The meninges have traditionally been viewed as specialized membranes surrounding and protecting the adult brain from injury. However, there is increasing evidence that the fetal meninges play important roles during brain development. Through the release of diffusible factors, the meninges influence the proliferative and migratory behavior of neural progenitors and neurons in the forebrain and hindbrain. Meningeal cells also secrete and organize the pial basement membrane, a critical anchor point for the radially-oriented fibers of neuroepithelial stem cells. With its emerging role in brain development, the potential that defects in meningeal development may underlie certain congenital brain abnormalities in humans should be considered. In this review, we will discuss what is known about assembly of the fetal meninges and review the role of meningeal-derived proteins in mouse and human brain development.
Origin and Structure of the Fetal Meninges
The first, primitive layer of meningeal cells is identifiable early in neural development: in chick, at HH15 (Embryonic day or E2) [1], in mouse between E9-E10 [2] and in human, Carnegie stage 15 or ~4th gestational week [3]. In the forebrain, this initial layer of meningeal cells is part of a wave of rostrally migrating cranial neural crest cells originating from the diencephalic neural crest [4]. In contrast, the meninges surrounding the midbrain, hindbrain, and spinal cord originate from the cephalic and somatic mesoderm [5-7] (Fig. 1A). This first group of meningeal cells ultimately becomes part of the leptomeninges, consisting of the two inner layers - the pia and arachnoid. In embryos, the leptomeninges are a loose network of cells lying in close contact with the surface of the brain, adjacent to the glial limitans, and intermingled with blood vessels of the perineural vascular plexus (Fig. 1B). The leptomeninges are first identifiable in human embryos between stages 17 and 18 [3] and ~E13 in mouse [2]. The outermost meningeal layer, the dura, forms between the leptomeninges and the calvarial mesenchyme, from which the calvarial bones will eventually form (Fig. 1B). Cells of the cephalic dura are first seen in mouse embryos at ~E14 [8] coinciding with the initial apical expansion of the calvarial bones [9,10].
Figure 1.
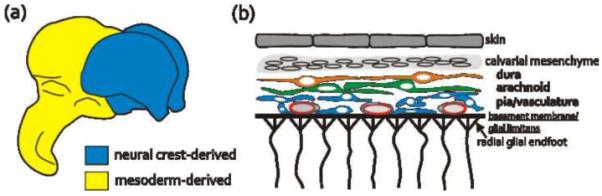
Origin and structure of the fetal meninges
(a) The meninges surrounding the forebrain are neural crest-derived (blue) whereas the meninges covering the rest of the brain and spinal cord originate from the somatic mesoderm.
(b) The pial meningeal cells and blood vessels are in close contact with the pial basement membrane, the attachment point for radial glial endfeet. The two outer meningeal layers, the arachnoid and dural layers, are single layers of cells beneath the calvarial mesenchyme.
Very little is known about regulation of meningeal development. Some insights into meningeal assembly came from studies showing that loss of the transcription factor Foxc1 disrupts formation of the forebrain meninges. A spontaneously occurring Foxc1 mouse mutant (the congenital hydrocephalus or ch mutant) was noted to have marked thinning of the meningeal layer in the initial reports describing these mice [11,12]. Years later, analysis of the ch mutant and mice with targeted disruption of Foxc1 (Foxc1-lacZ) confirmed that forebrain arachnoid and dural layers were absent except over the ventral surface [13, 14*, 15**]. While the exact role of Foxc1 in meningeal development is unclear, the meningeal phenotype in a less affected Foxc1 hypomorphic mutant (Foxc1-hith) [8] suggests that it regulates meningeal cell migration. In Foxc1 hypomorphs, the meninges are missing only from the more dorsal aspects of the forebrain and even in the Foxc1 hypomorph-null hybrid (Foxc1hith/lacz) the arachnoid and dural layers extend partially around the forebrain [15**]. This suggests that, at least in the forebrain, meningeal development may involve a ventral-dorsal wave of meningeal cell migration and that Foxc1 is critical for this process.
Secreted factors from the meninges regulate neural migration and positioning
The meninges, with their close proximately to the developing brain, are strategically positioned to provide short-range cues to neural cells located in the outer layer of developing brain structures. One example of this type of signal is Cxcl12 (aka SDF-1), which mediates chemoattraction of multiple cell types via binding to its receptors Cxcr4 and Cxcr7 (for review see [16]). In the forebrain and cerebellum specifically, meningeal-derived Cxcl12 plays an important role in neuronal migration and cell positioning.
Subpial migratory routes regulated by Cxcl12 are a common theme in development
During forebrain development rapid, tangential dispersion of neuronal subtypes in the subpial marginal zone (MZ) is a recurring program for distributing cells over long distances. Cajal-Retzius (CR) cells are the first to utilize this migratory route, originating from the edges of the cortex (the cortical hem, pallial-subpallial border and septum) then migrating tangentially in the MZ to cover the cortex [17-19] (Fig 2A). CR cells remain in the MZ throughout cortical development where they produce and secrete the glycoprotein reelin, an important migratory modulator critical for correct laminar positioning of neurons in the cerebral cortex (for review see [20]. Removal of the meninges from early forebrain explants halts migration of CR cells within the MZ, indicating that the meninges produce chemoattractive signals for CR cells [21**], and inhibitors of Cxcr4 signaling blocks CR cell chemotaxis toward the meninges in vitro [21**,22]. Cxcl12 and Cxcr4 null mutants have incomplete reductions in CR cell positioning in the forebrain marginal zone [21**, 22, 23], indicating that other factors from the meninges or the brain may also be involved in regulating CR cell migration in the MZ.
Figure 2.
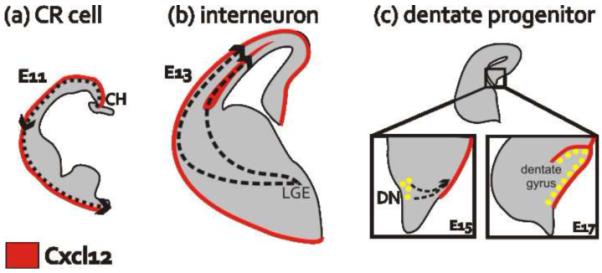
Subpial migratory routes mediated by meningeal-derived Cxcl12
(a) Beginning at E11 in the mouse telencephalon, some CR cells originate in the midline cortical hem (CH) then migrate at the periphery, adjacent to the meninges, to cover the entire surface of the forebrain.
(b) Starting at E13 interneurons migrate from their birthplace in the lateral ganglionic eminence (LGE) to the cortex where they utilize two Cxcl12-lined migratory streams, a subpial route and a deeper path in the SVZ.
(c) At the beginning of dentate morphogenesis (E15), dentate progenitors migrate away from the neuroepithelium at the dentate notch (DN) toward the Cxcl12-enriched meninges. Two days later, the dentate progenitors are arranged in a subpial neurogenic niche.
Slightly later in forebrain development, meningeal Cxcl12 guides tangential migration of inhibitory interneurons within the dorsal forebrain. Interneurons are born in the ventral forebrain and subsequently form two migratory streams as they enter the dorsal forebrain: the MZ stream adjacent to the Cxcl12-expressing meninges and a deeper route through the subventricular zone (SVZ) where Cxcl12 is expressed by SVZ intermediate progenitors [24-26] (Fig. 2B). After exiting the stream, interneurons migrate radially to their final position in the forming cerebral cortex. Loss of responsiveness to Cxcl12 mediates exiting of interneurons from the migratory stream [26], and the interneuron migratory streams in Cxcr4, Cxcr7 and Cxcl12 null mutants are disorganized with premature exit from the streams into the cortical plate [23-28]. Disrupted interneuron migration caused by loss of Cxcl12 signaling negatively impacts interneuron number and positioning in the adult cortex and ultimately affects the inhibitory tone in cortical circuits [25-28].
Meningeal Cxcl12 mediates interaction of neural progenitors with stem cell niches
Both homing and retention of cells in a specific location is frequently mediated by a strong chemoattractive signal like Cxcl12. In addition to the example of CR cells and interneurons (discussed above) there are two instances where Cxcl12 regulates the localization of neural precursors cells adjacent to the meninges, possibly to maintain their proximity to the appropriate stem cell niche. In both the developing cerebellum and dentate gyrus, granule cell progenitors are positioned within a neurogenic zone by meningeal Cxcl12. During cerebellar development, granule cell progenitors (GCP) proliferate in the outer external granule cell layer (EGL) at the edge of the cerebellar folia, then move into the inner EGL as post-mitotic cells and finally migrate inward on Bergmann radial glia to their final position in the internal granule cell layer [29]. In the cerebellum of both Cxcl12-null and Cxcr4-null mouse mutants, the GCP are displaced inward and the proliferative capacity of the progenitors is diminished [30,31], indicating that meningeal Cxcl12 is involved in retaining GPCs in the superficial layer of the EGL. In addition, there is evidence in cerebellar GCPs that Cxcl12 cooperates with Sonic Hedgehog as a mitogenic stimulus in these cells while they are adjacent to the meninges [32].
Similarly, during dentate gyrus morphogenesis, Cxcr4-expressing granule cell progenitors migrate from the dentate primordium to the forming dentate gyrus along the Cxcl12-enriched meninges (Fig. 2C). This migratory stream also functions as a temporary, subpial layer of proliferating dentate progenitors [33*]. In Cxcr4-null mice the migratory stream is disrupted and the transient neurogenic zone fails to form adequately [33*, 34, 35]. This results in premature differentiation of dentate granule cell precursors and the resulting post-mitotic granule cells are found ectopically within the migratory stream. Thus, Cxcl12- signaling in both the cerebellum and dentate gyrus is required for proper positioning of granule cell progenitors, and ultimately, for maintaining their proliferative capacity either due to direct Cxcl12 signaling in the progenitors or in cooperation with other niche-derived factors, e.g. Sonic Hedgehog.
Retinoic acid produced by the meninges regulates forebrain and hindbrain development
Retinoic acid (RA) is a lipophilic, small molecule critical for early events in CNS development, including neural tube closure and early forebrain patterning [36,37]. The meninges express high levels of RA synthesizing enzymes [38,39] and have proven to be in important source of this morphogen during brain development.
Neocortical development starts with an expansion phase where radial neural progenitors go through symmetric, self-renewing divisions. As cortical neuron generation begins, progenitors switch to asymmetric divisions to produce neurons directly or indirectly through intermediate progenitor populations (see review see [40]). The identity or even the existence of molecules responsible for the “neurogenic switch” is a longstanding topic of debate, but recent work using the meninges-deficient Foxc1 mutants indicates that retinoic acid made by the meninges is a major part of the switch driving the neurogenic decision [15**] (Fig. 3B). In Foxc1 mutants, there is an overabundance of symmetric divisions at the expense of neuron production resulting in a long, thin neocortex. The subpopulation of meningeal cells expressing RA synthesizing enzymes are partially or completely missing in the various Foxc1 mutants, indicating a local deficiency in RA production. Indeed, supplementation with exogenous RA rescues the neuron generation defects in the Foxc1 mutants. Interestingly, RA synthesizing cells appear in the forebrain meninges in a lateral-to-medial direction between E12-E13, corresponding to both the timing and the lateral-to-medial birth of the first cortical projection neurons. This, in conjunction with the Foxc1 mutant phenotype, is compelling evidence that RA from the meninges is an important component of the neurogenic switch.
Figure 3.
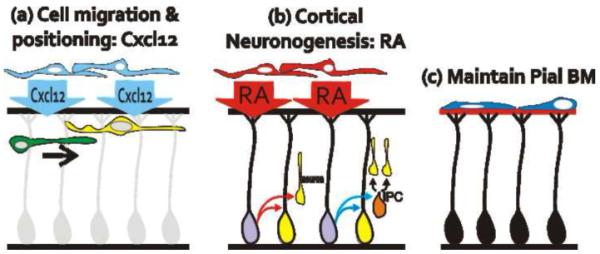
Three main functions of the meninges during brain development.
(a) Through release of the chemokine Cxcl12, the meninges regulate cell migration and positioning of multiple neuronal populations throughout development.
(b) During cortical development, RA produced by the meninges induces neural progenitors in the cerebral cortex to produce neurons directly or indirectly through an intermediate progenitor cell (IPC).
(c) Meningeal fibroblasts in the inner pial layer organize and maintain the BM, a critical attachment point for radial glial endfeet
Meningeal-derived RA may also play a role in anterior hindbrain development. RA signaling, as indicated by the RAREhasp68-lacZ reporter line, in the developing anterior hindbrain is found in neurons migrating from the dorsal precerebellar neuroepithelium to form the ventral pontine nuclei [41]. This dorsal-to-ventral migratory stream is located at the surface of the hindbrain, immediately below the RA-producing meninges. Though meninges-free hindbrain explants indicate that RA signaling in pontine nuclei neurons is dependent on RA from the meninges, it is unclear mechanistically how RA regulates the migration or maturation of this cell population.
Maintenance of the pial basement membrane by the meninges
Immediately below the pial meningeal layer is the extracellular matrix-enriched basement membrane (BM). The pial BM acts as both an anchor point for the endfeet of radial processes that originate from neuronal progenitors cells residing in the ventricular zone (VZ) and as a physical barrier to migrating neurons. During both cortical and cerebellar development, the radial processes provide a migratory scaffold for neurons that helps ensure correct cellular layering. Genetic ablation of components of the pial BM [42,43] or the proteins that mediate cell-ECM attachment [44-47] leads to loss of pial BM integrity, radial endfeet detachment, and disruption of cortical and cerebellar histogenesis. Premature detachment of radial endfeet also leads to increased neuronal progenitor cell death and, subsequently, reduced production of cortical neurons [48].
The first studies connecting the meninges to BM maintenance used focal, chemical ablation of the meninges from the surface of the cerebellar cortex to show breakdown of the pial BM followed by BM restoration when meningeal cells repopulated the lesion site [49]. The meninges may regulate pial BM integrity via cell-ECM adhesion, as indicated by the breakdown in the BM following conditional ablation of focal adhesion kinase from meningeal fibroblasts [46*]. Further studies in which ECM and cell-ECM proteins are conditionally ablated from the meninges will shed light onto the specific function of the meninges in pial BM maintenance.
More recently, BM defects in the neocortex have been observed in conjunction with cellular defects in the meninges, as is the case with the Foxc1 mutants [8,50] and in Zic1/3 double mutants [51]. In Foxc1-null mutants, there is a progressive breakdown in the pial BM from mid-corticogenesis onward resulting in severe disruption in the radial glial scaffold and cortical organization [50]. Zic1 and Zic3 are zinc-finger transcription factors that are expressed in the meninges in addition to subsets of cells in the developing forebrain [52]. Zic1/3 mutants have reduced expression of meningeal genes, like Cxcl12 and Foxc1, and meningeal-derived laminin [51]. In these mutants, the pial BM is disrupted and there is evidence of radial glial endfeet detachment. Collectively this suggests that primary meningeal defects can lead to disruption of the pial BM, and ultimately, lead to defects in cerebral cortical development.
Meninges and neurodevelopmental disorders: potential connection?
Meningeal-derived factors are involved in several, critical developmental events in the brain. This raises the possibility that defects in meningeal development or function, either through genetic mutation or through damage to the meninges in utero, may underlie certain neurodevelopmental disorders in humans. Recently, some cases of Dandy-Walker Syndrome, characterized by cerebellar hypoplasia and hydrocephaly, were linked to FOXC1 heterozygosity with small chromosomal deletions (6p25.3-FOXC1) or loss-of-function mutations [53**]. In Foxc1-null mouse embryos, aberrant migration and differentiation of cells originating from the rhombic lip and roof plate, two transient hindbrain structures that supply cells to the developing cerebellum, underlies the defects in cerebellar development. Unlike the forebrain meninges, the meninges over the hindbrain appear intact, suggesting that Foxc1 in hindbrain meninges regulates expression of secreted factors required for cerebellar development. In addition, almost half the 6p25.3-FOXC1 patients showed evidence of cortical meningeal defects, specifically defects in the midline; this is the same area of the meninges that is affected in Foxc1 hypomorph mutants, which like humans with FOXC1 mutations retain some Foxc1 protein expression, suggesting that the midline cortical meninges are uniquely vulnerable to loss of Foxc1.
Disruption of the meninges or meningeal-derived proteins in mice often results in disorganized cytoarchitecture and ectopic neuronal populations, most often in the cerebral cortex. This type of cortical dysplasia in mouse mutants resembles the type 2 lissencephalies seen generally in the cortex associated with congenital muscular dystrophies (CMDs) and more focally in sporadic cortical dysplasia seen in some individuals with intractable epilepsy. In the case of CMDs, like Walker-Warburg Syndrome, Fukuyama Muscular Dystrophy and Muscle-Eye-Brain disease, loss of pial BM integrity and emigration of neural tissue into the overlying meninges is observed in post-mortem analysis [54,55]. Though cytologic defects in the meninges have not been systematically examined in CMD patients or in animal models of CMD, it is possible that the genetic defects underlying CMDs, which include aberrant glycoslyation of α-dystroglycan, disrupt meningeal maintenance of the BM. Unlike CMDs, focal cortical dysplasias encompass a wide variety of cortical defects, including heterotopic cell clusters and disorganized cortical layering, seen in both radiological and histological studies of epileptic patients (for review see [56]. The heterogeneity of this disorder suggests a varied etiology, including the potential that focal meningeal defects, caused by in utero infection or hemorrhage, may disrupt developmental processes in the underlying cortical region.
Conclusions
Studies over the last few years have made clear that the meninges are far more than strictly a protective layer in the adult tasked with the resorption of circulating CSF. It is now clear that the forebrain and hindbrain meninges serve as a signaling center coordinating developmental events between the cortex and the skull by releasing a variety of secreted factors that instruct surrounding tissues in the course of their developmental programs. In the case of the forebrain, where the meninges is of cranial neural crest origin, the meninges have likely played an important role during the evolution of the vertebrate head helping to coordinate the structure of the skull and the brain. It is also clear that disruption of meningeal function either generally or focally can lead to significant disruption of brain development. Future work will inevitably expand our understanding of the human syndromes where brain malformations are caused primarily by defects in meningeal development.
Acknowledgements
This work was supported by NIDA R01 DA017627 (SJP), the Glenn W. Johnson Memorial Endowment (SJP) and NINDS K99 NS070920 (JAS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Etchevers HC, Couly G, Vincent C, Le Douarin NM. Anterior cephalic neural crest is required for forebrain viability. Development. 1999;126:3533–3543. doi: 10.1242/dev.126.16.3533. [DOI] [PubMed] [Google Scholar]
- 2.McLone DG, Bondareff W. Developmental morphology of the subarachnoid space and contiguous structures in the mouse. Am J Anat. 1975;142:273–293. doi: 10.1002/aja.1001420302. [DOI] [PubMed] [Google Scholar]
- 3.O’Rahilly R, Müller F. The meninges in human development. J Neuropathol Exp Neurol. 1986;45:588–608. [PubMed] [Google Scholar]
- 4.Couly G, Le Douarin N. Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev Biol. 1987;120:198–214. doi: 10.1016/0012-1606(87)90118-7. [DOI] [PubMed] [Google Scholar]
- 5.Bagnall K, Higgins S, Sanders E. The contribution made by cells from a single somite to tissues within a body segment and assessment of their integration with similar cells from adjacent segments. Development. 1989;107:931–943. doi: 10.1242/dev.107.4.931. [DOI] [PubMed] [Google Scholar]
- 6.Couly G, Coltey P, Le Douarin N. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development. 1992;114:1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- 8.Zarbalis K, Siegenthaler JA, Choe Y, May SR, Peterson AS, Pleasure SJ. Cortical dysplasia and skull defects in mice with a Foxc1 allele reveal the role of meningeal differentiation in regulating cortical development. Proc Natl Acad Sci U S A. 2007;104:14002–14007. doi: 10.1073/pnas.0702618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice D, Aberg T, Chan Y, Tang Z, Kettunen P, Pakarinen L, Maxson R, Thesleff I. Integration of FGF and TWIST in calvarial bone and suture development. Development. 2000;127:1845–1855. doi: 10.1242/dev.127.9.1845. [DOI] [PubMed] [Google Scholar]
- 10.Iseki S, Wilkie A, Morriss-Kay G. Fgfr1 and Fgfr2 have distinct differentiation- and proliferation-related roles in the developing mouse skull vault. Development. 1999;126:5611–5620. doi: 10.1242/dev.126.24.5611. [DOI] [PubMed] [Google Scholar]
- 11.Gruneberg H. Congenital hydrocephalus in the mouse, a case of spurious pleiotropism. Journal of Genetics. 1943;45:1–21. Edited by. [Google Scholar]
- 12.Green M. The developmental effects of congenital hydrocephalus (ch) in the mouse. Dev Biol. 1970;23:585–608. doi: 10.1016/0012-1606(70)90142-9. [DOI] [PubMed] [Google Scholar]
- 13.Vivatbutsiri P, Ichinose S, Hytonen M, Sainio K, Eto K, Iseki S. Impaired meningeal development in association with apical expansion of calvarial bone osteogenesis in the Foxc1 mutant. J Anat. 2008;212:603–611. doi: 10.1111/j.1469-7580.2008.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Kume T, Deng KY, Winfrey V, Gould DB, Walter MA, Hogan BL. The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell. 1998;93:985–996. doi: 10.1016/s0092-8674(00)81204-0. Identified Foxc1 as critical for normal brain, skeletal, and eye development.
- 15**.Siegenthaler J, Ashique A, Zarbalis K, Patterson K, Hecht J, Kane M, Folias A, Choe Y, May S, Kume T, et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. Showed that the meninges regulate cortical neurogenesis through the release of RA.
- 16.Ratajczak M, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida M, Assimacopoulos S, Jones K, Grove E. Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development. 2006;133:537–545. doi: 10.1242/dev.02209. [DOI] [PubMed] [Google Scholar]
- 18.Bielle F, Griveau A, Narboux-Nême N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- 19.Zhao C, Guan W, Pleasure S. A transgenic marker mouse line labels Cajal-Retzius cells from the cortical hem and thalamocortical axons. Brain Res. 2006;1077:48–53. doi: 10.1016/j.brainres.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 20.Frotscher M, Chai X, Bock H, Haas C, Förster E, Zhao S. Role of Reelin in the development and maintenance of cortical lamination. J Neural Transm. 2009;116:1451–1455. doi: 10.1007/s00702-009-0228-7. [DOI] [PubMed] [Google Scholar]
- 21**.Borrell V, Marin O. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci. 2006;9:1284–1293. doi: 10.1038/nn1764. Using explant and genetic ablation, showed that the meninges are required for Cajal-Retzius cell migration and the Cxcl12 released by the meninges is the primary chmemoattractant.
- 22.Paredes MF, Li G, Berger O, Baraban SC, Pleasure SJ. Stromal-derived factor-1 (CXCL12) regulates laminar position of Cajal-Retzius cells in normal and dysplastic brains. J Neurosci. 2006;26:9404–9412. doi: 10.1523/JNEUROSCI.2575-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stumm R, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Höllt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiveron M, Rossel M, Moepps B, Zhang Y, Seidenfaden R, Favor J, König N, Cremer H. Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. J Neurosci. 2006;26:13273–13278. doi: 10.1523/JNEUROSCI.4162-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Bendito G, Sanchez-Alcaniz JA, Pla R, Borrell V, Pico E, Valdeolmillos M, Marin O. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J Neurosci. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Adesnik H, Li J, Long J, Nicoll RA, Rubenstein JL, Pleasure SJ. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by Cxcl12/Cxcr4 signaling. J Neurosci. 2008;28:1085–1098. doi: 10.1523/JNEUROSCI.4602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Li G, Stanco A, Long J, Crawford D, Potter G, Pleasure S, Behrens T, Rubenstein J. CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron. doi: 10.1016/j.neuron.2010.12.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sánchez-Alcañiz J, Haege S, Mueller W, Pla R, Mackay F, Schulz S, López-Bendito G, Stumm R, Marín O. Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron. doi: 10.1016/j.neuron.2010.12.006. in press. [DOI] [PubMed] [Google Scholar]
- 29.Chizhikov V, Millen K. Development and malformations of the cerebellum in mice. Mol Genet Metab. 2003;80:54–65. doi: 10.1016/j.ymgme.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Ma Q, Jones D, Borghesani P, Segal R, Nagasawa T, Kishimoto T, Bronson R, Springer T. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt L. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein R, Rubin J, Gibson H, DeHaan E, Alvarez-Hernandez X, Segal R, Luster A. SDF-1 alpha induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development. 2001;128:1971–1981. doi: 10.1242/dev.128.11.1971. [DOI] [PubMed] [Google Scholar]
- 33**.Li G, Kataoka H, Coughlin S, Pleasure S. Identification of a transient subpial neurogenic zone in the developing dentate gyrus and its regulation by Cxcl12 and reelin signaling. Development. 2009;136:327–335. doi: 10.1242/dev.025742. Outlines the critical morphogenic steps in dentate gyrus development, including the requirement of Cxcl12 from the meninges for dentate progenitor migration and formation of a neurogenic niche.
- 34.Lu M, Grove E, Miller R. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci U S A. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagri A, Gurney T, He X, Zou Y, Littman D, Tessier-Lavigne M, Pleasure S. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- 36.Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 37.Ribes V, Wang Z, Dolle P, Niederreither K. Retinaldehyde dehydrogenase 2 (RALDH2)-mediated retinoic acid synthesis regulates early mouse embryonic forebrain development by controlling FGF and sonic hedgehog signaling. Development. 2006;133:351–361. doi: 10.1242/dev.02204. [DOI] [PubMed] [Google Scholar]
- 38.Romand R, Kondo T, Cammas L, Hashino E, Dolle P. Dynamic expression of the retinoic acid-synthesizing enzyme retinol dehydrogenase 10 (rdh10) in the developing mouse brain and sensory organs. J Comp Neurol. 2008;508:879–892. doi: 10.1002/cne.21707. [DOI] [PubMed] [Google Scholar]
- 39.Luo T, Wagner E, Grun F, Drager UC. Retinoic acid signaling in the brain marks formation of optic projections, maturation of the dorsal telencephalon, and function of limbic sites. J Comp Neurol. 2004;470:297–316. doi: 10.1002/cne.20013. [DOI] [PubMed] [Google Scholar]
- 40.Noctor S, Martinez-Cerdeño V, Kriegstein A. Neural stem and progenitor cells in cortical development. Novartis Found Symp. 2007;288:59–73. discussion 73-58, 96-58. [PubMed] [Google Scholar]
- 41.Zhang J, Smith D, Yamamoto M, Ma L, McCaffery P. The meninges is a source of retinoic acid for the late-developing hindbrain. J Neurosci. 2003;23:7610–7620. doi: 10.1523/JNEUROSCI.23-20-07610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halfter W, Dong S, Yip Y, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci. 2002;22:6029–6040. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costell M, Gustafsson E, Aszódi A, Mörgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fässler R. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Georges-Labouesse E, Mark M, Messaddeq N, Gansmüller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 45.Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny J, Müller U. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 46*.Beggs H, Schahin-Reed D, Zang K, Goebbels S, Nave K, Gorski J, Jones K, Sretavan D, Reichardt L. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. Demonstrates that signaling downstream of integrins is required in the meninges themselves for organization of the pial basement membrane.
- 47.Satz J, Ostendorf A, Hou S, Turner A, Kusano H, Lee J, Turk R, Nguyen H, Ross-Barta S, Westra S, et al. Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J Neurosci. 2010;30:14560–14572. doi: 10.1523/JNEUROSCI.3247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radakovits R, Barros C, Belvindrah R, Patton B, Müller U. Regulation of radial glial survival by signals from the meninges. J Neurosci. 2009;29:7694–7705. doi: 10.1523/JNEUROSCI.5537-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sievers J, Phlemann F, Gude S, Berry M. Meningeal cells organize the superficial glial limitans of the cerebellum and produce both the interstitial matrix and basement membrane. Journal of Neurocytology. 1994;23:135–149. doi: 10.1007/BF01183867. [DOI] [PubMed] [Google Scholar]
- 50.Hecht J, Siegenthaler J, Patterson K, Pleasure S. Primary cellular meningeal defects cause neocortical dysplasia and dyslamination. Ann Neurol. 2010;68:454–464. doi: 10.1002/ana.22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoue T, Ogawa M, Mikoshiba K, Aruga J. Zic deficiency in the cortical marginal zone and meninges results in cortical lamination defects resembling those in type II lissencephaly. J Neurosci. 2008;28:4712–4725. doi: 10.1523/JNEUROSCI.5735-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inoue T, Ota M, Ogawa M, Mikoshiba K, Aruga J. Zic1 and Zic3 regulate medial forebrain development through expansion of neuronal progenitors. J Neurosci. 2007;27:5461–5473. doi: 10.1523/JNEUROSCI.4046-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53**.Aldinger K, Lehmann O, Hudgins L, Chizhikov V, Bassuk A, Ades L, Krantz I, Dobyns W, Millen K. FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformation. Nat Genet. 2009;41:1037–1042. doi: 10.1038/ng.422. Identifies mutations in Foxc1 mutations in individuals with Dandy-Walker malformation, in which cerebellar development is affected, providing the first link between defects in the meninges to a neurodevelopmental disorder.
- 54.Nakano I, Funahashi M, Takada K, Toda T. Are breaches in the glia limitans the primary cause of the micropolygyria in Fukuyama-type congenital muscular dystrophy (FCMD)? Pathological study of the cerebral cortex of an FCMD fetus. Acta Neuropathol. 1996;91:313–321. doi: 10.1007/s004010050431. [DOI] [PubMed] [Google Scholar]
- 55.Saito Y, Murayama S, Kawai M, Nakano I. Breached cerebral glia limitans-basal lamina complex in Fukuyama-type congenital muscular dystrophy. Acta Neuropathol. 1999;98:330–336. doi: 10.1007/s004010051089. [DOI] [PubMed] [Google Scholar]
- 56.Blümcke I, Vinters H, Armstrong D, Aronica E, Thom M, Spreafico R. Malformations of cortical development and epilepsies: neuropathological findings with emphasis on focal cortical dysplasia. Epileptic Disord. 2009;11:181–193. doi: 10.1684/epd.2009.0261. [DOI] [PubMed] [Google Scholar]


