in positive margin rates between surgical techniques.
In another study, Rozet et al. [17] reported no statistically significant differences with respect to pathological outcomes and complication rates for LRP vs RARP among patients with comparable preoperative characteristics. Interestingly, their positive surgical margin rate was similar to those observed in the present study: 19.5% for RARP and 15.8% for LRP compared to 19.5% and 13.0% in the present series, respectively. Ahlering et al. [18] have previously compared single surgeon RRP and RARP pathological outcomes and complication rates. Positive surgical margin rates were 16.7% for RARP and 20% for RRP. In a study performed by Smith et al. [19], the overall incidence of positive surgical margins was lower for RARP compared to RRP (15% vs 35%) and the positive margin rate for pT2 disease was 9.4% and. 24.1% for RARP and RRP, respectively.
Berryhill et al. [5] published an extensive review comparing RARP with LRP and open surgery and reported weighted mean positive margin rates of 12.5%, 19.6% and 23.5% for RARP, LRP and RRP, respectively. This study was limited by selection bias and may not reflect lower positive margin rates usually achieved by experienced surgeons. For example, Hernandez et al. [20] reported data that experienced surgeons have a positive surgical margin rate of less than 6%, even when there is extraprostatic extension in the region of the neurovascular bundle.
One important issue that has not been previously addressed by other studies comparing RRP with LRP or RARP is the comparison of positive margin rate separately for pT2- and pT3 disease. In our series, the positive margin rate did not statistically differ for pT2 disease when comparing the three techniques. However, there were higher positive surgical margins rates associated with minimally-invasive techniques (LRP and RARP) compared to RRP when analysis was confined to patients with stage pT3 disease. One explanation for this finding may be that, for locally advanced disease, tactile feedback can provide important information with respect to resection margins and intra-operative decision-making. Another explanation may be related to the learning curve associated with LRP and RARP in our cohort of patients.
There are limitations to the present study. First, we did not investigate functional outcomes and perioperative complication rates associated with the three surgical techniques. However, this has been the subject of previous studies and was not the primary goal of the present study [21–23]. Second, follow-up for biochemical outcome was limited; extended follow-up was not available because of the relatively newer utilization of RARP for treatment of prostate cancer compared to RRP. Third, a selection bias may be present for the type of surgical technique offered to each patient, as well as for the different patient referral patterns that may exist for each surgeon. Only a randomized study incorporating all three surgical approaches for radical prostatectomy would be likely to produce better matching of preoperative variables.
In conclusion, RRP, LRP and RARP produce excellent pathological and biochemical outcomes. In the propensity score matched cohorts of the present study, the overall positive SM rate was higher for the RARP group (19.5%) compared to LRP (13.0%) and RRP (14.4%) but did not differ significantly with regard to pT2 disease. The higher positive surgical margin rate for RARP was not associated with worse biochemical recurrence-free outcomes with limited follow-up. Further prospective studies with more extended follow-up are warranted to determine whether RRP and LRP remain comparable to RRP with respect to long-term clinical outcomes.
Acknowledgments
This study was supported by the National Institutes of Health/National Cancer Institute SPORE Grant P50CA58236.
Abbreviations
- LRP
laparoscopic radical prostatectomy
- RARP
robot-assisted radical prostatectomy
- RRP
radical retropubic prostatectomy
Footnotes
CONFLICT OF INTEREST
None declared.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Han M, Partin AW, Chan DY, Walsh PC. An evaluation of the decreasing incidence of positive surgical margins in a large retropubic prostatectomy series. J Urol. 2004;171:23–6. doi: 10.1097/01.ju.0000098604.09395.27. [DOI] [PubMed] [Google Scholar]
- 3.Walsh PC. Nerve sparing radical prostatectomy for early stage prostate cancer. Semin Oncol. 1988;15:351–8. [PubMed] [Google Scholar]
- 4.Zorn KC, Gofrit ON, Orvieto MA, et al. Da Vinci robot error and failure rates: single institution experience on a single three-arm robot unit of more than 700 consecutive robot-assisted laparoscopic radical prostatectomies. J Endourol. 2007;21:1341–4. doi: 10.1089/end.2006.0455. [DOI] [PubMed] [Google Scholar]
- 5.Berryhill R, Jr, Jhaveri J, Yadav R, et al. Robotic prostatectomy: a review of outcomes compared with laparoscopic and open approaches. Urology. 2008;72:15–23. doi: 10.1016/j.urology.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 6.Binder J, Kramer W. Robotically-assisted laparoscopic radical prostatectomy. BJU Int. 2001;87:408–10. doi: 10.1046/j.1464-410x.2001.00115.x. [DOI] [PubMed] [Google Scholar]
- 7.Rubin DB. On principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf. 2004;13:855–7. doi: 10.1002/pds.968. [DOI] [PubMed] [Google Scholar]
- 8.Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. 1996;52:249–64. [PubMed] [Google Scholar]
- 9.Guillonneau B, Cathelineau X, Doublet JD, Baumert H, Vallancien G. Laparoscopic radical prostatectomy: assessment after 550 procedures. Crit Rev Oncol Hematol. 2002;43:123–33. doi: 10.1016/s1040-8428(02)00024-0. [DOI] [PubMed] [Google Scholar]
- 10.Descazeaud A, Peyromaure M, Zerbib M. Will robotic surgery become the gold standard for radical prostatectomy? Eur Urol. 2007;51:9–11. doi: 10.1016/j.eururo.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Ahlering TE, Skarecky D, Lee D, Clayman RV. Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: initial experience with laparoscopic radical prostatectomy. J Urol. 2003;170:1738–41. doi: 10.1097/01.ju.0000092881.24608.5e. [DOI] [PubMed] [Google Scholar]
- 12.Rocco B, Djavan B. Robotic prostatectomy: facts or fiction? Lancet. 2007;369:723–4. doi: 10.1016/S0140-6736(07)60336-5. [DOI] [PubMed] [Google Scholar]
- 13.Gamboa AJ, Santos RT, Sargent ER, et al. Long-term impact of a robot assisted laparoscopic prostatectomy mini fellowship training program on postgraduate urological practice patterns. J Urol. 2009;181:778–82. doi: 10.1016/j.juro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Laurila TA, Huang W, Jarrad DF. Robotic-assisted laparascopic and radical retropublic prostatectomy generate similar positive margin rates in low and intermediate risk patients. Urol Oncol. 2008;27:529–33. doi: 10.1016/j.urolonc.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Herrell SD, Smith JA., Jr Robotic-assisted laparoscopic prostatectomy: what is the learning curve? Urology. 2005;66 (5 Suppl):105–7. doi: 10.1016/j.urology.2005.06.084. [DOI] [PubMed] [Google Scholar]
- 16.Vickers AJ, Savage CJ, Hruza M, et al. The surgical learning curve for laparoscopic radical prostatectomy: a retrospective cohort study. Lancet Oncol. 2009;10:475–80. doi: 10.1016/S1470-2045(09)70079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rozet F, Harmon J, Cathelineau X, Barret E, Vallancien G. Robot-assisted versus pure laparoscopic radical prostatectomy. World J Urol. 2006;24:171–9. doi: 10.1007/s00345-006-0065-3. [DOI] [PubMed] [Google Scholar]
- 18.Ahlering TE, Woo D, Eichel L, Lee DI, Edwards R, Skarecky DW. Robot-assisted versus open radical prostatectomy: a comparison of one surgeon’s outcomes. Urology. 2004;63:819–22. doi: 10.1016/j.urology.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Smith JA, Jr, Chan RC, Chang SS, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007;178:2385–9. doi: 10.1016/j.juro.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez DJ, Epstein JI, Trock BJ, Tsuzuki T, Carter HB, Walsh PC. Radical retropubic prostatectomy. How often do experienced surgeons have positive surgical margins when there is extraprostatic extension in the region of the neurovascular bundle? J Urol. 2005;173:446–9. doi: 10.1097/01.ju.0000151135.80249.c9. [DOI] [PubMed] [Google Scholar]
- 21.Menon M, Muhletaler F, Campos M, Peabody JO. Assessment of early continence after reconstruction of the periprostatic tissues in patients undergoing computer assisted (robotic) prostatectomy: results of a 2 group parallel randomized controlled trial. J Urol. 2008;180:1018–23. doi: 10.1016/j.juro.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 22.Wagner AA, Link RE, Trock BJ, Sullivan W, Pavlovich CP. Comparison of open and laparoscopic radical prostatectomy outcomes from a surgeon’s early experience. Urology. 2007;70:667–71. doi: 10.1016/j.urology.2007.06.1104. [DOI] [PubMed] [Google Scholar]
- 23.Lein M, Stibane I, Mansour R, et al. Complications, urinary continence, and oncologic outcome of 1000 laparoscopic transperitoneal radical prostatectomies-experience at the Charite Hospital Berlin, Campus Mitte. Eur Urol. 2006;50:1278–82. doi: 10.1016/j.eururo.2006.06.023. [DOI] [PubMed] [Google Scholar]