Abstract
Background
Trauma is a leading cause of death and although the gut is recognized as the “motor” of post-traumatic systemic inflammatory response syndrome and multiple organ failure, studies on the gastrointestinal tract are few. Our objectives were to create a precisely controllable tissue injury model in which gastrointestinal motility, systemic inflammation and wound fluid can be analyzed.
Methods
A non-narcotic murine trauma model was developed by the subcutaneous dorsal trans-implantation of a devitalized donor syngeneic harvested tissue-bone matrix (TBX), which was precisely adjusted to % total body weight and studied after 21 hrs. Gastrointestinal transit histograms were plotted after the oral administration of non-digestible FITC-dextran and geometric centers calculated. Organ bath evaluated jejunal circular muscle contractility. Multiplex electrochemiluminescence measurements of serum and TBX wound fluid inflammatory mediators were performed.
Key Results
Increasing TBX amounts progressively delayed transit, whereas TBX heat denaturation or decellularization prevented ileus and death. In the TBX17.5% model, jejunal muscle contractility was suppressed and a systemic inflammatory response developed as significant serum elevations in IL-6, keratinocyte cytokine and IL-10 compared to sham. Additionally, inflammatory responses within the wound fluid showed elevated levels of preformed IL-1β and TNF-α, whereas, 21 hours after implantation IL-1β, IL-6 and keratinocyte cytokine were significantly increased in the wound.
Conclusions & Inferences
A novel donor tissue-bone matrix trauma model was developed that is precisely adjustable and recapitulates important clinical phenomena. The non-narcotic model demonstrated that increasing tissue injury progressively caused ileus, initiated a systemic inflammatory response and developed inflammatory changes within the wound.
Keywords: trauma, inflammation, ileus, tissue injury, wound fluid, gastrointestinal motility
INTRODUCTION
Trauma is the most common cause of death among individuals between 1 and 45 years of age in the US and the main cause of early mortality among the population worldwide. Yearly, nearly 40 million people suffer from a traumatic injury with over 150,000 deaths occurring in the United States with motor vehicle accidents being the most common cause. Additionally, traumatic injury is all too frequently a consequence of active military combat service and acts of civilian terror. Annually, health care costs directly related to trauma are approximately $117 billion and only heart disease ranks higher. Thus, the human, medical, and financial toll of trauma is staggering making the elucidation of the underlying pathophysiological mechanisms of trauma crucial to effective therapeutic intervention (CDC Injury Factbook - 2006).
Blast-related injury as a result of improvised explosive devices is the main mechanism of injury on the modern battlefield and in civilian acts of terror. The emergence of advanced body and vehicular armor has shifted the anatomy of wounding to the extremities, with nearly 80% of personnel wounded during combat or acts of terror having one or more of their extremities injured. High-energy blast wounds are notoriously complex, characterized by massive zones of limb and life-threatening injury and gross contamination by bacteria, devitalized tissue, and retained metal and composite materials. Treatments near the traumatic incident, the rapid evacuation of casualties and utilization of advanced medical technologies have improved the initial survival of service members during wartime operations. The overall impact of these systematic advances has been an unparalleled increase the number of casualties with devastating wounds, which progress to developing a systemic inflammatory response, gastrointestinal ileus, vasodilatation, increased microvascular permeability, fluid sequestration, generalized tissue hypoxia, activation of clotting mechanisms, and microcirculatory plugging leading to remote organ damage.
Traumatic injury triggers a highly complex cascade of acute metabolic, endocrine, and immune responses that involve recruitment of platelets, coagulation factors, neutrophils, B cells and T cells to the site of injury, modulated by a profound system-wide release of circulating pro-inflammatory cytokines. From a teleological perspective, the ultimate outcome of the patient is governed by the ability to achieve a delicate balance in pro-inflammatory and anti-inflammatory host responses. When there is disequilibrium resulting in an exaggerated production of pro-inflammatory circulating cytokines, the systemic inflammatory response syndrome (SIRS) emerges, which can lead to multiple organ dysfunction syndrome (MODS) with a mortality rate of nearly a third in severely wounded patients. Moreover, a systemic inflammatory response may result in a maladaptive compensatory anti-inflammatory response syndrome (CARS) with its characteristic increased risk of infection associated with CARS-related immunosuppression (1).
Cytokine-mediated dysregulation of the inflammatory response to severe trauma is a well recognized component of SIRS and MODS. There is little doubt that the gut plays a determining role in the pathophysiology of the body’s response to trauma(2). The gut appears to be particularly vulnerable to developing dysfunctional activity, including gastric stasis and severe gastrointestinal ileus. This is clinically evident by the general inability to enterally feed the severely injured trauma patient which subsequently plays a major detrimental role in limiting vital nutritional support for the critically ill. Moreover, the trauma induced gut stasis leads to bacterial overgrowth and disruption of the mucosal barrier resulting in bacterial translocation and systemic infection. This complication further incites an infections systemic inflammatory response through massive cytokine release, which further exacerbates local enteric dysfunction and potentiates remote organ dysfunction threatening the hosts ability to survive (3).
Presently, the mechanisms which cause severe gastrointestinal stasis after peripheral traumatic injury are virtually unknown. Mechanistic insights into traumatic shock have been gained through models of systemic hemorrhage combined with laparotomy. But the subsequent molecular and functional responses in this model are appropriately interpreted as a modulation of the ischemia/reperfusion response by minor tissue injury. Short term studies have used systemic hemorrhage combined with femur fracture, but after resuscitation there is an obligatory use of analgesics in these models, which confounds the interpretation of the mechanistic data. Unfortunately the use of analgesics introduces a myriad of incalculable pharmacological effects on immune, lung and gastrointestinal functions. Additionally, current experimental models consisting of soft tissue injury and orthopaedic fractures are difficult to standardize, and the notoriously confounding variable of pain is very challenging to mechanistically isolate (4). Furthermore, it is problematic to analyze and determine the role of wound fluid biomarkers in these models, as importantly the wound precipitates SIRS and MODS.
Currently, there is no reliable, precisely adjustable resuscitation animal model of peripheral traumatic injury in which to investigate the molecular mechanisms which cause severe gastrointestinal stasis. Therefore, the objective of this study was to develop a novel peripheral tissue trauma prototype that would overcome the limitations and confounding variables of existing models and which would begin to elucidate the molecular and functional mechanisms which cause gastrointestinal stasis. We demonstrate in this manuscript that this can be successfully accomplished by the subcutaneous dorsal trans-implantation of a syngeneic harvested tissue-bone matrix (TBX) into a naïve host mouse.
METHODS
Tissue-Bone Matrix Model and Groups
The experimental design used in this study was approved by the Institutional Animal Use and Care Committee of the University of Pittsburgh. Male C57/BL6 mice (Jackson Laboratories, Bar Harbor, ME, age, 8–10 wks; weight 20 to 30 g) were used. The trauma model consisted of the subcutaneous dorsal trans-implantation of a devitalized syngeneic tissue-bone matrix (TBX = minced rib cage, long bones and overlying skeletal muscle) into a subcutaneous pouch covering most of the back of a naïve recipient mouse anesthetized with isofluorane inhalation (3%) through a small cutaneous dorsal/caudal slit of 1 cm. The TBX was prepared by the sterile harvesting and blade mincing of the skinless legs and rib cage of a syngeneic donor mouse in a 5% penicillin/streptomycin antibiotic solution (% of harvested TBX weight). The sterility of the TBX was microscopically confirmed at the time point of implantation and at sacrifice by tissue culturing a 25 mg sample of the engrafted TBX in DMEM for 24 hours. We constructed eight groups of 4–6 animals each for this study: unoperated control, TBX17.5% sham and recipients receiving TBX5%, 10%, 15%, 17.5%, 20% and 25% (% body weight). TBX sham animals consisted of anesthesia and a subcutaneous installation into a back pouch of a volume matched penicillin/streptomycin antibiotic solution 5% into the dorsal subcutaneous space. Additionally, groups of mice were trans-implanted with heat inactivated TBX17.5% by heating the TBX to 90° C for 2 hours before implantation or with mild peracetic acid decellularization of the TBX20%. Animals were sacrificed 21 hours after syngeneic TBX trans-implantation under 3% isoflurane inhalation anesthesia and cardiac bleed.
In vivo Gastrointestinal Transit
To determine the effects of TBX on in vivo gastrointestinal motility, we measured the aboral transit of non-digestible, non-absorbable fluorescein isothiocyanate-labeled dextran with an average molecular mass of 70 kDa (FD70)(Sigma), as described previously (5;6). In brief, each mouse was orally fed a 10μl bolus of FD70 and then after a period of 75 min of gastrointestinal transit, the animal was sacrificed by isoflurane inhalation overdose. The entire gastrointestinal tract from lower esophageal sphincter to anus was excised and divided into 15 segments: stomach, small intestine (divided into 10 segment of equal length), cecum, and colon (divided into 3 segments of equal length). Each segment was opened in a 2 ml tube with 800μl of saline, vortexed to release the intra-lumenal FD70 tracer, cleared by centrifugation (10,000 rpm for 10 min) and fluorometrically assayed for FD70 signal in a plate reader (Molecular Device). The distribution of the FD70 along the gastrointestinal tract was plotted in a distribution histogram and quantified by calculating the geometric center (GC) using the following formula: GC = Σ(% of total fluorescent signal per segment × segment number)/100.
In vitro Jejunal Circular Muscle Contraction
To determine the effects of TBX17.5% which produced significant ileus on the intestinal contractile unit, we evaluated the contractile effects of the muscarinic agonist bethanechol on mid-jejunal full thickness circular smooth muscle strips in vitro using a standard organ bath. Briefly, mucosa-free individual circular muscle strips (1.5 mm wide) were mounted horizontally in organ bath chambers perfused with a prewarmed (37 °C), pH adjusted and oxygenated modified Krebs solution. After equilibration, normalized spontaneous and muscarinic dose-dependent intestinal smooth muscle contractile responses (bethanechol, 0.3 μM to 300 μM) were assessed by measuring the integral area under the contraction over a period of 10 minutes.
Multiplex Electrochemiluminescence Measurement of Inflammatory Mediators
Blood samples from naïve controls, sham and TBX17.5% implanted mice were harvested by cardiac puncture at the time of sacrifice and immediately cold centrifuged to obtain serum, which were then aliquoted and stored at −80°C. The supernatant fluid from freshly harvested TBX and wound TBX collected 21 hours after trans-implantation was obtained by cold centrifugation (5000 rpm) of the minced TBX, which was then aliquoted and stored at −80°C. Each serum and TBX fluid was analyzed for concentrations of IFN-γ, IL-1β, IL-10, IL-12 p70, IL-6, KC/CXCL1 and TNF-α. Samples were thawed on ice and filtered using Ultrafree-MC Centrifugal Filter Units with Microporous Membrane (d=0.65μm) from Millipore (Billerica, MA) for 5 minutes in a refrigerated centrifuge (4°C) at 10000g to remove all the solid and clotted material. Additionally, due to high concentration of analytes in wound fluid, these samples were 10-fold diluted prior to analysis. Mouse Proinflamatory-7 Ultrasensitive Kit (Meso Scale Discovery, Gaithersburg, MD) was used for assessing chemokine and cytokine concentrations. The assay employs a sandwich immunoassay format where capture antibodies are coated on multi-array plates. Cytokine levels are quantified using a cytokine-specific Detection Antibody labeled with MSD SULFO-TAGTM reagent (electrochemiluminescence reaction) and analyzed on the Sector Imager SI6000 (Meso Scale Discovery, Gaithersburg, MD).
Data Analysis
Data analysis was performed using the MSD Discovery Workbench TM2006 MSD_3_0_18 software (Meso Scale Discovery, Gaithersburg, MD) and Microsoft Excel. Results are reported as mean ± standard error (SEM unless otherwise specified. Statistical significance was determined using Student t-test or analysis of variance (ANOVA). EZ Analyze add-in for Microsoft Excel was used for F-test and Bonferroni correction applied for posthoc group comparisons where appropriate. Two-tailed p values of < 0.05 were considered statistically significant.
RESULTS
Transplanted Syngeneic Subcutaneous Tissue Injury Model
Recognizing that direct injury would require the use of gut altering analgesics, we “transplanted” the injury by harvesting syngeneic tissue and underlying bone from the skinned legs and rib cage of a syngeneic donor mouse, mincing it with blades and implanting it into the subcutaneous dorsal space of a host mouse. The tissue-bone matrix (TBX) was prepared and confirmed sterile at the time point of implantation and at sacrifice.
This transplanted syngeneic tissue injury model was reliably constructed and the amount of injury could be precisely adjusted by implanting specific amounts of the sterile TBX based on the body weight of the host mouse (5–25%). The organ systems of the injury-implanted host mouse were then allowed to respond over time to the damaged tissue without the confounding effects of analgesics. The injury-implanted host mice receiving TBX lacked behavioral indicators of stress for the first six hours after recovering from anesthesia. However, over the next 10–12 hours >10% TBX mice progressively became less mobile and developed a huddled and hunched posture with rapid breathing. Approximately 16 hours after trans-implantation the TBX20% mice would ambulate minimally upon prodding and were sacrificed. In contrast, as expected sham surgery with antibiotic injection did not overtly alter the behavior of the mice after recovery from anesthesia compared to controls.
Tissue-Bone Matrix Induction of Gastrointestinal Ileus
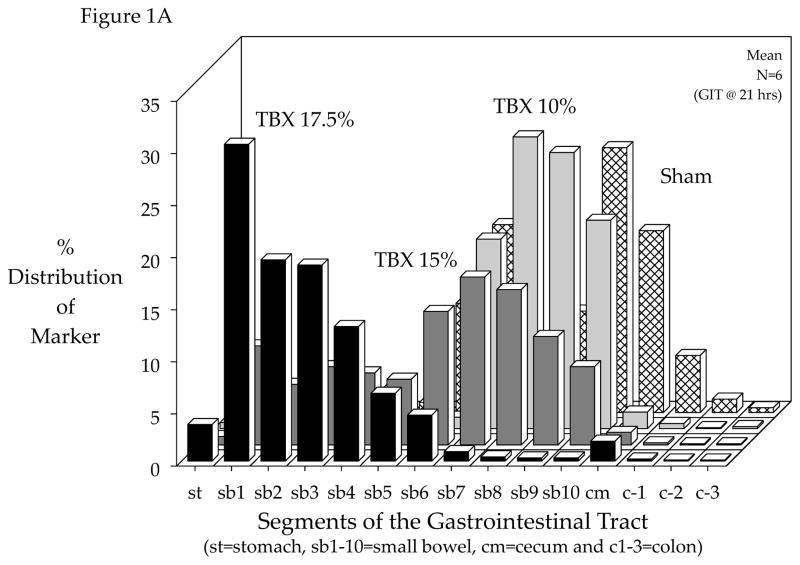
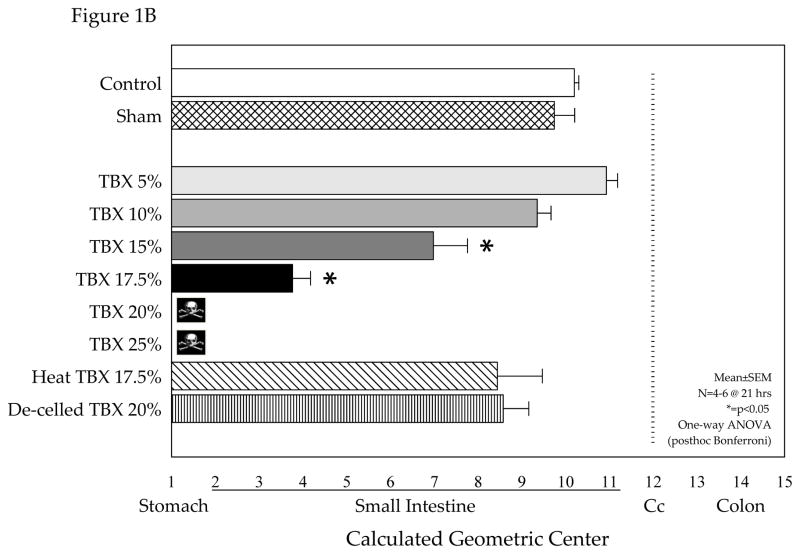
Although the response of any organ system to the implanted tissue injury can be studied with this model, in this study we focused on the gastrointestinal tract because of its key role in the trauma patient. Utilizing the syngeneic TBX model without confounding pharmacological effects, we determined the effect of graded tissue injury on gastrointestinal motility after 21 hours of implantation. As shown in Figure 1A, in sham mice the FD70 motility marker progressed to the distal regions of the small bowel, cecum and proximal colon after 75 minutes. In contrast, increasing graded amounts of TBX contributed to an ever mounting delay in gastrointestinal transit, which became noticeable in mice implanted with TBX15%. In TBX17.5% implanted mice, the liquid fluorescent marker after 75 minutes demonstrated 3.5% of the marker retained in the stomach and a severe delay in intestinal motility. Calculating the geometric center from the individual transit distributions statistically demonstrated that TBX implantation of 15% or higher resulted in a significant delay in gastrointestinal transit (Figure 1B). Interestingly, the host mouse response to TBX of greater than 17.5% of its body weight produced lethality within 21 hours.
Figure 1.
Effect of devitalized tissue injury on gastrointestinal transit. Panel 1-A: Increasing graded amounts of tissue injury produced by the subcutaneous dorsal trans-implantation of a tissue-bone matrix (TBX) into a host caused a progressive delay in gastrointestinal transit as measured by the gastrointestinal distribution of an orally fed bolus of a non-digestible, non-absorbable FITC-dextran (70 kD) after 75 minutes. Sham implantation with subcutaneous administration of antibiotics did not delay gastrointestinal transit. Increasing the amount of tissue injury based on % body weight caused a marked delay in transit in TBX15% and TBX17.5% groups of mice measure 21 hours after implantation. Panel 1B: Calculated geometric center values from gastrointestinal transit data demonstrates a progressive delay in gastrointestinal transit with increasing graded amounts of tissue injury (TBX) after 21 hours of exposure with a significant transit delay using TBX15% and a severe stasis using TBX17.5%. Trans-implantation of TBX in amounts greater than 17.5% was lethal to the mice (N=4). Experimental manipulation of the TBX before trans-implantation by heat inactivation or de-cellularization completely prevented the delay in transit caused by TBX17.5% and the observed mortality with TBX20%, respectively.
The TBX model, in addition to being precisely adjustable and free of confounding drug effects, can be uniquely manipulated by technically modifying the implanted TBX to investigate the deleterious inciting components within the TBX. We have observed that heat inactivation of the TBX17.5% (90° C for 2 hours) before implantation completely removes the interactive response of producing ileus (Figure 1B). And, likewise, mild peracetic acid decellularization of the TBX20% prevented the development of gastrointestinal ileus and death (Figure 1B). These studies indicate that the stripped matrix itself is not an inciting factor, but it remains to be determined if its degradation products by active cellular components might play a significant role.
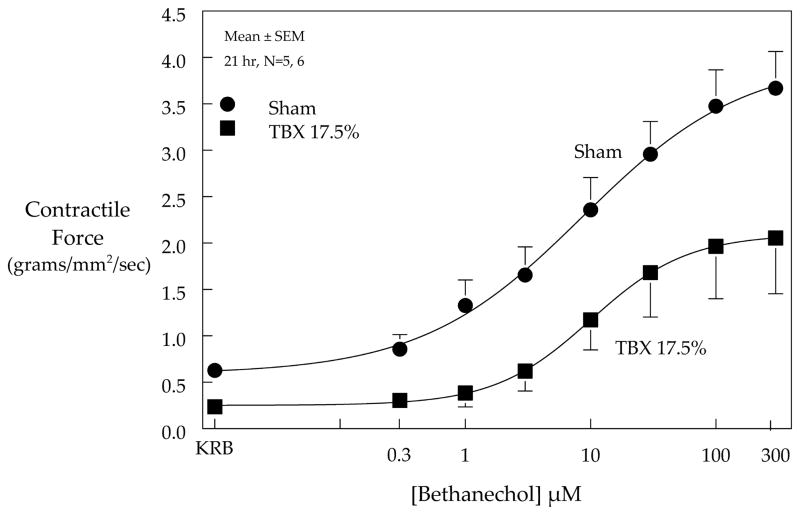
We next performed experiments to further investigate the effects of the peripheral tissue trauma induced ileus on in vitro jejunal circular smooth muscle contractions using standard organ bath techniques. As seen in Figure 2, spontaneous circular muscle contractions generated from sham treated mice were robust, whereas spontaneous contractions from TBX mice harvested after 21 hours were comparatively diminished (0.63±0.089 vs. 0.23±0.052 mg/mm2/sec, p<0.05). Likewise, bethanechol stimulated contractions were also markedly suppressed by the implanted TBX (bethanechol 100μM: 3.48±0.392 vs. 1.96±0.564, p<0.05). Thus, through a series of events peripheral tissue injury caused a sustained suppression in smooth muscle contractility which would appear to have contributed to the development of gastrointestinal ileus.
Figure 2.
Jejunal circular smooth muscle spontaneous and bethanechol stimulated contractions recorded from sham and TBX17.5% groups of mice. The recorded spontaneous muscle activity quantified over a period of 10 minutes was significantly diminished after subcutaneous implantation of the TBX for 21 hours compared to sham treated mice (0.63±0.089 vs. 0.23±0.052 mg/mm2/sec, p<0.05). Similarly, bethanechol dose-response curve generated from the tissue injury mice was also markedly suppressed compared to sham (bethanechol 100μM: 3.48±0.392 vs. 1.96±0.564, p<0.05).
Tissue-Bone Matrix Induction of a Systemic Inflammatory Response
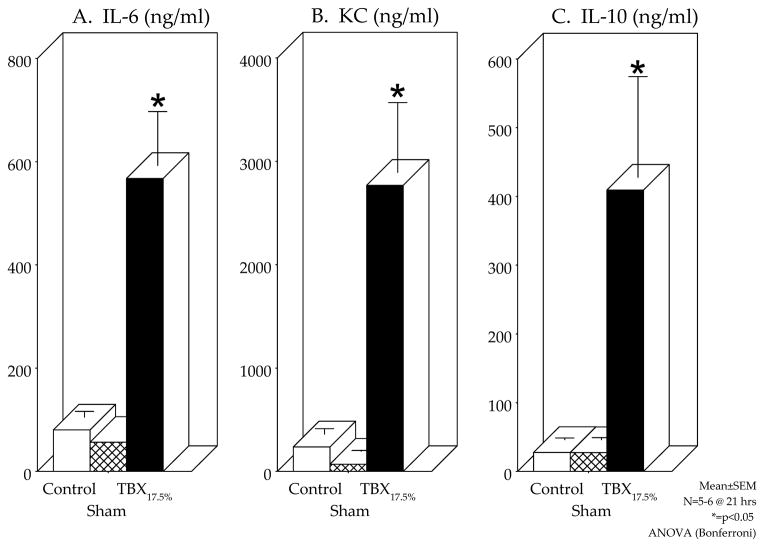
A key clinical feature of the trauma patient is the development of the detrimental systemic inflammatory response syndrome. Therefore, we sought to determine the systemic serum expression of certain prototypical inflammatory mediators (IL-1β, IL-6, IL-10, IL-12p70, IFN-γ, keratinocyte cytokine and TNF-α) in the TBX17.5% model after 21 hours of post-injury implantation. Electrochemiluminescence detection of inflammatory proteins demonstrated that the subcutaneous implanted injured tissue caused significant serum elevations in IL-6, keratinocyte cytokine (KC) and IL-10 compared to control and the sham surgical groups (Figure 3). As may have been expected for TNF-α, particular cytokines (IL-1β, IL-12p70, IFN-γ and TNF-α) were not significantly altered at this 21 hour time point after TBX implantation. Hence, the TBX host develops a systemic inflammatory response at a late time point for a select group of mediators.
Figure 3.
Serum inflammatory proteins induced by the subcutaneous dorsal trans-implantation of a tissue-bone matrix (TBX17.5%) into a host caused a systemic inflammatory response measured 21 hours after TBX implantation. Electrochemiluminescence detection of inflammatory proteins demonstrated that the implanted injured tissue caused significant serum elevations in IL-6 (Panel A), keratinocyte cytokine (KC) (Panel B) and anti-inflammatory mediator IL-10 (Panel C) compared to control and the sham surgical groups.
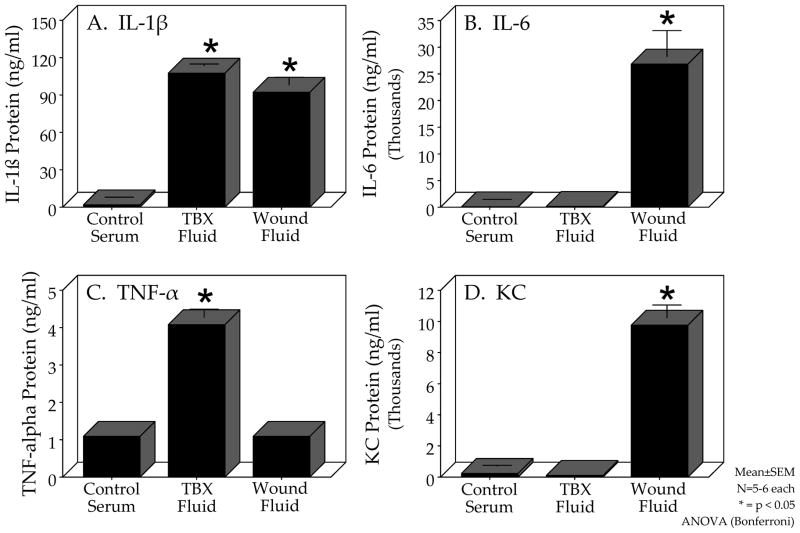
Inflammatory Responses within the Tissue-Bone Matrix Wound Fluid
Understanding the molecular and cellular interactions between the host and the traumatic wound is crucial to developing therapeutic interventions. In addition to the previously mentioned advantages of the TBX model, we can also show that this mouse model is uniquely suited for wound fluid analysis. As seen in Figure 4, the centrifuged supernatant from control serum, freshly minced TBX fluid and trans-implanted wound fluid obtained from the re-harvested TBX17.5% after 21 hours can be analyzed by 7-plex electrochemi-luminescence for inflammatory mediators. Interestingly, three different patterns of cytokine expression were detected compared to normal mouse serum. Figure 4A shows that IL-1β exhibited increased levels in both the fresh TBX supernatant and in the re-harvested TBX wound fluid. The measurement of TNF-α showed an increased presence only in the fresh TBX supernatant (Figure 4C). IL-6 and keratinocyte cytokine were significantly increased only in the re-harvested TBX17.5% after 21 hours of implantation indicating that these cytokines were generated only after implantation(Figure 4B and 4D).
Figure 4.
Comparative expression of inflammatory proteins in control serum, freshly isolated TBX17.5% fluid and re-harvested TBX17.5% wound fluid 21 hours after subcutaneous dorsal trans-implantation demonstrated three patterns of cytokine expression. Electrochemiluminescence detection of the inflammatory protein IL-1β (Panel A) was significantly increased in the freshly isolated TBX fluid and in the re-harvested TBX wound fluid compared to control serum. Panels B and D show that IL-6 and keratinocyte cytokine (KC) levels were only increased in the re-harvested TBX wound fluid compared to control serum and freshly isolated TBX fluid. On the other hand, TNF-α levels shown in Panel C demonstrated a significant increase in TNF-α only in the freshly isolated TBX fluid compared to control serum and the re-harvested TBX wound fluid after 21 hours of trans-implantation.
DISCUSSION
Clinical outcomes and survival rates for trauma patients have incrementally increased over the past decade primarily due to advances in trauma systems, including timing and capability of initial response, rapid transport, effective resuscitation and damage control, and supportive critical care. Traumatic death, however, remains a major clinical challenge because, unfortunately, no specific therapies currently exist for post-traumatic multiple organ failure(7). Therefore, the elucidation of the underlying pathophysiological mechanisms of trauma, particularly those related to dysregulated inflammatory responses (SIRS, MODS and CARS) is crucial to the development of new and effective therapeutic interventions. A reliable animal model of peripheral traumatic injury, particularly one that reproduces the contributory effects of the gastrointestinal tract on systemic inflammatory responses, and one which can be precisely regulated to study these pathophysiological mechanisms, has thus far been elusive. A novel donor tissue-bone matrix trauma model was developed in the current study that is precisely adjustable, that recapitulates clinically relevant biology, and one that provides an important means to study systemic and organ-specific molecular and functional effects of trauma.
The gastrointestinal tract plays a determining role in the high morbidity and mortality of the trauma patient (2;8). However, studies of the effects of trauma on the gastrointestinal tract and secondary systemic effects have been hampered by the obligatory use of post-traumatic analgesics in existing experimental models, which are known to have major untoward pharmacological effects on multiple organ systems, especially gastrointestinal functions of epithelial secretion and motility (9;10). The clinically phenomenon of gastrointestinal stasis and the systemic release of numerous gut-derived inflammatory mediators following trauma is well established. These post-traumatic events compromise the patient’s nutritional status, increase the risk of aspiration pneumonitis, and contribute to gastrointestinal bacterial overgrowth and mucosal breakdown, which are critical second hit events in the development of a deleterious systemic inflammatory response.
To our knowledge, the specific effect of tissue injury on gastrointestinal motility has not been previously investigated. This is in large part due to the lack of a reliable, adjustable and long term animal model of tissue trauma to study the gastrointestinal tract. Clearly, a variety of trauma/hemorrhage models exist with the traumatic injury in most studies consisting of a laparotomy or bone fracture(4). The disadvantage of these current animal models is, although the degree of wounding is a critical element in trauma models, the degree of tissue trauma is often limited. In addition, all current trauma/hemorrhage experimental models require analgesia to humanely study animals long-term after resuscitation – which has major confounding multi-organ system effects, particularly on gastrointestinal motor and secretory functions.
The first aim of this study was to successfully develop a reliable, adjustable and IACUC-approved animal model of tissue trauma that can be studied over the short- and long-term, which does not require the use of analgesics. We accomplished this by constructing a precisely graded tissue injury mouse model, which consisted of “transplanting” specific amounts of a sterile syngeneic injured tissue into a subcutaneous dorsal pouch of a host mouse. Interestingly, trans-implanted tissue-bone matrix (TBX) of greater than 17.5% of the animal’s body weight was lethal to the animal after an apparent behavioral full recovery from the initial implantation procedure. Clearly, within the time frame of this study, the animals did not severely decompensate from a loss of nutrition, but their rapid breathing pattern suggested the development of an acute lung failure. Therefore, the TBX model could be a reliable graded injury model for studying the pulmonary effects of tissue injury, as well.
Importantly, we utilized the novel experimental TBX mouse model to reliably recapitulate the frequent clinical problem of gastrointestinal stasis in trauma patients. The data showed that increasing graded amounts of TBX over 10% of the animal’s body weight caused an ever mounting delay in liquid gastrointestinal transit. Furthermore, the peripherally implanted damaged tissue resulted in a significant sustained suppression in isolated jejunal circular muscle contractions through as of yet unknown mechanisms. Severe gastrointestinal stasis occurs in a significant number of trauma patients causing increased morbidity and mortality. Although, nutritional support via the enteral route is preferred in the critically ill trauma patient,, ICU patients fed naso-gastrically achieve only about half of their nutritional goals(11). Furthermore, gastrointestinal stasis is frequently associated with an increased risk of gastrointestinal bacterial overgrowth, aspiration of gastric contents, and secondary pneumonia (12). Hence, an experimental model to specifically address the mechanisms of trauma- induced gastrointestinal stasis is greatly needed.
Another noteworthy mechanistic advantage to this novel injury model is the ability to alter the trans-implanted TBX before implantation. Here, we show that heat denaturation of the TBX proteins completely eliminated the inciting deleterious effects of the TBX. It is well known that cytosolic and nuclear proteins released extracellularly from injured tissue can elicit a potent inflammatory response. In other organ systems, studies have shown that the innate immune system and the endothelium are activated by a spectrum of intracellular molecules including: IL-1α, IL-1β, heat shock proteins, urine acid, ATP, S100 proteins, high-mobility group box-1 protein, histones and DNA-RNA-protein complexes) (13;14), some of which exert their effects in part via specific pattern-recognition receptors, like the Toll-like receptors(13;15). These first stage events subsequently activate coagulation factors, initiate platelet aggregation and generate cytokines and chemokines which recruit neutrophils, monocytes, B-cells and T-cells to the site of injury. All of which contributes to the development of a systemic inflammatory response that potentially progresses to multiple organ failure.
Similarly, trans-implantation of the isolated extracellular matrix of the TBX also eliminated the lethality and intestinal motility effects in the model. However, it remains to be determined if the degradation products of the extracellular matrix by active cellular components might generate significant inflammatory agents which could participate in the tissue injury response. Hyaluronic acid is a ubiquitous molecule and a major component of the extracellular matrix which is synthesized into an extensive polymer. It is known that during tissue injury, inflammation and clearance of cellular debris, fragmented hyaluronan is released into the circulation by increased matrix metalloproteinase activity (16). Indeed, serum elevations of hyaluronan have been measured in many inflammatory disorders and fragmented extracellular matrix components (hyaluronan and fibronectin) themselves have been shown to function as pro-inflammatory stimuli to macrophages and the endothelium (17–19). Recently, it has been shown that the inflammatory events of the degraded extracellular matrix are mediated through Toll-like receptors, TLR2 and TLR4, utilizing the MyD88 dependent pathway and NF-κB activation (20;21).
Clinically, severe trauma results in the development of the systemic inflammatory response syndrome, which can further cause multiple organ failure. In the TBX model, we measured significant elevations in serum IL-6, keratinocyte cytokine and the anti-inflammatory cytokine IL-10 twenty-one hours after exposure to tissue injury. It is known that serum IL-6 and IL-10 levels provide significant prognostic correlation and are independent risk factors of morbidity and mortality in trauma patients (22;23). In contrast, at this particular time point, we did not observe increases in serum IL-1β, IL-12p70, IFN-γ or TNF-α. However, the expression of various inflammatory mediators is known to be time dependent and serum analysis at earlier time points may have exhibited increases. Hence, a detailed temporal profile of specific inflammatory mediators would be of interest, when a rational mechanism for the cause of gastrointestinal stasis can be proposed. Comparative studies utilizing vascularized damaged tissue would also be note worth, as regions of vascularize tissue could potentially display a different array of inflammatory mediators.
Understanding the molecular and cellular interactions between the host and the traumatic wound is also crucial to developing therapeutic interventions for limiting the deleterious systemic effects of trauma, and also for promoting tissue repair and regeneration. In addition to the previously mentioned advantages of the TBX model, we can also show that this mouse model is uniquely suited for investigating mediators within sterile wound fluid. Interestingly, IL-1β and TNF-α were both significantly expressed in the freshly prepared TBX fluid indicating that they were present in a preformed state. In contrast to TNF-α, IL-6 and keratinocyte cytokine were generated within the TBX wound fluid only after implantation, while IL-1β was consistently present. The cytokine and chemokine protein expression patterns in the wound fluid may be predictive of wound healing, as inflammatory dysregulation has been shown within the serum and wound fluid of patients with delayed wound healing (22). Additional studies combining the TBX model and clinical data should allow the development of a wound biomarker panel which could predict wound healing outcome as well as estimate the impact of tissue injury on various organ systems, in particular the gastrointestinal tract. Considerable work has been done analyzing post-traumatic bronchoalveolar lavage fluid and these studies have also shown higher levels of TNF-α, IL-1β and IL-6, though primarily in hemorrhagic shock models of various species (24). The cellular origins of specific inflammatory mediators of trauma are still unclear in both clinical and experimental realms. In this unique model, whether the inflammatory mediators were of donor or host origin remains to be determined, but future studies could definitively determine this by trans-implanting genetically modified TBX harvested from specific transgenic mice or utilizing specific transgenic host mice.
In summary, we have constructed and characterized a novel donor tissue-bone matrix trauma animal model that is precisely controllable and reproducible, which recapitulates many important clinical phenomena and can capitalize on the genetic tools available in a mouse model. Importantly, the model does not require the obligatory use of narcotics as in other models, and therefore lacks the incalculable confounding pharmacological effects of narcotics on immune, lung and gastrointestinal functions thereby, for the first time, allowing the study of post-traumatic gastrointestinal motility. Uniquely in this model, the devitalized injured tissue and wound fluid can be experimentally manipulated and may provide definitive investigations into the deleterious systemic effects of tissue injury, and provide mechanistic insights into wound healing. Finally, this innovative model offers the capability to study the long-term molecular and functional events of tissue trauma-induced compensatory anti-inflammatory response syndrome in a precise manner, as well as, multiple two-hit scenarios of trauma.
Acknowledgments
The authors wish to thank Dr. Annette Wilson for her technical assistance in the decellularization of the TBX, the National Institutes of Health for the following funding: (R01-GM58241, R01-DK068610, P50-GM-53789, and DK02488), the Congressional Combat Wound Initiative Program, and the Henry M. Jackson Foundation for the Advancement of Military Medicine. Both civilian and military personnel involved in this study are dedicated to improving the lives of those who have been placed in harm’s way for the good of our nation.
This study was supported by the National Institutes of Health (R01-GM58241, R01-DK068610, P50-GM-53789, and DK02488), the Congressionally-sponsored Combat Wound Initiative Program, and the Henry M. Jackson Foundation for the Advancement of Military Medicine.
ABBREVIATIONS
- IL-1β
interleukin-1beta
- IL-6
interleukin-6
- IL-10
interleukin 10
- KC
kerotinocyte cytokine
- TNF-α
tumor necrosis factor-alpha
- TBX
tissue-bone matrix
- FD70
FITC-dextran
- SIRS
systemic inflammatory response syndrome
- MODS
multiple organ dysfunction syndrome
- CARS
compensatory anti-inflammatory response syndrome
Footnotes
Takeshi Tsukamoto: performed components of the research, analyzed the data and participated in the design of the study.
Vlado Antonic: performed components of the research, analyzed the data and contributed to writing the manuscript.
Ihab I. El Hajj: performed components of the research and analyzed the data.
Alexander Stojadinovic: participated in the study design and data interpretation, contributed essential reagents/tools and shared in writing the manuscript.
David G. Binion: participated in the study design and data interpretation, contributed essential reagents/tools and shared in writing the manuscript.
Mina J. Izadjoo: participated in the study design and data interpretation, contributed essential reagents/tools and shared in writing the manuscript.
Hiroyuki Yokota: participated in the study design and shared in editing the manuscript.
Hans Christoph Pape: participated in the study design, contributed essential reagents/tools and shared in writing the manuscript.
Anthony J. Bauer: designed the study, performed components of the research, analyzed and interpreted the data, and shared in writing the manuscript.
Competing Interests: the authors have no competing interests.
The views expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense or the United States Government. This effort was supported, in part, by the congressionally funded Combat Wound Initiative Program. Some of the authors are a military service member (or employee of the U.S. Government). This work was prepared as part of official duties. We certify: 1. that all individuals who qualify as authors have been listed; 2. that each has participated in the conception and design of this work, the analysis of data (when applicable), the writing of the document, and/or the approval of the submission of this version; 3. that the document represents valid work; 4. that if we used information derived from another source, we obtained all necessary approvals to use it and made appropriate acknowledgements in the document; and, 5. that each author takes public responsibility for it.
References
- 1.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36(6):691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract. The “undrained abscess” of multiple organ failure. Annals of Surgery. 1993;218(2):111–119. doi: 10.1097/00000658-199308000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deitch EA. The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Arch Surg. 1990;125(3):403–404. doi: 10.1001/archsurg.1990.01410150125024. [DOI] [PubMed] [Google Scholar]
- 4.Tsukamoto T, Pape HC. Animal models for trauma research: what are the options? Shock. 2009;31(1):3–10. doi: 10.1097/SHK.0b013e31817fdabf. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt J, Stoffels B, Moore BA, Chanthaphavong RS, Mazie AR, Buchholz BM, et al. Proinflammatory role of leukocyte-derived Egr-1 in the development of murine postoperative ileus. Gastroenterology. 2008;135(3):926–936. doi: 10.1053/j.gastro.2008.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoffels B, Schmidt J, Nakao A, Nazir A, Chanthaphavong RS, Bauer AJ. Role of interleukin 10 in murine postoperative ileus. Gut. 2009;58(5):648–660. doi: 10.1136/gut.2008.153288. [DOI] [PubMed] [Google Scholar]
- 7.Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. [Review] [56 refs] Injury. 2009;40(9):912–918. doi: 10.1016/j.injury.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Soeters PB, Luyer MD, Greve JW, Buurman WA. The significance of bowel permeability. Current Opinion in Clinical Nutrition & Metabolic Care. 2007;10(5):632–638. doi: 10.1097/MCO.0b013e3282a0780e. [DOI] [PubMed] [Google Scholar]
- 9.Bauer AJ, Sarr MG, Szurszewski JH. Opioids inhibit neuromuscular transmission in circular muscle of human and baboon jejunum. Gastroenterology. 1991;101(4):970–976. doi: 10.1016/0016-5085(91)90723-x. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt J, Stoffels B, Nazir A, Dehaven-Hudkins DL, Bauer AJ. Alvimopan and COX-2 inhibition reverse opioid and inflammatory components of postoperative ileus. Neurogastroenterol Motil. 2008;20(6):689–699. doi: 10.1111/j.1365-2982.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- 11.Stapleton RD, Jones N, Heyland DK. Feeding critically ill patients: what is the optimal amount of energy? Crit Care Med. 2007;35(9 Suppl):S535–S540. doi: 10.1097/01.CCM.0000279204.24648.44. [DOI] [PubMed] [Google Scholar]
- 12.Chapman M, Fraser R, Vozzo R, Bryant L, Tam W, Nguyen N, et al. Antro-pyloro-duodenal motor responses to gastric and duodenal nutrient in critically ill patients. Gut. 2005;54(10):1384–1390. doi: 10.1136/gut.2005.065672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flohe SB, Bangen JM, Flohe S, Agrawal H, Bergmann K, Schade FU. Origin of immunomodulation after soft tissue trauma: potential involvement of extracellular heat-shock proteins. Shock. 2007;27(5):494–502. doi: 10.1097/shk.0b013e31802dec51. [DOI] [PubMed] [Google Scholar]
- 14.Bitto A, Barone M, David A, Polito F, Familiari D, Monaco F, et al. High mobility group box-1 expression correlates with poor outcome in lung injury patients. Pharmacological Research. 2010;61(2):116–120. doi: 10.1016/j.phrs.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. Extracellular histones are major mediators of death in sepsis. Nature Medicine. 2009;15(11):1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P, Ba ZF, Biondo A, Chaudry I. Liver endothelial cell dysfunction occurs early following hemorrhagic shock and persists despite crystalloid resuscitation. J Surg Res. 1996;63(1):241–247. doi: 10.1006/jsre.1996.0255. [DOI] [PubMed] [Google Scholar]
- 17.McKee CM, Lowenstein CJ, Horton MR, Wu J, Bao C, Chin BY, et al. Hyaluronan fragments induce nitric-oxide synthase in murine macrophages through a nuclear factor kappaB-dependent mechanism. Journal of Biological Chemistry. 1997;272(12):8013–8018. doi: 10.1074/jbc.272.12.8013. [DOI] [PubMed] [Google Scholar]
- 18.Rockey DC, Chung JJ, McKee CM, Noble PW. Stimulation of inducible nitric oxide synthase in rat liver by hyaluronan fragments. Hepatology. 1998;27(1):86–92. doi: 10.1002/hep.510270115. [DOI] [PubMed] [Google Scholar]
- 19.Bot PT, Hoefer IE, Piek JJ, Pasterkamp G. Hyaluronic acid: targeting immune modulatory components of the extracellular matrix in atherosclerosis. Curr Med Chem. 2008;15(8):786–791. doi: 10.2174/092986708783955554. [DOI] [PubMed] [Google Scholar]
- 20.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nature Medicine. 2005;11(11):1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 21.Levy RM, Prince JM, Yang R, Mollen KP, Liao H, Watson GA, et al. Systemic inflammation and remote organ damage following bilateral femur fracture requires Toll-like receptor 4. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2006;291(4):R970–R976. doi: 10.1152/ajpregu.00793.2005. [DOI] [PubMed] [Google Scholar]
- 22.Hawksworth JS, Stojadinovic A, Gage FA, Tadaki DK, Perdue PW, Forsberg J, et al. Inflammatory biomarkers in combat wound healing. Annals of Surgery. 2009;250(6):1002–1007. doi: 10.1097/sla.0b013e3181b248d9. [DOI] [PubMed] [Google Scholar]
- 23.Nast-Kolb DM, Waydhas CM, Gippner-Steppert CP, Schneider I, Trupka AM, Ruchholtz SM, et al. Indicators of the Posttraumatic Inflammatory Response Correlate with Organ Failure in Patients with Multiple Injuries. Journal of Trauma-Injury Infection & Critical Care. 1997;42(3):446–455. doi: 10.1097/00005373-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Tasoulis MK, Livaditi O, Stamatakos M, Stefanaki C, Paneris P, Prigouris P, et al. High concentrations of reactive oxygen species in the BAL fluid are correlated with lung injury in rabbits after hemorrhagic shock and resuscitation. Tohoku Journal of Experimental Medicine. 2009;219(3):193–199. doi: 10.1620/tjem.219.193. [DOI] [PubMed] [Google Scholar]