Abstract
Somatic genital reflexes such as ejaculation and vaginocervical contractions are produced through the striated muscles associated with the genitalia. The coordination of these reflexes is surprisingly complex and involves a number of lumbosacral spinal and supraspinal systems. The rat model has proved to be an excellent source of information regarding these mechanisms, and many parallels to research in humans can be drawn. An understanding of the spinal systems involving the lumbosacral spinal cord, both efferent and afferent, has been generated through decades of research. Spinal and supraspinal mechanisms of descending excitation, through a spinal ejaculation generator in the lumbar spinal cord and thalamus, and descending inhibition, through the ventrolateral medulla, have been identified and characterized both anatomically and physiologically. In addition, delineation of the neural circuits whereby ascending genitosensory information regarding the regulation of somatic genital reflexes is relayed supraspinally has also been the topic of recent investigation. Lastly, the importance of the “social neuropeptides” oxytocin and vasopressin in the regulation of somatic genital reflexes, and associated sociosexual behaviors, is emerging. This work not only has implications for understanding how nervous systems generate sexual behavior, but also provides treatment targets for sexual dysfunction in people.
Keywords: gentialia, penis, copulation, lordosis, vagina, sexual behavior
Introduction
While advances in our knowledge of the supraspinal and spinal control of the genital musculature have been made, the neural circuits and neurochemistry underlying the regulation of somatic genital reflexes such as ejaculation in men, and vaginocervical contractions in women, have not been fully elucidated. In both men and women, internally or externally derived stimulation results in rhythmic contractions of genital muscles often associated with orgasm (Argiolas and Melis, 2003). All of these processes are reflexive and under the control of somatic spinal efferents (Giuliano and Clement, 2005; McKenna, 2002; Temel et al., 2005). However, these efferents are regulated by both excitatory and inhibitory control from numerous spinal and supraspinal sites. Coordination of the various circuits controlling genital reflexes has been studied extensively in men, with recent interest in defining homologous circuits in women. From this work, a general understanding of the discrete processes underlying genital reflex control has been established. First, the autonomic nervous system plays the key role in providing the necessary signals for increased blood flow to the genitalia for both men (erection) and women (genital engorgement) as well as providing signals for the production of secretive fluids used for lubrication and seminal transfer (ejaculation: emission phase; McKenna, 2002; Temel et al., 2005; Yang and Jiang, 2009). Second, and the focus of this review, the somatic nervous system provides the signals for rhythmic contractions of the genital musculature in both men (ejaculation: expulsion phase) and women (vaginocervical contractions; Giuliano and Clement, 2005; Levin, 1998; Yang and Jiang, 2009). There is a surprisingly complex integration of ascending sensory information from the genitalia with both descending excitatory and inhibitory inputs to the spinal motor neurons. Non-human animal models, in particular the rat, have been particularly informative in elucidating the basic anatomy and physiology of these circuits (Pfaus et al., 2003). An understanding of the anatomy and physiology of these circuits, with a special emphasis on social neuropeptides, is an important area of research with significant implications for our understanding of basic sexual processes as well as sexual dysfunction in people.
The striated genital musculature and associated motoneurons
The pelvic muscles associated with the genitalia in both humans and rats include the striated perineal muscles m. bulbospongiosus (also known as the m. bulbocavernosus), m. ischiocavernosus, and m. levator ani (Blaivas et al., 1981; deGroat and Booth, 1980; Holmes et al., 1991; Rand and Breedlove, 1987). These muscles, in particular the bulbospongiosus, provide the rhythmic contractions associated with orgasm and the expulsion of semen during ejaculation in males in both humans and rats (Ertekin and Reel, 1976; Gerstenberg et al., 1990; Hart and Melese-D'Hospital, 1983; Holmes et al., 1991; Sachs, 1982). In females, despite the “vestigial” description some authors have applied (Arakawa et al., 2010; Fishman and Breedlove, 1988), these muscles are found to produce rhythmic contractions during orgasm in both humans and rats (Giraldi et al., 2004; McKenna and Nadelhaft, 1989; Meston et al., 2004; Vodusek et al., 1983).
The perineal muscles are innervated by the pelvic and pudendal nerves (Pacheco et al., 1989; Pastelin et al., 2008) from the lower lumbar and upper sacral divisions of the spinal cord (de Araujo et al., 1982; Katagiri et al., 1986; Roppolo et al., 1985) in both humans and rats. In rats, the lumbosacral spinal motoneuron pools associated with these nerves are referred to as the dorsomedial nucleus (DM; also referred to as the spinal nucleus of the bulbocavernosus) and the dorsolateral nucleus (DL) of the L5-S1 ventral horn (Collins et al., 1991; Katagiri et al., 1986; McKenna and Nadelhaft, 1986; Peshori et al., 1995; Schroder, 1980). The DM and DL are considered homologues of Onuf's nucleus in humans (Breedlove and Arnold, 1980; Roppolo et al., 1985; Schroder, 1981). The DM is an androgen sensitive sexually dimorphic structure with more numerous and larger cells in males in both humans (Forger and Breedlove, 1986) and rats (Breedlove and Arnold, 1980; Freeman et al., 1995; Katagiri et al., 1986).
The DM and DL nuclei are active during ejaculation in rats (Clement et al., 2007; Giuliano et al., 2007). Interneurons within the spinal cord appear to connect primary afferent somatosensory information with the DM and DL (Collins et al., 1991; Peshori et al., 1995; Wiedey et al., 2008), but a number of descending projections from other parts of the spinal cord, as well as from supraspinal sites, provide both excitatory and inhibitory drive to these motoneuron pools (Allard et al., 2005; Coolen, 2005; Marson and McKenna, 1990; Wagner and Clemens, 1991). These excitatory and inhibitory drives compete at the level of the spinal cord motoneuronal pools to regulate somatic genital reflexes.
The ejaculation generator and descending excitatory circuits
A hypothesized ejaculation generator, constituting a central pattern generator for the muscles of ejaculation, has been described in the lumbar spinal cord of rats, and appears to be present in both sexes (Carro-Juarez and Rodriguez-Manzo, 2006). These galanin-immunoreactive neurons, referred to as lumbar spinothalamic (LSt) neurons, express ejaculation induced Fos (Truitt et al., 2003) and project to both a thalamic region (the parvocellular subparafascicular thalamic nucleus; SPFpc) that expresses ejaculation-induced Fos (Coolen et al., 2003a; Coolen et al., 2003b), and the autonomic nuclei responsible for the emission phase of ejaculation (Xu et al., 2006), and the DM and DL (Newton, 1993; Xu et al., 2006). This anatomical connectivity suggests a central role in the relay of sensory information from the genitals, in combination with the coordination of the emission and expulsion phase of ejaculation. In fact, lesions of these lumbar spinothalamic cells abolish the ability of male rats to ejaculate, while leaving other aspects of sexual behavior intact (Truitt and Coolen, 2002). In addition, electrical stimulation of LSt cells in male rats produces both the emission and expulsion phase of ejaculation in a coordinated fashion, as measured by elicited seminal vesicle and bulbospongiosus contractions (Borgdorff et al., 2008). Interestingly, vaginocervical stimulation does not induce Fos expression in these cells in female rats indicating that there may be a discrete sexual dimorphism in the organization of this circuit (Truitt, Shipley et al. 2003). It is also possible that descending excitation to the somatic motoneurons is relayed from these LSt cells, through the thalamus, to other forebrain regions with descending input to the DM and DL spinal motoneuronal pools.
The paraventricular hypothalamic nucleus (PVN) has direct and indirect connections to the DM and DL (Tang et al., 1999; Wagner and Clemens, 1991). The PVN has been previously implicated in the control of genital reflexes, and is most likely a source of descending excitatory input to the genital musculature. Lesions of both magnocellular and parvocellular PVN cells increase ejaculation latency in rats (Liu et al., 1997), though lesions restricted to the parvocellular PVN do not (Ackerman et al., 1997). Importantly, lesions of the PVN have more dramatic effects on penile reflexes mediated by the autonomic nervous system rather than somatic efferents (Ackerman et al., 1997; Chen et al., 1997; Eaton et al., 1991; Liu et al., 1997), and it has been difficult to tease apart these two components of sexual behavior with regard to descending PVN projections.
Descending inhibition via the nucleus paragigantocellularis
The nPGi of the ventrolateral medulla is the hypothesized source of descending inhibition to genital reflexes In humans, the homologous structure is referred to as the nucleus paragigantocellularis lateralis (Zec and Kinney, 2001) and is also believed to be associated with descending inhibition of genital reflexes (Johnson, 2006). Numerous lines of evidence suggest that the nPGi is the primary source of descending inhibition of genital reflexes in rats and humans.
The nPGi sends direct descending androgen sensitive projections to the DM and DL in both male and female rats (Hamson et al., 2004; Hermann et al., 2003; Marson and Carson 3rd, 1999; Marson and McKenna, 1996; Tang et al., 1999). Electrolytic (Yells et al., 1992; Yells et al., 1994) or neurotoxic (Normandin and Murphy, 2011) lesions of the nPGi in male rats consistently result in the facilitation of sexual behavior, as indicated by a decrease in mount and intromission frequency, ejaculation latency, and an increase the number of ejaculations to satiety. nPGi lesions also decrease the latency to- and increase the number of ex copula erections (Marson et al., 1992; Marson and McKenna, 1990). Similarly, electrical stimulation of nPGi neurons in male rats produces increased firing latency and decreased amplitude of firing in the DM (Johnson and Hubscher, 1998), consistent with the role of the nPGi as a source of tonic descending inhibition of genital reflexes.
The role of the nPGi in female sexual behavior has not received as much attention. However, anatomical evidence suggests that the nPGi is important for sexual behavior in females. Retrograde trans-synaptic tracing from rat clitoris (Marson and Murphy, 2006), vagina (Marson and Murphy, 2006), and cervix (Lee and Erskine, 2000) produces extensive labeling in the nPGi of females. In addition, a number of brain regions associated with sexual behavior project to the nPGi of female rats, including the medial preoptic area of the hypothalamus (MPOA), PVN, and periaqueductal gray (PAG; Marson and Foley, 2004; Marson and Murphy, 2006; Murphy and Hoffman, 2001; Normandin and Murphy, 2008), suggesting a role for the nPGi in female sexual behavior. Our laboratory has recently reported that excitotoxic lesions of the nPGi in female rats left most sexual behaviors intact, though curiously, lesioned females spent less time mating, and had longer ejaculation-return latencies as compared to baseline, suggesting that the reinforcing value of sexual behavior had been altered (Normandin and Murphy, 2011). To test this hypothesis, subsequent experiments were performed using conditioned place-preference for artificial vaginocervical stimulation (aVCS). While both nPGi lesioned and non-lesioned females formed a conditioned place preference for aCVS, the amount of time lesioned females spent in the non-reinforced chamber versus the reinforced chamber did not differ, indicating a weakened CPP for aVCS. These data suggest that nPGi lesions produce dysregulation of genital function that feeds back on reward systems (Normandin and Murphy, 2011). Further testing of the effect of nPGi lesions on the physiology of the pelvic muscles in females is needed to confirm this hypothesis.
How the central nervous system regulates descending inhibition of genital reflexes via the nPGi has received little attention. However, some clues regarding the neuroanatomy of these circuits, and how sex hormones may potentially influence them, have been found. Using the retrograde tracer Fluorogold we found that a multitude of regions throughout the neuraxis project to the nPGi (Normandin and Murphy, 2008), which is not surprising for a brainstem reticular region. To resolve which nPGi-projecting regions are most important for the regulation of genital reflexes, we combined our tracing with sexual behavior-induced Fos expression and found that only a few hypothalamic regions, such as the MPOA, PVN, and perifornical nucleus projected to the nPGi and were active during sexual behavior (Normandin and Murphy, 2008). This is particularly interesting as the MPOA is known to be critical for the expression of sexual behavior (Balthazart and Ball, 2007; Hull et al., 1995; Sakuma, 2008). In addition, we found that MPOA-nPGi projecting cell numbers were sexually dimorphic, with a much larger number of MPOA-nPGi projecting cells in males. The location of estrogen and androgen receptors in relation to MPOA-nPGi projections was also examined. We found that males have a greater percentage of MPOA-nPGi projecting cells that contain androgen receptors than females (Normandin and Murphy, 2008), indicating that androgenic stimulation of the MPOA is critical in male sexual behavior. How the MPOA, or any of the other regions mentioned, influence nPGi activity remains to be elucidated, but given the pro-sexual role of the MPOA, a working hypothesis might be that MPOA projections to the nPGi would inhibit nPGi activity, allowing for the disinhibition of genital reflexes.
Ascending genitosensation
Despite decades of research on sexual behavior circuits in mammalian models, a complete description of neural targets receiving genitosensory information is unavailable. The rat model has been studied most extensively with regard to genitosensory circuits. The somatosensory afferents from the genitalia travel along the sensory branches of the pelvic, pudendal, and hypogastric nerves (Dail et al., 1985; Nunez et al., 1986; Peters et al., 1987; Purinton et al., 1976) terminating within the dorsal horn of the lumbosacral spinal cord (Martin-Alguacil et al., 2008; Nunez et al., 1986), with collaterals traveling to supraspinal sites. Specific nervous system regions receiving genitosensory information have been described in rats. Important sites within the spinal cord (LSt) and thalamus (SPFpc) that receive genitosensory information, and that are important for the regulation of sexual response, have been described (Coolen et al., 2003a; Truitt and Coolen, 2002). Medullary reticular cells also receive genitosensory input in male rats as evidenced by electrophysiological recordings in conjunction with stimulation of the penis (Hubscher and Johnson, 1996) through multiple ascending pathways (Hubscher et al., 2010). In addition, in female rats, transections of the pelvic nerve reduce Fos expression induced by vaginocervical stimulation or mating in the MPOA, bed nucleus of the stria terminalis, ventromedial hypothalamus, and medial amygdala (Pfaus et al., 2006; Rowe and Erskine, 1993), indicating that genitosensory afferents in female rats are important in regulating those regions.
We recently used a novel anterograde trans-synaptic viral tracer, herpes simplex virus 1 strain 129 (H129) in order to provide a full map of the functional genitosensory pathway in rats. The H129 virus has been found to infect neurons in the region where it is injected, replicate in infected cells, travel along axons in an anterograde fashion, and release from axon terminals to synaptically connected neurons, continuing this process indefinitely (Garner and LaVail, 1999; Zemanick et al., 1991). We injected H129 into the penis and vagina of rats; six days later, animals were euthanized and transcardially perfused with fixative. Brains were removed, sectioned at 25μm, and immunohistochemically stained for H129 (rabbit anti-HSV-1, 1:300,000; Dako) using a peroxidase method with diaminobenzadine as the chromagen, then counterstained with cresyl violet.
Table 1 provides a summary of H129 labeling in supraspinal sites in both males and females. Unsurprisingly, we found that genitosensory information is provided to the lumbosacral spinal cord (Figure 1) in both sexes, lending support to the well-established role of the lumbosacral spinal cord in the regulation of somatic genital reflexes. We also observed H129 labeling in the ventrolateral medulla (including the nPGi) in both sexes (Figure 2), indicating that genitosensory information could be directly modulating descending inhibition of somatic genital reflexes via the nPGi. Within the PAG, males exhibited H129 labeling in both the lateral and ventrolateral portions, with only lateral labeling observed in females. This result is interesting in the context of the PAG as a known integrator of cardiovascular, nociceptive and sexual behavior (Holstege, 1992; Holstege and Georgiadis, 2004; Murphy et al., 1999; Vanderhorst and Holstege, 1995). Thalamic H129 labeling was observed in the SPFpc nucleus in male but not females. As copulation-induced Fos expression in the SPFpc has been observed in both sexes (Coolen et al., 2003a), our results suggest that in females, such Fos expression is reflective of motor output rather than sensory input. We also observed prominent labeling within the PVN in both sexes (Figure 3; see next section for discussion of this finding), and labeling in the central amygdala was only observed in males (Figure 3). The central amygdala plays a role in anxiety/arousal (McEwen, 2007) and this result suggests that such activity could be modulated by genitosensory information. Interestingly, ventro-orbital cortical (VO) labeling was only observed in males (Figure 4). The VO has been shown to be active during sexual behavior in men as evidenced by functional magnetic resonance imaging (Holstege et al., 2003; Hu et al., 2008; Karama et al., 2002; Walter et al., 2008) and might represent a sexual-behavior specific percept, or modulator of executive function, in both rats and humans. The sex differences within our functional genitosensory maps imply that responses to genitosensory stimuli could be very different for males versus females. Indeed, well-established phenomenon regarding neuroendocrine changes in rats as a result of sexual behavior exist. For example, vaginocervical stimulation or mating in female rats produces pregnancy-promoting changes to neuroendocrine function with the establishment of pseudopregnancy (Castro-Vasquez and Carreno, 1981).
Table 1. Abundance of H129 labeling in supraspinal sites of male and female rats as a result of genital inoculation.
| Region | Male | Female |
|---|---|---|
| VO | +++ | - |
| AIC | ++ | - |
| M1/S1 | + | + |
| BNST | +++ | - |
| CeM | +++ | - |
| VP | ++ | - |
| MPG | ++ | - |
| LPG | ++ | - |
| PVN | +++ | +++ |
| LH | +++ | + |
| MCLH | - | + |
| PeF | +++ | ++ |
| Sub | +++ | - |
| ZI | +++ | - |
| VPM | ++ | - |
| VA | +++ | - |
| SPFpc | ++ | - |
| SNR | ++ | - |
| PF | + | + |
| LPAG | + | + |
| VLPAG | +++ | - |
| RPC | +++ | +++ |
| RMC | +++ | +++ |
| IMLF | ++ | + |
| PL | + | + |
| DpMe | + | - |
| DpG | ++ | - |
| CnF | + | - |
| VLTg | ++ | - |
| PB | ++ | - |
| NRM | +++ | +++ |
| DMTg | ++ | - |
| LC | +++ | +++ |
| Mo5 | - | ++ |
| A5 | - | ++ |
| nPGi | +++ | +++ |
| PnC | ++ | - |
| Sol | +++ | - |
| PCRt | ++ | - |
The relative density of H129 labeling in each region as represented by the crosses is as follows: + = a few cells, ++ = 5 to 25 cells, +++ = >25 cells. VO = ventral orbital cortex, AIC = anterior insular cortex, M1/S1 = primary somatomotor cortex, BNST = bed nucleus of the stria terminalis, CeM = central amygdala, VP = ventral posterior thalamic nucleus, MPG = medial globus pallidus, LPG = lateral globus pallidus, PVN = paraventricular hypothalamic nucleus, LH = lateral hypothalamus, MCLH = magnocellular nucleus of the lateral hypothalamus, PeF = perifornical nucleus, Sub = subicular nucleus, ZI = zona incerta, VPM = posteromedial ventral nucleus of the thalamus, VA = ventral anterior thalamic nucleus, SPFpc = parvocellular subparafascicular nucleus, SNR = substantia nigra, PF = paraflocculus, LPAG = lateral periaqueductal gray, VLPAG = ventrolateral periaqueductal gray, RPC = parvocellular red nucleus, RMC = magnocellular red nucleus, IMLF = interstitial nucleus of the medial longitudinal fasciculus, PL = paralemniscal nucleus, DpMe = deep mesencephalic nucleus, DpG = deep gray layer of the superior colliculus, CnF = cuniform nucleus, VLTg = ventrolateral tegmental area, PB = parabrachial nucleus, NRM = nucleus raphe magnus, DMTg = dorsomedial tegmental area, LC = locus coeruleus, Mo5 = motor trigeminal nucleus, A5 = A5 noradrenergic cell group, nPGi = nucleus paragigantocellularis, PnC = caudal pontine reticular nucleus, Sol = solitary nucleus, PCRt = parvocellular reticular nucleus.
Figure 1. Photomicrographs of lumbosacral spinal cord regions receiving genitosensory information in a male and female rat.
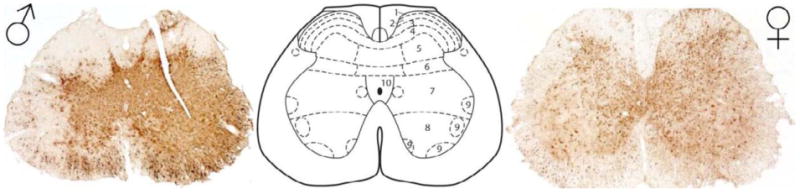
Photomicrographs of H129 labeling in the lumbosacral spinal cord of a male (left) and female (right) rat, as a result of genital inoculation. Note the robust labeling within most spinal cord lamina. The center panel is a spinal atlas diagram provided for reference purposes (Paxinos and Watson, 2005), 1-10 = spinal cord lamina 1 through 10.
Figure 2. Photomicrographs of brainstem regions receiving genitosensory information in a male and female rat.
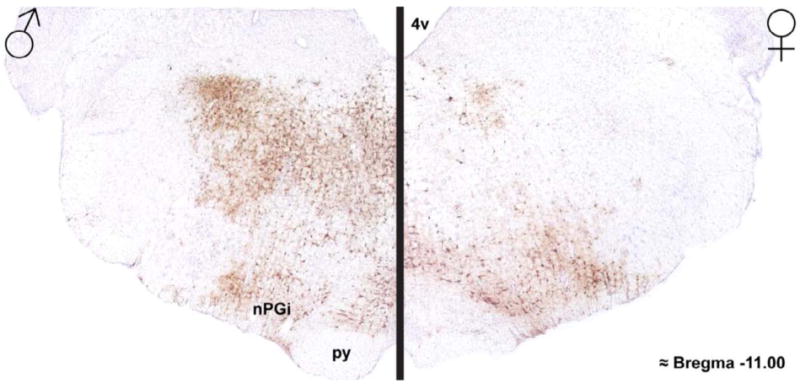
Photomicrographs of H129 labeling in the brainstem of a male (left) and female (right) rat, as a result of genital inoculation. Note the robust labeling within the rostroventrolateral medulla in both sexes, and more dorsal brainstem regions in males. Discrete labeling within the nPGi was observed in both sexes. 4v = 4th ventricle, nPGi = nucleus paragigantocellularis, py = pyramidal tract.
Figure 3. Photomicrographs of diencephalic regions receiving genitosensory information in a male and female rat.
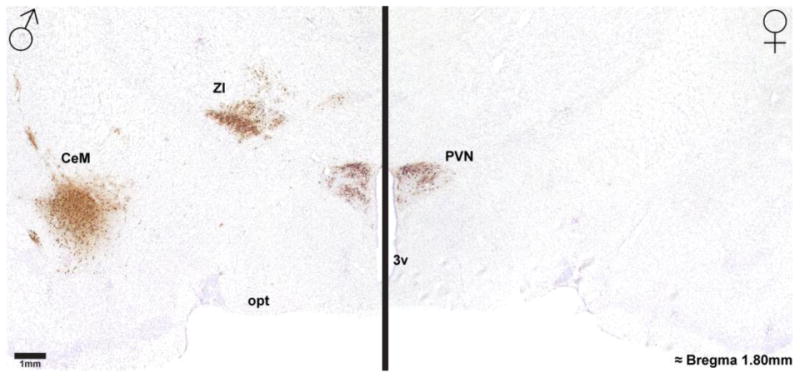
Photomicrographs of H129 labeling in the diencephalon of a male (left) and female (right) rat, as a result of genital inoculation, show robust labeling in the thalamus and central amygdala in a male but not a female rat. Note that both sexes show robust H129 labeling in the paraventricular hypothalamic nucleus (PVN). 3v = 3rd ventricle, CeM = central amygdala, opt = optic tract, ZI = zona incerta.
Figure 4. Photomicrograph of a cortical region receiving genitosensory information in a male rat.
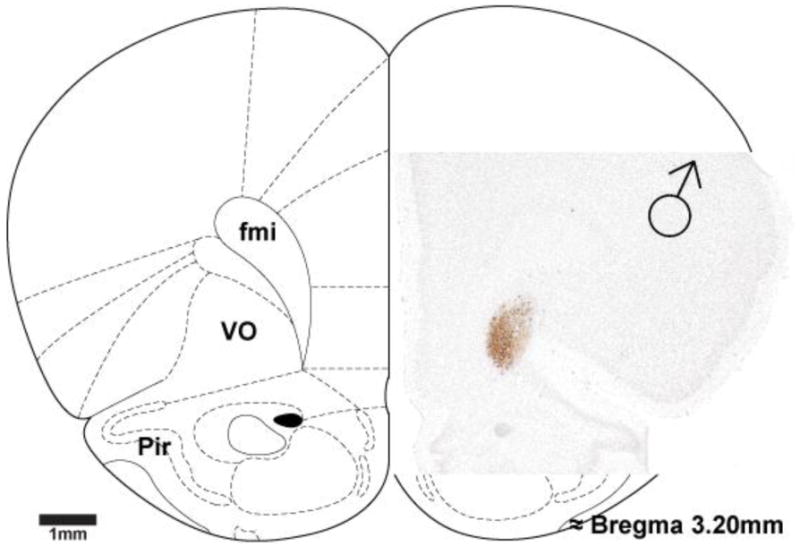
Photomicrograph overlaid on rat brain atlas image (Paxinos and Watson, 1997) of H129 labeling in the cerebral cortex of a male rat, as a result of genital inoculation, shows discrete labeling in ventro-orbital granular cells. fmi = forceps minor of the corpus callosum, Pir = piriform cortex, VO = ventro-orbital cortex.
Social neuropeptide contributions to somatic genital reflexes
The neuropeptides/hormones oxytocin (OT) and arginine vasopressin (AVP) have both peripheral and central actions in mammals, including humans. In both rats and humans, OT and AVP are found in the PVN with projections to the posterior pituitary, as well as central brain regions (Hawthorn et al., 1985; Nilaver et al., 1980; Sofroniew, 1980; Swaab et al., 1975; Swanson and Sawchenko, 1980). OT has long been known to be important in the initiation of vaginal muscle contractions during birth (de Geest et al., 1985), as well as for its facilitatory role in milk ejection during breast feeding (Freund-Mercier et al., 1988). AVP (also known as antidiuretic hormone) plays a key role in water retention with its effects on the kidney (Jard et al., 1984).
In addition to these peripheral actions, there has been increasing interest in recent years of the central role of OT and AVP in the control of social behavior in mammals. OT has been linked to the control of social behavior in mammals (Ackerman et al., 1997; Consiglio and Lucion, 1996; Donaldson and Young, 2008; Phelps et al., 2010; Ross and Young, 2009; Veenema and Neumann, 2008), with special focus on the role of OT in the formation of pair-bonds (Hammock and Young, 2006; Macdonald and Macdonald, 2010; Young et al., 2005), as well as its role in sexual behavior (Baskerville and Douglas, 2008). For example, in both humans and rats, OT is released into the blood as a result of sexual behavior (Carmichael et al., 1987; Ivell et al., 1997; Stoneham et al., 1985). AVP has also been linked to social behavior in mammals (Donaldson and Young, 2008; Heinrichs et al., 2009), including pair-bonding (Hammock and Young, 2006), as well as aggression (Gobrogge et al., 2009).
OT has also been implicated in the control of genital reflexes. Systemic or intracerebroventricular OT administration decreases ejaculation latency and the post-ejaculatory interval in male rats (Arletti et al., 1985) and intracerebroventricular OT antagonsists block ejaculation (Argiolas et al., 1988). The effect of OT on ejaculation is also more pronounced in sexually “sluggish” male rats than in those with vigorous sexual responses (Arletti et al., 1990). Moreover, PVN OT cells exhibit sexual behavior-induced Fos immunoreactivity (Witt and Insel, 1994) in male rats. Interestingly, lesions of parvocellular PVN neurons in male rats reduces OT expression in the spinal cord, but does not affect ejaculation, but rather decreases the amount of semen emitted (Ackerman et al., 1997). Consistent with this result, PVN OT cells are only marginally labeled after trans-synaptic retrograde tracer injection into the bulbospongiosus muscle in male rats (Tang et al., 1999), suggesting that PVN OT is not involved in the control of the somatic genital reflexes, but rather autonomic genital reflexes. However, other work has found that peripheral OT antagonist administration to male rats reduces BS muscle contractions, and administration of the OT antagonist to the spinal cord at the L6 level, but not the T13 level, reduces bulbospongiosus muscle contractions and ejaculation (Clement et al., 2008). Intranasal or I.V. administration of OT to men does not alter ejaculation latency or semen quantity (Byrne et al., 2003; Walch et al., 2001), although there is one case report of anorgasmia in a man being effectively treated with intranasal OT (Ishak et al., 2008). From these seemingly inconsistent results, it appears that there are subsets of PVN OT neurons associated with autonomic genital reflexes, and somatic genital reflexes.
We have shown that the PVN provides robust projections (that are active during sexual behavior) to the nPGi (Normandin and Murphy, 2008) and that the PVN receives genitosensory input (as described in the preceding section). This places the PVN in a unique functional anatomical position to release OT and/or AVP as a result of sexual behavior to regulate both sociosexual behavior and genital reflexes.
To explore the possibility of the PVN as an integrator of genitosensory input and behavioral output, tissue from our intra-genital H129- and intra nPGi FG-injected animals were immunohistochemically stained for H129 (rabbit anti-HSV-1, 1:300,000; Dako) or FG (rabbit anti-FG, 1:30,000; Chemicon) with a fluorescent-labeled secondary antibody (Cy2 goat anti-rabbit; Jackson Immunoresearch) and OT (mouse anti-oxytocin, 1:100,000; Millipore) or AVP (guinea pig anti-vasopressin, 1:40,000; Peninsula) with a fluorescent-labeled secondary antibody (Texas Red giant anti-mouse or guinea pig; Jackson Immunoresearch).
The pattern of labeling of PVN cells that project to the nPGi or receive genitosensory information was remarkably similar in both sexes (Figure 5). As our tracing was conducted in separate animals, we cannot definitively say that the same PVN cells project to both the nPGi and receive genitosensory information. However, given that the pattern of labeling of the two circuits is so strikingly similar throughout the PVN, it is likely such labeling represents the same cells.
Figure 5. Photomicrographs of PVN cells projecting to the nPGi in comparison to PVN cell receiving genitosensory information in a male and female rat.
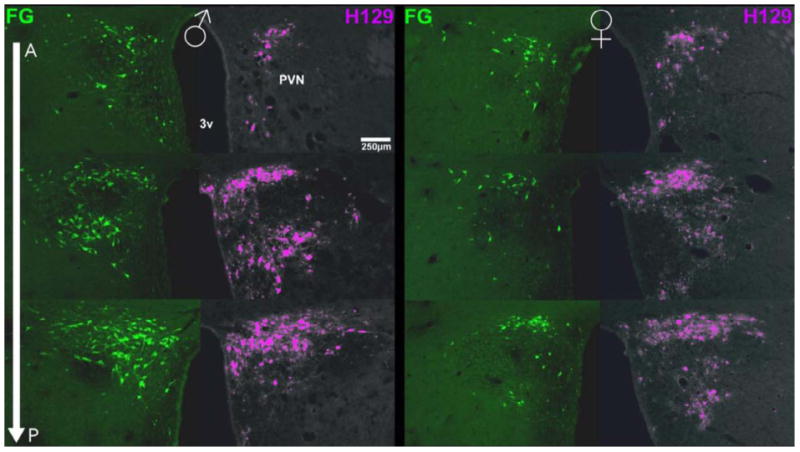
Photomicrographs of the PVN indicate that the number of FG+ cells (green) increased moving caudally through the PVN in a male (left) and a female (right) rat. The number of H129+ cells (pseudocolor purple) also increased moving caudally through the PVN in a male and a female rat. Note the concordance of labeling between those cells projecting to the nPGi (FG+) and those receiving genitosensory information (H129+). A-P = anterior-posterior, 3V = third ventricle, FG = Fluorogold, H129 = herpes simplex virus strain 129
In the more caudal sections of the PVN, some FG labeled cells co-localized with OT labeled cells in both sexes (Figure 6), and such co-localization occurred in mostly parvocellular and some magnocellular cells. Such co-localization implies that OT could be a modulator of genital reflexes through the nPGi. Inhibition of male rat sexual behavior by the selective serotonin reuptake inhibitor fluoxetine can be reversed by administration of OT (Cantor et al., 1999), and it is possible that such an effect could be mediated by the circuit we have delineated here. Indeed, OT fibers are present in the nPGi in both sexes (unpublished observation), and PVN OT cells also project to the spinal cord motor neurons involved in genital reflexes in male rats (Tang et al., 1998) as previously mentioned.
Figure 6. Photomicrographs of PVN cells projecting to the nPGi and co-localization with oxytocin in a male and female rat.
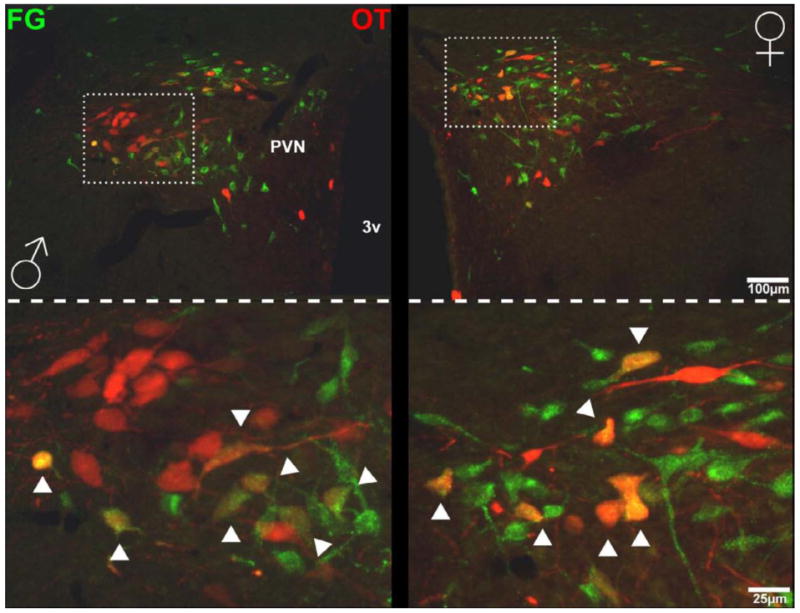
Photomicrographs of the PVN at a lower magnification (top) and higher magnification (bottom) show that FG+ cells (green) co-localized with oxytocin cells (red) in a male (left) and a female (right) rat. Arrows indicate co-localization. 3V = third ventricle, FG = Fluorogold, OT = oxytocin.
H129 labeled cells in the caudal portion of the PVN co-localized with OT (Figure 7), and these cells appear to co-localize with OT to a greater degree in females. In contrast to OT co-localization with PVN cells projecting to the nPGi, OT co-localization with PVN cells receiving genitosensory information was primarily magnocellular. The difference in the co-localization of OT with our tracers within different subsets of PVN cells implies separate functional connectivity. It is possible that the magnocellular OT cells that receive genitosensory information further project to forebrain regions involved in social behavior, whereas the parvocellular OT cells projecting to the nPGi participate in the regulation of somatic genital reflexes. Supporting this idea, Ross et al. (2009) found that in voles, magnocellular OT cells project to the nucleus accumbens. In addition, OT in the accumbens is critical for the expression of mating-induced pair-bonding in female voles (Liu and Wang, 2003) and magnocellular oxytocinergic PVN cells that receive genitosensory information may therefore represent the functional anatomical link between mating and pair-bonding.
Figure 7. Photomicrographs of PVN that receive genitosensory information and co-localization with oxytocin in a male and female rat.
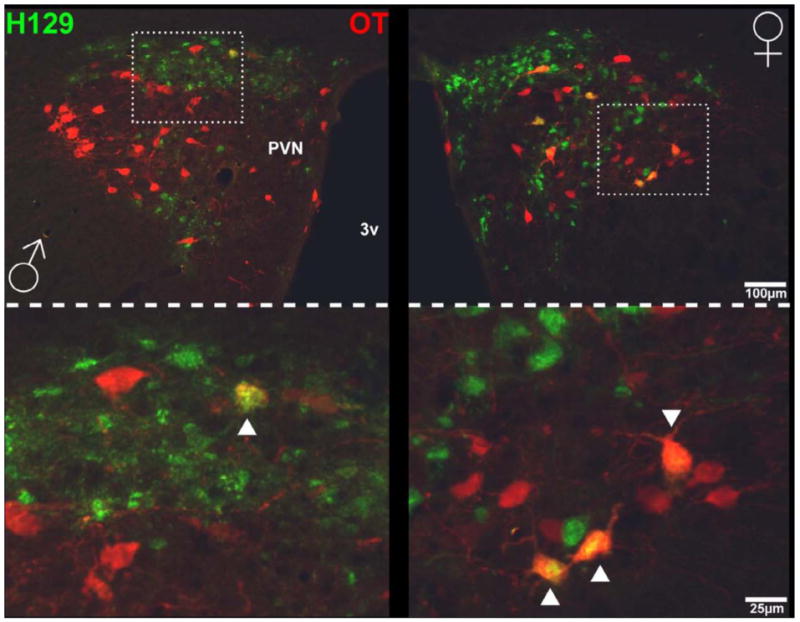
Photomicrographs of the PVN at a lower magnification (top) and higher magnification (bottom) show that H129+ cells (green) co-localized with oxytocin cells (red) in a male (left) and a female (right) rat. Arrows indicate co-localization. 3V = third ventricle, H129 = herpes simplex virus strain 129, OT = oxytocin.
Neither FG labeled nor H129 labeled cells in the PVN co-localized with AVP in either sex with the exception of one or two cells. This result is not surprising given that AVP fibers are not found in the nPGi (unpublished observations), and no evidence of vasopressinergic modulation of genital reflexes has been described. However, AVP is an important mediator of mating-induced pair-bonding in male voles (Liu et al., 2001) and one might expect that PVN cells receiving genitosensory information might contain AVP, which we did not observe. This implies that there is another mediator between genitosensation and AVP release.
The majority of PVN cells projecting to the nPGi and PVN cells receiving genitosensory information did not contain OT. This is an important finding suggesting that these circuits, with respect to the PVN, also have other neurochemical components the need to be characterized.
Conclusion
Sexual dysfunction is a common problem in both men and women. Up to 31% of men and 43% of women will experience sexual dysfunction in their lifetime (Laumann et al., 1999). Such dysfunctions can include loss of sex drive, premature ejaculation, delayed ejaculation, anorgasmia, involuntary vaginal spasms, and vaginal pain during intercourse (Breiner, 2004). These dysfunctions have a large impact on fertility and quality of life experiences. These sexual dysfunctions all have an underlying dysregulation of genital reflexes in common. By identifying the regions of the brain that regulate somatic genital reflexes, we can provide new insights and treatment targets for people with sexual dysfunction.
The lumbosacral spinal cord is the final source of output to the genital musculature, and activity at the level of the lumbosacral motoneuron pools is influenced by a number of descending brain systems to regulate somatic genital reflexes. The LSt cells of the spinal cord, in conjunction with the SPFpc of the thalamus, appear to coordinate the rhythmic contractions of the genital musculature through these lumbosacral targets. In addition, these lumbosacral targets are under tonic inhibition provided by the nPGi, which in turn is receives projections from the hypothalamus and PAG in a sexually dimorphic pattern. Ascending genitosensory information appears to directly influence these lumbosacral targets but also ascends to many regions associated with the regulation of sexual behavior, including the OT-and AVP-containing neurons in the PVN. These data are summarized in Figure 8. The neural/neurochemical regions described may prove to be important targets for treating sexual dysfunction in people.
Figure 8. Schematic of the ins and outs of somatic genital reflexes in rats.
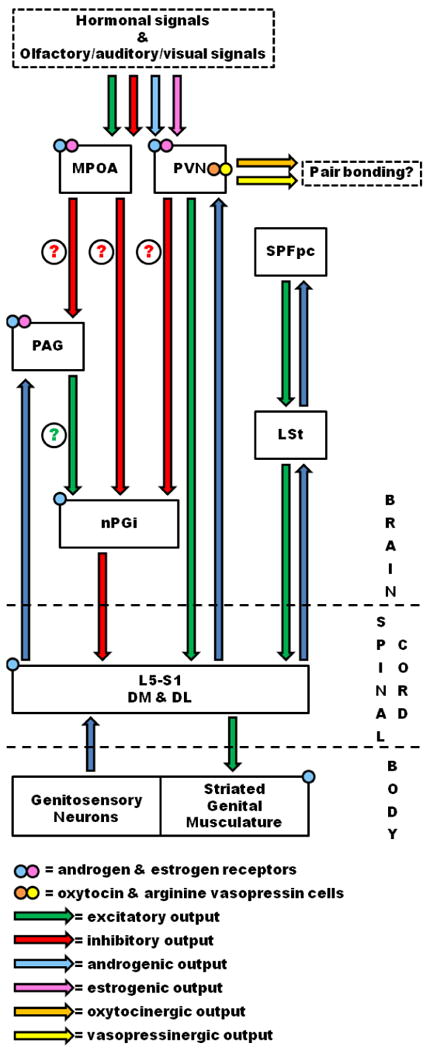
The schematic illustrates the inputs, outputs, and relative physiology of the brain, spinal cord, and somatic regions discussed in the text.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman AE, Lange GM, Clemens LG. Effects of paraventricular lesions on sex behavior and seminal emission in male rats. Physiol Behav. 1997;63:49–53. doi: 10.1016/s0031-9384(97)00386-7. [DOI] [PubMed] [Google Scholar]
- Allard J, Truitt WA, McKenna KE, Coolen LM. Spinal cord control of ejaculation. World J Urol. 2005;23:119–126. doi: 10.1007/s00345-004-0494-9. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Collu M, Gessa GL, Melis MR, Serra G. The oxytocin antagonist d(CH2)5Tyr(Me)-Orn8-vasotocin inhibits male copulatory behaviour in rats. Eur J Pharmacol. 1988;149:389–392. doi: 10.1016/0014-2999(88)90675-9. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR. The neurophysiology of the sexual cycle. J Endocrinol Invest. 2003;26:20–22. [PubMed] [Google Scholar]
- Arletti R, Bazzani C, Castelli M, Bertolini A. Oxytocin improves male copulatory performance in rats. Horm Behav. 1985;19:14–20. doi: 10.1016/0018-506x(85)90002-9. [DOI] [PubMed] [Google Scholar]
- Arletti R, Benelli A, Bertolini A. Sexual behavior of aging male rats is stimulated by oxytocin. Eur J Pharmacol. 1990;179:377–381. doi: 10.1016/0014-2999(90)90178-9. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville TA, Douglas AJ. Interactions between dopamine and oxytocin in the control of sexual behaviour. Prog Brain Res. 2008;170:277–290. doi: 10.1016/S0079-6123(08)00423-8. [DOI] [PubMed] [Google Scholar]
- Blaivas JG, Zayed AA, Labib KB. The bulbocavernosus reflex in urology: a prospective study of 299 patients. J Urol. 1981;126:197–199. doi: 10.1016/s0022-5347(17)54445-6. [DOI] [PubMed] [Google Scholar]
- Borgdorff AJ, Bernabe J, Denys P, Alexandre L, Giuliano F. Ejaculation elicited by microstimulation of lumbar spinothalamic neurons. Eur Urol. 2008;54:449–456. doi: 10.1016/j.eururo.2008.03.043. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Breiner SJ. Male sexual dysfunction. JAMA. 2004;292:2722. doi: 10.1001/jama.292.22.2722-a. author reply 2722-2723. [DOI] [PubMed] [Google Scholar]
- Byrne MM, Rolf C, Depenbusch M, Cooper TG, Nieschlag E. Lack of effect of a single i.v. dose of oxytocin on sperm output in severely oligozoospermic men. Hum Reprod. 2003;18:2098–2102. doi: 10.1093/humrep/deg416. [DOI] [PubMed] [Google Scholar]
- Cantor JM, Binik YM, Pfaus JG. Chronic fluoxetine inhibits sexual behavior in the male rat: reversal with oxytocin. Psychopharmacology (Berl) 1999;144:355–362. doi: 10.1007/s002130051018. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Carro-Juarez M, Rodriguez-Manzo G. Evidence for the presence of the spinal pattern generator involved in the control of the genital ejaculatory pattern in the female rat. Brain Res. 2006;1084:54–60. doi: 10.1016/j.brainres.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Castro-Vasquez A, Carreno NB. A study of the sensory requirements for eliciting the pseudopregnancy response. Brain Res. 1981;230:205–220. doi: 10.1016/0006-8993(81)90402-9. [DOI] [PubMed] [Google Scholar]
- Chen KK, Chan SH, Chang LS, Chan JY. Participation of paraventricular nucleus of hypothalamus in central regulation of penile erection in the rat. J Urol. 1997;158:238–244. doi: 10.1097/00005392-199707000-00078. [DOI] [PubMed] [Google Scholar]
- Clement P, Bernabe J, Gengo P, Denys P, Laurin M, Alexandre L, Giuliano F. Supraspinal site of action for the inhibition of ejaculatory reflex by dapoxetine. Eur Urol. 2007;51:825–832. doi: 10.1016/j.eururo.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Clement P, Peeters M, Bernabe J, Denys P, Alexandre L, Giuliano F. Brain oxytocin receptors mediate ejaculation elicited by 7-hydroxy-2-(di-N-propylamino) tetralin (7-OH-DPAT) in anaesthetized rats. Br J Pharmacol. 2008;154:1150–1159. doi: 10.1038/bjp.2008.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WF, 3rd, Erichsen JT, Rose RD. Pudendal motor and premotor neurons in the male rat: a WGA transneuronal study. J Comp Neurol. 1991;308:28–41. doi: 10.1002/cne.903080104. [DOI] [PubMed] [Google Scholar]
- Consiglio AR, Lucion AB. Lesion of hypothalamic paraventricular nucleus and maternal aggressive behavior in female rats. Physiol Behav. 1996;59:591–596. doi: 10.1016/0031-9384(95)02117-5. [DOI] [PubMed] [Google Scholar]
- Coolen LM. Neural control of ejaculation. J Comp Neurol. 2005;493:39–45. doi: 10.1002/cne.20784. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Veening JG, Petersen DW, Shipley MT. Parvocellular subparafascicular thalamic nucleus in the rat: anatomical and functional compartmentalization. J Comp Neurol. 2003a;463:117–131. doi: 10.1002/cne.10740. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Veening JG, Wells AB, Shipley MT. Afferent connections of the parvocellular subparafascicular thalamic nucleus in the rat: evidence for functional subdivisions. J Comp Neurol. 2003b;463:132–156. doi: 10.1002/cne.10739. [DOI] [PubMed] [Google Scholar]
- Dail WG, Manzanares K, Moll MA, Minorsky N. The hypogastric nerve innervates a population of penile neurons in the pelvic plexus. Neuroscience. 1985;16:1041–1046. doi: 10.1016/0306-4522(85)90114-9. [DOI] [PubMed] [Google Scholar]
- de Araujo CG, Schmidt RA, Tanagho EA. Neural pathways to lower urinary tract identified by retrograde axonal transport of horseradish peroxidase. Urology. 1982;19:290–295. doi: 10.1016/0090-4295(82)90502-7. [DOI] [PubMed] [Google Scholar]
- de Geest K, Thiery M, Piron-Possuyt G, Vanden Driessche R. Plasma oxytocin in human pregnancy and parturition. J Perinat Med. 1985;13:3–13. doi: 10.1515/jpme.1985.13.1.3. [DOI] [PubMed] [Google Scholar]
- deGroat WC, Booth AM. Physiology of male sexual function. Ann Intern Med. 1980;92:329–331. doi: 10.7326/0003-4819-92-2-329. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Markowski VP, Lumley LA, Thompson JT, Moses J, Hull EM. D2 receptors in the paraventricular nucleus regulate genital responses and copulation in male rats. Pharmacol Biochem Behav. 1991;39:177–181. doi: 10.1016/0091-3057(91)90418-2. [DOI] [PubMed] [Google Scholar]
- Ertekin C, Reel F. Bulbocavernosus reflex in normal men and in patients with neurogenic bladder and/or impotence. J Neurol Sci. 1976;28:1–15. doi: 10.1016/0022-510x(76)90044-7. [DOI] [PubMed] [Google Scholar]
- Forger NG, Breedlove SM. Sexual dimorphism in human and canine spinal cord: role of early androgen. Proc Natl Acad Sci U S A. 1986;83:7527–7531. doi: 10.1073/pnas.83.19.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LM, Padgett BA, Prins GS, Breedlove SM. Distribution of androgen receptor immunoreactivity in the spinal cord of wild-type, androgen-insensitive and gonadectomized male rats. Journal of neurobiology. 1995;27:51–59. doi: 10.1002/neu.480270106. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Moos F, Poulain DA, Richard P, Rodriguez F, Theodosis DT, Vincent JD. Role of central oxytocin in the control of the milk ejection reflex. Brain Res Bull. 1988;20:737–741. doi: 10.1016/0361-9230(88)90085-8. [DOI] [PubMed] [Google Scholar]
- Garner JA, LaVail JH. Differential anterograde transport of HSV type 1 viral strains in the murine optic pathway. J Neurovirol. 1999;5:140–150. doi: 10.3109/13550289909021996. [DOI] [PubMed] [Google Scholar]
- Gerstenberg TC, Levin RJ, Wagner G. Erection and ejaculation in man. Assessment of the electromyographic activity of the bulbocavernosus and ischiocavernosus muscles. Br J Urol. 1990;65:395–402. doi: 10.1111/j.1464-410x.1990.tb14764.x. [DOI] [PubMed] [Google Scholar]
- Giraldi A, Marson L, Nappi R, Pfaus J, Traish AM, Vardi Y, Goldstein I. Physiology of female sexual function: animal models. J Sex Med. 2004;1:237–253. doi: 10.1111/j.1743-6109.04037.x. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Bernabe J, Gengo P, Alexandre L, Clement P. Effect of acute dapoxetine administration on the pudendal motoneuron reflex in anesthetized rats: comparison with paroxetine. J Urol. 2007;177:386–389. doi: 10.1016/j.juro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Clement P. Neuroanatomy and physiology of ejaculation. Annu Rev Sex Res. 2005;16:190–216. [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A. 2009;106:19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond B Biol Sci. 2006;361:2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamson DK, Jones BA, Watson NV. Distribution of androgen receptor immunoreactivity in the brainstem of male rats. Neuroscience. 2004;127:797–803. doi: 10.1016/j.neuroscience.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Hart BL, Melese-D'Hospital PY. Penile mechanisms and the role of the striated penile muscles in penile reflexes. Physiol Behav. 1983;31:807–813. doi: 10.1016/0031-9384(83)90277-9. [DOI] [PubMed] [Google Scholar]
- Hawthorn J, Ang VT, Jenkins JS. Effects of lesions in the hypothalamic paraventricular, supraoptic and suprachiasmatic nuclei on vasopressin and oxytocin in rat brain and spinal cord. Brain Res. 1985;346:51–57. doi: 10.1016/0006-8993(85)91093-5. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Holmes GM, Rogers RC, Beattie MS, Bresnahan JC. Descending spinal projections from the rostral gigantocellular reticular nuclei complex. J Comp Neurol. 2003;455:210–221. doi: 10.1002/cne.10455. [DOI] [PubMed] [Google Scholar]
- Holmes GM, Chapple WD, Leipheimer RE, Sachs BD. Electromyographic analysis of male rat perineal muscles during copulation and reflexive erections. Physiol Behav. 1991;49:1235–1246. doi: 10.1016/0031-9384(91)90357-t. [DOI] [PubMed] [Google Scholar]
- Holstege G. The emotional motor system. Eur J Morphol. 1992;30:67–79. [PubMed] [Google Scholar]
- Holstege G, Georgiadis JR. The emotional brain: neural correlates of cat sexual behavior and human male ejaculation. Progress in brain research. 2004;143:39–45. doi: 10.1016/S0079-6123(03)43004-5. [DOI] [PubMed] [Google Scholar]
- Holstege G, Georgiadis JR, Paans AM, Meiners LC, van der Graaf FH, Reinders AA. Brain activation during human male ejaculation. J Neurosci. 2003;23:9185–9193. doi: 10.1523/JNEUROSCI.23-27-09185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SH, Wei N, Wang QD, Yan LQ, Wei EQ, Zhang MM, Hu JB, Huang ML, Zhou WH, Xu Y. Patterns of brain activation during visually evoked sexual arousal differ between homosexual and heterosexual men. AJNR Am J Neuroradiol. 2008;29:1890–1896. doi: 10.3174/ajnr.A1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubscher CH, Johnson RD. Responses of medullary reticular formation neurons to input from the male genitalia. J Neurophysiol. 1996;76:2474–2482. doi: 10.1152/jn.1996.76.4.2474. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Reed WR, Kaddumi EG, Armstrong JE, Johnson RD. Select spinal lesions reveal multiple ascending pathways in the rat conveying input from the male genitalia. J Physiol. 2010;588:1073–1083. doi: 10.1113/jphysiol.2009.186544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak WW, Berman DS, Peters A. Male anorgasmia treated with oxytocin. J Sex Med. 2008;5:1022–1024. doi: 10.1111/j.1743-6109.2007.00691.x. [DOI] [PubMed] [Google Scholar]
- Ivell R, Balvers M, Rust W, Bathgate R, Einspanier A. Oxytocin and male reproductive function. Adv Exp Med Biol. 1997;424:253–264. doi: 10.1007/978-1-4615-5913-9_47. [DOI] [PubMed] [Google Scholar]
- Jard S, Butlen D, Cantau B, Guillon G, Marie J, Roy C. The mechanisms of action of antidiuretic hormone. Adv Nephrol Necker Hosp. 1984;13:163–177. [PubMed] [Google Scholar]
- Johnson RD. Descending pathways modulating the spinal circuitry for ejaculation: effects of chronic spinal cord injury. Prog Brain Res. 2006;152:415–426. doi: 10.1016/S0079-6123(05)52028-4. [DOI] [PubMed] [Google Scholar]
- Johnson RD, Hubscher CH. Brainstem microstimulation differentially inhibits pudendal motoneuron reflex inputs. Neuroreport. 1998;9:341–345. doi: 10.1097/00001756-199801260-00030. [DOI] [PubMed] [Google Scholar]
- Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, Joubert S, Beauregard M. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T, Gibson SJ, Su HC, Polak JM. Composition and central projections of the pudendal nerve in the rat investigated by combined peptide immunocytochemistry and retrograde fluorescent labelling. Brain Res. 1986;372:313–322. doi: 10.1016/0006-8993(86)91139-x. [DOI] [PubMed] [Google Scholar]
- Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537–544. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- Lee JW, Erskine MS. Pseudorabies virus tracing of neural pathways between the uterine cervix and CNS: effects of survival time, estrogen treatment, rhizotomy, and pelvic nerve transection. J Comp Neurol. 2000;418:484–503. [PubMed] [Google Scholar]
- Levin RJ. Sex and the human female reproductive tract--what really happens during and after coitus. Int J Impot Res. 1998;10 1:S14–21. [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Liu YC, Salamone JD, Sachs BD. Impaired sexual response after lesions of the paraventricular nucleus of the hypothalamus in male rats. Behav Neurosci. 1997;111:1361–1367. doi: 10.1037//0735-7044.111.6.1361. [DOI] [PubMed] [Google Scholar]
- Macdonald K, Macdonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry. 2010;18:1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- Marson L, Carson CC., 3rd Central Nervous System Innervation of the Penis, Prostate, and Perineal Muscles: A Transneuronal Tracing Study. Mol Urol. 1999;3:43–50. [PubMed] [Google Scholar]
- Marson L, Foley KA. Identification of neural pathways involved in genital reflexes in the female: a combined anterograde and retrograde tracing study. Neuroscience. 2004;127:723–736. doi: 10.1016/j.neuroscience.2004.04.063. [DOI] [PubMed] [Google Scholar]
- Marson L, List MS, McKenna KE. Lesions of the nucleus paragigantocellularis alter ex copula penile reflexes. Brain Res. 1992;592:187–192. doi: 10.1016/0006-8993(92)91675-5. [DOI] [PubMed] [Google Scholar]
- Marson L, McKenna KE. The identification of a brainstem site controlling spinal sexual reflexes in male rats. Brain Res. 1990;515:303–308. doi: 10.1016/0006-8993(90)90611-e. [DOI] [PubMed] [Google Scholar]
- Marson L, McKenna KE. CNS cell groups involved in the control of the ischiocavernosus and bulbospongiosus muscles: a transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1996;374:161–179. doi: 10.1002/(SICI)1096-9861(19961014)374:2<161::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Marson L, Murphy AZ. Identification of neural circuits involved in female genital responses in the rat: a dual virus and anterograde tracing study. Am J Physiol Regul Integr Comp Physiol. 2006;291:R419–428. doi: 10.1152/ajpregu.00864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Alguacil N, Schober JM, Sengelaub DR, Pfaff DW, Shelley DN. Clitoral sexual arousal: neuronal tracing study from the clitoris through the spinal tracts. J Urol. 2008;180:1241–1248. doi: 10.1016/j.juro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McKenna KE. The neurophysiology of female sexual function. World J Urol. 2002;20:93–100. doi: 10.1007/s00345-002-0270-7. [DOI] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The pudendo-pudendal reflex in male and female rats. J Auton Nerv Syst. 1989;27:67–77. doi: 10.1016/0165-1838(89)90130-6. [DOI] [PubMed] [Google Scholar]
- Meston CM, Levin RJ, Sipski ML, Hull EM, Heiman JR. Women's orgasm. Annu Rev Sex Res. 2004;15:173–257. [PubMed] [Google Scholar]
- Murphy AZ, Hoffman GE. Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: a potential circuit for the initiation of male sexual behavior. J Comp Neurol. 2001;438:191–212. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Rizvi TA, Ennis M, Shipley MT. The organization of preoptic-medullary circuits in the male rat: evidence for interconnectivity of neural structures involved in reproductive behavior, antinociception and cardiovascular regulation. Neuroscience. 1999;91:1103–1116. doi: 10.1016/s0306-4522(98)00677-0. [DOI] [PubMed] [Google Scholar]
- Newton BW. Galanin immunoreactivity in rat spinal lamina IX: emphasis on sexually dimorphic regions. Peptides. 1993;14:955–969. doi: 10.1016/0196-9781(93)90072-o. [DOI] [PubMed] [Google Scholar]
- Nilaver G, Zimmerman EA, Wilkins J, Michaels J, Hoffman D, Silverman AJ. Magnocellular hypothalamic projections to the lower brain stem and spinal cord of the rat. Immunocytochemical evidence for predominance of the oxytocin-neurophysin system compared to the vasopressin-neurophysin system. Neuroendocrinology. 1980;30:150–158. doi: 10.1159/000122991. [DOI] [PubMed] [Google Scholar]
- Normandin JJ, Murphy AZ. Nucleus paragigantocellularis afferents in male and female rats: organization, gonadal steroid receptor expression, and activation during sexual behavior. J Comp Neurol. 2008;508:771–794. doi: 10.1002/cne.21704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normandin JJ, Murphy AZ. Excitotoxic lesions of the nucleus paragigantocellularis facilitate male sexual behavior but attenuate female sexual behavior in rats. Neuroscience. 2011;175:212–223. doi: 10.1016/j.neuroscience.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez R, Gross GH, Sachs BD. Origin and central projections of rat dorsal penile nerve: possible direct projection to autonomic and somatic neurons by primary afferents of nonmuscle origin. J Comp Neurol. 1986;247:417–429. doi: 10.1002/cne.902470402. [DOI] [PubMed] [Google Scholar]
- Pacheco P, Martinez-Gomez M, Whipple B, Beyer C, Komisaruk BR. Somato-motor components of the pelvic and pudendal nerves of the female rat. Brain Res. 1989;490:85–94. doi: 10.1016/0006-8993(89)90433-2. [DOI] [PubMed] [Google Scholar]
- Pastelin CF, Zempoalteca R, Pacheco P, Downie JW, Cruz Y. Sensory and somatomotor components of the “sensory branch” of the pudendal nerve in the male rat. Brain Res. 2008;1222:149–155. doi: 10.1016/j.brainres.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 3rd ed. Academic Press; San Diego: 1997. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 5th ed. Elsevier Academic Press; Amsterdam ; Boston: 2005. [Google Scholar]
- Peshori KR, Erichsen JT, Collins WF., 3rd Differences in the connectivity of rat pudendal motor nuclei as revealed by retrograde transneuronal transport of wheat germ agglutinin. J Comp Neurol. 1995;353:119–128. doi: 10.1002/cne.903530111. [DOI] [PubMed] [Google Scholar]
- Peters LC, Kristal MB, Komisaruk BR. Sensory innervation of the external and internal genitalia of the female rat. Brain Res. 1987;408:199–204. doi: 10.1016/0006-8993(87)90372-6. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Kippin TE, Coria-Avila G. What can animal models tell us about human sexual response? Annu Rev Sex Res. 2003;14:1–63. [PubMed] [Google Scholar]
- Pfaus JG, Manitt C, Coopersmith CB. Effects of pelvic, pudendal, or hypogastric nerve cuts on Fos induction in the rat brain following vaginocervical stimulation. Physiol Behav. 2006;89:627–636. doi: 10.1016/j.physbeh.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Phelps SM, Campbell P, Zheng DJ, Ophir AG. Beating the boojum: comparative approaches to the neurobiology of social behavior. Neuropharmacology. 2010;58:17–28. doi: 10.1016/j.neuropharm.2009.06.043. [DOI] [PubMed] [Google Scholar]
- Purinton PT, Fletcher TF, Bradley WE. Innervation of pelvic viscera in the rat. Evoked potentials in nerves to bladder and penis (clitoris) Invest Urol. 1976;14:28–32. [PubMed] [Google Scholar]
- Rand MN, Breedlove SM. Ontogeny of functional innervation of bulbocavernosus muscles in male and female rats. Brain Res. 1987;430:150–152. doi: 10.1016/0165-3806(87)90186-6. [DOI] [PubMed] [Google Scholar]
- Roppolo JR, Nadelhaft I, de Groat WC. The organization of pudendal motoneurons and primary afferent projections in the spinal cord of the rhesus monkey revealed by horseradish peroxidase. J Comp Neurol. 1985;234:475–488. doi: 10.1002/cne.902340406. [DOI] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DW, Erskine MS. c-Fos proto-oncogene activity induced by mating in the preoptic area, hypothalamus and amygdala in the female rat: role of afferent input via the pelvic nerve. Brain Res. 1993;621:25–34. doi: 10.1016/0006-8993(93)90294-w. [DOI] [PubMed] [Google Scholar]
- Sachs BD. Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J Reprod Fertil. 1982;66:433–443. doi: 10.1530/jrf.0.0660433. [DOI] [PubMed] [Google Scholar]
- Sakuma Y. Neural substrates for sexual preference and motivation in the female and male rat. Ann N Y Acad Sci. 2008;1129:55–60. doi: 10.1196/annals.1417.009. [DOI] [PubMed] [Google Scholar]
- Schroder HD. Organization of the motoneurons innervating the pelvic muscles of the male rat. J Comp Neurol. 1980;192:567–587. doi: 10.1002/cne.901920313. [DOI] [PubMed] [Google Scholar]
- Schroder HD. Onuf's nucleus X: a morphological study of a human spinal nucleus. Anat Embryol (Berl) 1981;162:443–453. doi: 10.1007/BF00301870. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Projections from vasopressin, oxytocin, and neurophysin neurons to neural targets in the rat and human. J Histochem Cytochem. 1980;28:475–478. doi: 10.1177/28.5.7381192. [DOI] [PubMed] [Google Scholar]
- Stoneham MD, Everitt BJ, Hansen S, Lightman SL, Todd K. Oxytocin and sexual behaviour in the male rat and rabbit. J Endocrinol. 1985;107:97–106. doi: 10.1677/joe.0.1070097. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Pool CW, Nijveldt F. Immunofluorescence of vasopressin and oxytocin in the rat hypothalamo-neurohypophypopseal system. J Neural Transm. 1975;36:195–215. doi: 10.1007/BF01253126. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Tang Y, Rampin O, Calas A, Facchinetti P, Giuliano F. Oxytocinergic and serotonergic innervation of identified lumbosacral nuclei controlling penile erection in the male rat. Neuroscience. 1998;82:241–254. doi: 10.1016/s0306-4522(97)00290-x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Rampin O, Giuliano F, Ugolini G. Spinal and brain circuits to motoneurons of the bulbospongiosus muscle: retrograde transneuronal tracing with rabies virus. J Comp Neurol. 1999;414:167–192. [PubMed] [Google Scholar]
- Temel Y, Hafizi S, Beuls E, Visser-Vandewalle V. The supraspinal network in the control of erection. Expert Opin Ther Targets. 2005;9:941–954. doi: 10.1517/14728222.9.5.941. [DOI] [PubMed] [Google Scholar]
- Truitt WA, Coolen LM. Identification of a potential ejaculation generator in the spinal cord. Science. 2002;297:1566–1569. doi: 10.1126/science.1073885. [DOI] [PubMed] [Google Scholar]
- Truitt WA, Shipley MT, Veening JG, Coolen LM. Activation of a subset of lumbar spinothalamic neurons after copulatory behavior in male but not female rats. J Neurosci. 2003;23:325–331. doi: 10.1523/JNEUROSCI.23-01-00325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhorst VG, Holstege G. Caudal medullary pathways to lumbosacral motoneuronal cell groups in the cat: evidence for direct projections possibly representing the final common pathway for lordosis. The Journal of comparative neurology. 1995;359:457–475. doi: 10.1002/cne.903590308. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Vodusek DB, Janko M, Lokar J. Direct and reflex responses in perineal muscles on electrical stimulation. J Neurol Neurosurg Psychiatry. 1983;46:67–71. doi: 10.1136/jnnp.46.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CK, Clemens LG. Projections of the paraventricular nucleus of the hypothalamus to the sexually dimorphic lumbosacral region of the spinal cord. Brain Res. 1991;539:254–262. doi: 10.1016/0006-8993(91)91629-f. [DOI] [PubMed] [Google Scholar]
- Walch K, Eder R, Schindler A, Feichtinger W. The effect of single-dose oxytocin application on time to ejaculation and seminal parameters in men. J Assist Reprod Genet. 2001;18:655–659. doi: 10.1023/A:1013115301159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Bermpohl F, Mouras H, Schiltz K, Tempelmann C, Rotte M, Heinze HJ, Bogerts B, Northoff G. Distinguishing specific sexual and general emotional effects in fMRI-subcortical and cortical arousal during erotic picture viewing. Neuroimage. 2008;40:1482–1494. doi: 10.1016/j.neuroimage.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Wiedey J, Alexander MS, Marson L. Spinal neurons activated in response to pudendal or pelvic nerve stimulation in female rats. Brain Res. 2008;1197:106–114. doi: 10.1016/j.brainres.2007.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt DM, Insel TR. Increased Fos expression in oxytocin neurons following masculine sexual behavior. J Neuroendocrinol. 1994;6:13–18. doi: 10.1111/j.1365-2826.1994.tb00549.x. [DOI] [PubMed] [Google Scholar]
- Xu C, Giuliano F, Yaici ED, Conrath M, Trassard O, Benoit G, Verge D. Identification of lumbar spinal neurons controlling simultaneously the prostate and the bulbospongiosus muscles in the rat. Neuroscience. 2006;138:561–573. doi: 10.1016/j.neuroscience.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Yang CC, Jiang X. Clinical autonomic neurophysiology and the male sexual response: an overview. J Sex Med. 2009;63 3:221–228. doi: 10.1111/j.1743-6109.2008.01180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yells DP, Hendricks SE, Prendergast MA. Lesions of the nucleus paragigantocellularis: effects on mating behavior in male rats. Brain Res. 1992;596:73–79. doi: 10.1016/0006-8993(92)91534-l. [DOI] [PubMed] [Google Scholar]
- Yells DP, Prendergast MA, Hendricks SE, Nakamura M. Fluoxetine-induced inhibition of male rat copulatory behavior: modification by lesions of the nucleus paragigantocellularis. Pharmacol Biochem Behav. 1994;49:121–127. doi: 10.1016/0091-3057(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Young LJ, Murphy Young AZ, Hammock EA. Anatomy and neurochemistry of the pair bond. J Comp Neurol. 2005;493:51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
- Zec N, Kinney HC. Anatomic relationships of the human nucleus paragigantocellularis lateralis: a DiI labeling study. Auton Neurosci. 2001;89:110–124. doi: 10.1016/S1566-0702(01)00258-2. [DOI] [PubMed] [Google Scholar]
- Zemanick MC, Strick PL, Dix RD. Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain-dependent. Proc Natl Acad Sci U S A. 1991;88:8048–8051. doi: 10.1073/pnas.88.18.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]


