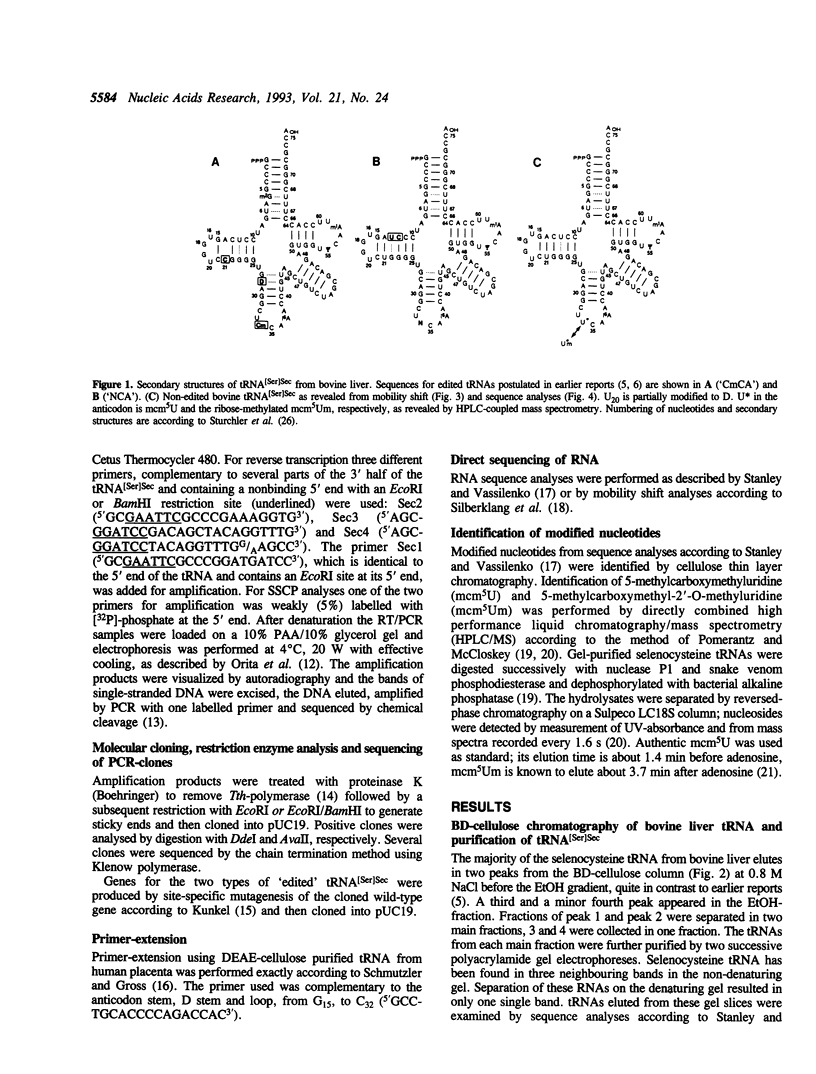
Abstract
It has been reported that selenocysteine tRNA from bovine liver is completely edited to two isoacceptor species, called tRNA([Ser]SecNCA) and tRNA([Ser]SecCmCa), which differ from the gene sequence. We used direct tRNA sequencing, mobility shift analyses, primer extension, restriction enzyme digestion and single strand conformational polymorphism (SSCP) analyses of products from reverse transcription coupled with polymerase chain reaction (RT/PCR), sequencing of RT/PCR products and HPLC-coupled mass spectrometry to reproduce this result and show here that editing of these tRNAs does not occur.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beier H., Lee M. C., Sekiya T., Kuchino Y., Nishimura S. Two nucleotides next to the anticodon of cytoplasmic rat tRNA(Asp) are likely generated by RNA editing. Nucleic Acids Res. 1992 Jun 11;20(11):2679–2683. doi: 10.1093/nar/20.11.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain P. F. Preparation and enzymatic hydrolysis of DNA and RNA for mass spectrometry. Methods Enzymol. 1990;193:782–790. doi: 10.1016/0076-6879(90)93450-y. [DOI] [PubMed] [Google Scholar]
- Crowe J. S., Cooper H. J., Smith M. A., Sims M. J., Parker D., Gewert D. Improved cloning efficiency of polymerase chain reaction (PCR) products after proteinase K digestion. Nucleic Acids Res. 1991 Jan 11;19(1):184–184. doi: 10.1093/nar/19.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. M., Choi I. S., Crain P. F., Hashizume T., Pomerantz S. C., Cruz R., Steer C. J., Hill K. E., Burk R. F., McCloskey J. A. Dietary selenium affects methylation of the wobble nucleoside in the anticodon of selenocysteine tRNA([Ser]Sec). J Biol Chem. 1993 Jul 5;268(19):14215–14223. [PubMed] [Google Scholar]
- Diamond A. M., Montero-Puerner Y., Lee B. J., Hatfield D. Selenocysteine inserting tRNAs are likely generated by tRNA editing. Nucleic Acids Res. 1990 Nov 25;18(22):6727–6727. doi: 10.1093/nar/18.22.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Dudock B., Hatfield D. Structure and properties of a bovine liver UGA suppressor serine tRNA with a tryptophan anticodon. Cell. 1981 Aug;25(2):497–506. doi: 10.1016/0092-8674(81)90068-4. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Gross H. J., Krupp G., Domdey H., Raba M., Jank P., Lossow C., Alberty H., Ramm K., Sänger H. L. Nucleotide sequence and secondary structure of citrus exocortis and chrysanthemum stunt viroid. Eur J Biochem. 1982 Jan;121(2):249–257. doi: 10.1111/j.1432-1033.1982.tb05779.x. [DOI] [PubMed] [Google Scholar]
- Hatfield D. L., Lee B. J., Price N. M., Stadtman T. C. Selenocysteyl-tRNA occurs in the diatom Thalassiosira and in the ciliate Tetrahymena. Mol Microbiol. 1991 May;5(5):1183–1186. doi: 10.1111/j.1365-2958.1991.tb01891.x. [DOI] [PubMed] [Google Scholar]
- Hatfield D. L., Smith D. W., Lee B. J., Worland P. J., Oroszlan S. Structure and function of suppressor tRNAs in higher eukaryotes. Crit Rev Biochem Mol Biol. 1990;25(2):71–96. doi: 10.3109/10409239009090606. [DOI] [PubMed] [Google Scholar]
- Hatfield D., Choi I. S., Mischke S., Owens L. D. Selenocysteyl-tRNAs recognize UGA in Beta vulgaris, a higher plant, and in Gliocladium virens, a filamentous fungus. Biochem Biophys Res Commun. 1992 Apr 15;184(1):254–259. doi: 10.1016/0006-291x(92)91186-t. [DOI] [PubMed] [Google Scholar]
- Hatfield D., Diamond A., Dudock B. Opal suppressor serine tRNAs from bovine liver form phosphoseryl-tRNA. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6215–6219. doi: 10.1073/pnas.79.20.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D., Lee B. J., Hampton L., Diamond A. M. Selenium induces changes in the selenocysteine tRNA[Ser]Sec population in mammalian cells. Nucleic Acids Res. 1991 Feb 25;19(4):939–943. doi: 10.1093/nar/19.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke A., Päbo S. Editing of a tRNA anticodon in marsupial mitochondria changes its codon recognition. Nucleic Acids Res. 1993 Apr 11;21(7):1523–1525. doi: 10.1093/nar/21.7.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Hoshino H., Harada F. Minor serine tRNA containing anticodon NCA (C4 RNA) from human and mouse cells. Biochem Int. 1983 Nov;7(5):635–645. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kuntzel B., Weissenbach J., Wolff R. E., Tumaitis-Kennedy T. D., Lane B. G., Dirheimer G. Presence of the methylester of 5-carboxymethyl uridine in the wobble position of the anticodon of tRNAIII Arg from brewer's yeast. Biochimie. 1975;57(1):61–70. doi: 10.1016/s0300-9084(75)80110-6. [DOI] [PubMed] [Google Scholar]
- Lonergan K. M., Gray M. W. Editing of transfer RNAs in Acanthamoeba castellanii mitochondria. Science. 1993 Feb 5;259(5096):812–816. doi: 10.1126/science.8430334. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Myers T. W., Gelfand D. H. Reverse transcription and DNA amplification by a Thermus thermophilus DNA polymerase. Biochemistry. 1991 Aug 6;30(31):7661–7666. doi: 10.1021/bi00245a001. [DOI] [PubMed] [Google Scholar]
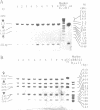
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Pomerantz S. C., McCloskey J. A. Analysis of RNA hydrolyzates by liquid chromatography-mass spectrometry. Methods Enzymol. 1990;193:796–824. doi: 10.1016/0076-6879(90)93452-q. [DOI] [PubMed] [Google Scholar]
- Roe B. A. Studies on human tRNA. I. The rapid, large scale isolation and partial fractionation of placenta and liver tRNA. Nucleic Acids Res. 1975 Jan;2(1):21–42. doi: 10.1093/nar/2.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutzler C., Gross H. J. Genes, variant genes, and pseudogenes of the human tRNA(Val) gene family are differentially expressed in HeLa cells and in human placenta. Nucleic Acids Res. 1990 Sep 11;18(17):5001–5008. doi: 10.1093/nar/18.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
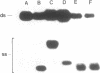
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
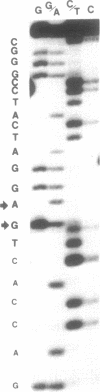
- Stanley J., Vassilenko S. A different approach to RNA sequencing. Nature. 1978 Jul 6;274(5666):87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]
- Sturchler C., Westhof E., Carbon P., Krol A. Unique secondary and tertiary structural features of the eucaryotic selenocysteine tRNA(Sec). Nucleic Acids Res. 1993 Mar 11;21(5):1073–1079. doi: 10.1093/nar/21.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]