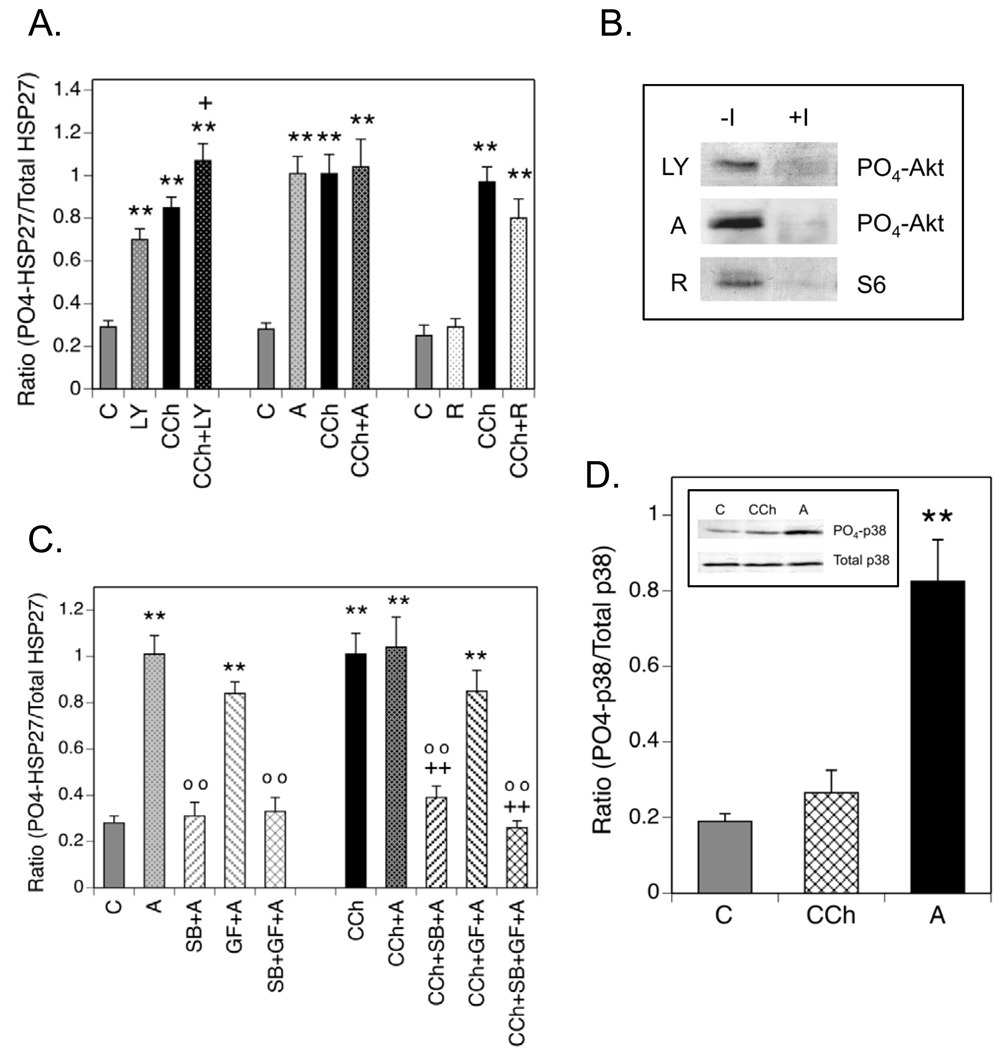
Fig. 6. Phosphorylation of HSP27 is Modulated by Inhibition of PI3-K or Akt.
(A.) Cells were incubated ± LY 294002 (LY, 50 µM), Akti-1/2 (A, 10 µM) or rapamycin (R, 0.1 µM) for 60 min prior to addition of CCh for 5 min. Control incubations (C) contained equal volumes of the vehicles for CCh and/or the inhibitors. (B.) To confirm the activities of LY, A and R, cells were incubated ± these protein kinase inhibitors for 60 min prior to addition of 1 mM CCh or a corresponding volume of serum-free DMEM for 5 min. Lysates were prepared, resolved with SDS-PAGE and immunoblotting was performed for phospho-(Ser-473)-Akt or phospho-(Ser-235/236)-S6 ribosomal protein. (C.) Cells were preincubated with Akti-1/2 alone or in combination with SB 203580 (SB, 10 µM) and/or GF 109203X (GF, 5 µM) for 60 min prior to addition of 1 mM CCh or an equal volume of serum-free DMEM for 5 min. Immunoblotting was performed on cell lysates for phospho-(Ser-82)- and total HSP27. (D.) SH-SY5Y cells were incubated under control (C) conditions or with 1 mM CCh for 5 min following a 60 min preincubation or with 10 µM Akti-1/2 (A) for 60 min. Cell lysates were prepared and immunoblotting was performed with a phospho-specific antibody directed against Thr-180/Tyr-182 in the activation domain of p38 MAPK or with an antibody that recognizes p38 MAPK independent of its phosphorylation state. The insert is a representative blot showing amounts of phospho- and total p38 MAPK in one experiment. Results shown are the mean ± SEM of normalized phosphorylation from 3–12 experiments. ** p< 0.01 as compared to the control value; +p< 0.05 as compared to the CCh value; ++p< 0.01 as compared to the CCh value; oo p< 0.01 as compared to the Akti-1/2 value.