Abstract
Objective
The aim of this report was to propose a novel measure of nonstationarity of EEG signals, named Shannon entropy of the peak frequency shifting (SEPFS). The feasibility of this method was documented comparing this measure with traditional time domain assessment of nonstationarity and its application to EEG data sets obtained from student-athletes before and after suffering a single episode of mild traumatic brain injury (mTBI) with age-matched normal controls.
Methods
Instead of assessing the power density distribution on the time-frequency plane, like previously proposed measures of signal nonstationarity, this new measure is based on the shift of the dominant frequency of the EEG signal over time. We applied SEPFS measure to assess the properties of EEG nonstationarity in subjects before and shortly after they suffered mTBI. Student–athletes at high risk for mTBI (n = 265) were tested prior to concussive episodes as a baseline. From this subject pool, 30 athletes who suffered from mTBI were re-tested on day 30 post-injury. Additional subjects pool (student-athletes without history of concussion, n=30) were recruited and test-retested within the same 30 day interval. Thirty-two channels EEG signals were acquired in sitting eyes closed condition.
Results
The results showed that the SEPFS values significantly decreased in subjects suffering from mTBI. Specifically, reduced EEG nonstationarity was observed in occipital, temporal and central brain areas, indicating the possibility of residual brain dysfunctions in concussed individuals. A similar but less statistically significant trend was observed using traditional time domain analysis of EEG nonstationarity.
Conclusions
The proposed measure has at least two merits of interest: (1) it is less affected by the limited resolution of time-frequency representation of the EEG signal; (2) it takes into account the neural characteristics of the EEG signal that have not been considered in previously proposed measures of nonstationarity.
Significance
This new method may potentially be used as a complementary tool to assess the alteration of brain functions as a result of mTBI.
Keywords: EEG, Nonstationarity, mild traumatic brain injury (mTBI)
1. Introduction
Mild traumatic brain injury (mTBI), otherwise known as concussion (Cantu 2006), is the most common head injury seen in athletics as well as in other activities of daily life. It can occur in a myriad of different situations including but not limited to athletic events, recreational outings, and transportation accidents where the brain accelerates and/or decelerates differentially in the skull. Current evidence suggests that attempts to classify mTBI as a traumatic event based upon clinical symptoms at the site of injury may be erroneous. However, advanced research methods may detect both behavioral (e.g., abnormal balance see: Cavanaugh et al., 2005a,b; 2006; Slobounov et al., 2007; 2008), neural [e.g., abnormal EEG (Thatcher et al., 1989; Slobounov et al., 2002; 2006a,b,c; fMRI (McAllister et al., 2001; Cheng et al., 2004; Ptito et al 2007; Slobounov et al., 2010; MRS (Bluml and Brooks, 2006; Goryunova et al., 2007); and DTI (Levin 2003; Bigler and Bazarian 2010) residual deficits far beyond the early post-injury10 days period.
That said there is still debate in the literature regarding the feasibility of EEG for clinical assessment of mTBI. For example, it was reported that no clear EEG features are unique to mTBI, especially late after injury (Nuwer et al., 2005). Therefore, further efforts are needed to explore unique features of EEG signals enabling the detection of functional brain abnormalities and classification of mTBI patients.
It is well-documented that EEG signals, as well as many other biological signals (ECG, EMG, etc), are not wide sense stationary (WSS). The nonstationarity assumes that temporal and spectral characteristics of the signal vary over time. There are several quantitative comparisons of a signal's nonstationarity in the literature with regards to neurological deficits. For example, it has been reported in animal studies that nonstationarity of an EEG signal can be altered after hypoxicischemic (HI) brain injury, especially in the upper delta and lower theta band as well as beta2 bands (Tong and Thakor, 2003).
The WSS is traditionally defined in the time domain, meaning that the first moment and second moment of the stochastic signal are invariant over the time. Thus, the nonstationarity in the time domain can be quantitatively measured by the variation of the first and second moments over time. In practice, this can be implemented by first estimating the epoch mean u and epoch standard deviation σ for each epoch, and then calculating the variation of u and σ across epochs.
| (1) |
| (2) |
Where, i is the epoch index, M is the number of epochs, N is the length of each epoch
Compared to the nonstationarity in the time domain, the nonstationarity in the spectrum domain of the EEG signal has been emphasized more recently, because of the corresponding neurophysiologic meanings of the EEG spectrum components. Even though the traditional timeaveraged spectrum of EEG based on WSS assumption is still widely used in the EEG analyses, it is not appropriate for quantifying the temporal variability in the spectrum of the nonstationary signals. Therefore, time-frequency analysis methods including Short-Time-Fourier-Transformation (STFT), Gabor transformation and Wavelet transformation (Gabor, 1946; Grossman and Morlet, 1984) have been utilized to estimate the temporal evolution of the spectrum. The time-frequency decomposition can provide clear images of the energy distribution on the time-frequency plane directly demonstrating the nonstationarity of the signal. However, these methods are unable to provide any quantitative measures of EEG nonstationarity. To overcome this limitation, several quantifications of the nonstationarity based on the time-frequency representation (TFR) have been recently introduced. For example, Tong et al., (2003) proposed time-frequency-complex (TFC) as a measure of how much the EEG signal is uniformly distributed on the time-frequency plane (see equation 1 for details).
| (3) |
Where tfr(ai,nΔt) is the time-frequency representation of non-stationary signal at frequency ai and time nΔt.
It was pointed out in their following paper (Tong et al., 2007) that the TFC is too general to differentiate whether the non-uniformity of the signal comes from the frequency or the time domain. To address this concern, three other quantifications have been introduced. These are the degree of stationarity (DS), Shannon entropy of marginal spectrum (SE) and Kullaback-Leibler Distance (KLD) between a TFR of certain frequency and a uniform distribution (Tong et al., 2007).
| (4) |
| (5) |
| (6) |
where
| (7) |
It is noteworthy that all of these four quantifications are based on the power density at individual frequency. It should be also noted that in addition to the spectrum power density, other spectral characteristics of non-stationary signals, including the peak frequency (PF), can vary over time, and, thus, can be used to evaluate nonstationarity. Moreover, various brain functional states may be dominated by certain EEG peak frequencies (PF) and altered due to cortical damage and/or cerebral functional deficits (Randolph and Miller, 1988; Holschneider and Leuchter, 2004; Tebano et al., 1988).
In this study, a new quantification of EEG nonstationarity based on peak frequency shifting, instead of power density, named Shannon Entropy of the peak frequency shifting (SEPFS) is introduced. We aimed to provide empirical evidence that SEPFS measures are sensitive towards alteration of brain functions induced by concussive blows. Therefore, it can be complementary to other advanced measures of EEG dynamics (i.e., EEG wavelet entropy, Slobounov et al., 2009), and utilized as a new measure of EEG nonstationarity that reveals additional information to conventional EEG analysis with regards to mTBI.
2. Methods
2.1. Subjects
A total of 265 subjects were initially recruited (baseline testing) for a multipurpose sport-related concussion study. All subjects were Pennsylvania State University athletes at high risk for traumatic brain injury (collegiate rugby, football, ice hockey and soccer players), aged between 18 and 25 years, male (n = 180, mean age − 21.3 years) and female (n = 85, mean age = 20.8 years). None of these subjects had a history of concussion at the time of baseline testing. In this report we included data from subjects who met the following inclusion criteria: (a) suffered a single episode of concussion within 12 months after baseline testing; (b) a concussive episode was a grade 1 mTBI (Cantu Data Driven Revised Concussion Grading Guideline, 2006); and (c) neuropsychological (NP) and EEG data were available from baseline testing. Thirty of the total mTBI subject pool met all of the inclusion criteria and their data obtained on day 30 +/- 3 days post-injury are included in this report. The initial diagnosis of mTBI was made on the field by certified athletic trainers (AT) based on commonly accepted clinical symptoms, such as: complaints of loss of concentration, dizziness, fatigue, headache, irritability, visual disturbances, and light sensitivity (Bryant and Harvey 1999). According to interviews with concussed subjects and AT reports, the majority of documented concussions occurred as a result of direct head-to-head and/or head-to-torso collision (often with the side of the head as a major site of impact) during the athletic events. All 30 subjects were clinically asymptomatic on day 7 after mTBI and were cleared for sport participation based upon neurological assessments (Cooperative Ataxia Rating Scale, World Federation of Neurology, Trouillas et al,, 1997) as well as clinical symptoms resolution.
In an addition, we recruited 30 age and sex-matched normal controls (Penn State student-athletes without history of concussion) and tested these subjects twice within the 30 days interval. This allowed us to conduct a cross-sectional study comparing normal controls and mTBI subjects as well as to assess the reproducibility of our EEG measures over time. All subjects signed an informed consent form and the protocol was approved by the Institutional Review Board of the Pennsylvania State University.
2.2. Neuropsychological (NP) assessments
The neuropsychological tests were administered at baseline testing and before EEG testing as standard paper and pencil tests. The subjects were seated at a table and administered the test battery by the tester. The subject was instructed to complete the tests as quickly and accurately as possible. The NP testing battery consisted of three segments: Subjective Symptom Rating Scale (e.g., Penn State University Standard Concussion Rating Scale) to assess mTBI symptom severity; Symbol Digit Substitution test to assess information processing speed and working memory; Trails “B” test to assess information processing speed and scanning ability (Randolph, 2001). Several other conventional NP tests, commonly used in concussion research (see Slobounov & Sebastianelli, 2006 for details in terms of its sensitivity to detect the brain dysfunctions induced by a concussive blow), were administered, including subjects' reported fatigue, the Hopkins Verbal Learning Test –Revised (HVLT) Stroop tests. All subjects were asymptomatic at the time of the post-injury testing (see Slobounov et al., 2009 for details of neuropsychological testing, see also Table 1 for details of NP testing under this study).
Table 1.
Neuropsychological Test Performance Variables including Reported Fatigue Scores prior to EEG testing for normal controls (NC) and mTBI subjects at day 30 post-injury
| Subjects Groups | NC | MTBI | ||
|---|---|---|---|---|
| M (SD) | M (SD) | t-test | p-value | |
| Reported fatigue: | ||||
| Beatty Test Fatigue Rating | ||||
| Cognitive fatigue | 9.3 (3.3) | 10.4 (3.7) | 2.05 | .071 |
| Physical fatigue | 9.9 (3.1) | 10.7 (4.2) | 2.05 | .086 |
| Total fatigue | 21.6 (5.1) | 22.3 (11.3) | 2.074 | .081 |
| Neuropsychological (NS) test performance: | ||||
| Trailmaking A | 31(4) | 30(6) | 1.93 | .071 |
| Trailmaking B | 88 (5) | 87(1) | 1.28 | .133 |
| Stroop Color-Word (CS) & Color-Word Interference (CW-I) | ||||
| CW total time | 51.9 (7.8) | 50.3 (6.1) | 3.25 | .077 |
| CW-I total time | 101.5 (25.5) | 101.4 (21.6) | 3.12 | .077 |
| CW total errors | 0.0 (0.0) | 0.3 (0.5) | 1.41 | >.10 |
| CW-I total errors | 1.5 (0.7) | 1.6(1.4) | 1.46 | .088 |
2.3. EEG recording and processing
Subjects were seated with eyes closed in an electrically shielded and dimly lit environment. The continuous EEG was recorded using Ag/AgCl electrodes mounted in a 19-channel spandex Electro-cap (Electro-cap International Inc., Eaton, OH). The electrical activity from the scalp was recorded at 19-sites: FP1, FP2, FZ, F3, F4, F7, F8, CZ, C3, C4, T3, T4, T5, T6, PZ, P3, P4, O1, O2, according to the International 10–20 system (Jasper, 1958). The ground electrode was located 10% anterior to FZ, linked earlobes served as references and electrode impedances were below 5kΩ. EEG signals were recorded using a programmable DC coupled broadband SynAmps amplifier (NeuroScan, Inc., El Paso, TX.). The EEG signals were amplified (gain 2500, accuracy 0.033/bit) with a recording range set for ±55 mV in the DC to 70-Hz frequency range. The EEG signals were digitized at 1000 Hz using 16-bit analog-to-digital converters. The EEG data were initially processed off-line using EEGLAB 5.03 (Delorme and Makeig, 2004) using Matlab open source toolbox (Mathworks, Natick, USA). Imported data were down sampled to 200 Hz to reduce computing time and epoched from 0 to 500 ms. After baseline normalization these epochs were automatically screened for unique, non-stereotypic artifacts using a probabilistic function within EEGLAB. This procedure allows the removal of epochs containing signal values exceeding 3 SD and controls for artifacts such as eye blinks, eye movements, heartbeats etc. Following this procedure at least 2 min of artifact free EEG signal were subjected to further analysis. Since the frequency band between 2Hz and 40 Hz was considered in this study, the frequency components outside of this range were filtered out. The continuous wavelet transform (CWT) coefficients of the filtered EEG signal at exponentially increased frequencies f1, f2… f63, where f1=2Hz, fn =2*1.05(n-1) were calculated.
2.4. Time-Frequency Representation of EEG
In this study, we used the CWT as a time-frequency representation of the EEG signal. The time-frequency resolution of CWT is normalized in such a way that the higher-frequency component of the signal has higher temporal resolution than the lower frequency resolution and the lower-frequency component has lower temporal resolution than higher frequency resolution. As a result of this normalization, the time-frequency characteristics of the signal can be represented more efficiently. The equation of CWT is shown as follows:
| (8) |
where ψ(·) is the wavelet function which can be considered as a band-pass filter, and τ is the translation in time, variable 1/a gives the frequency scale and τ gives the temporal localization. For the low-frequency component of the signal, a large scale parameter is chosen, the basis function ψ(·) is stretched, whereas for the high-frequency component, a small scale parameter is chosen, the basis function ψ(·) is contracted. The squared magnitude of the CWT coefficient, |CWTx(a,τ)|2, is used to present the energy distribution. For a given CWT, an exact relation between scale and frequency is:
| (9) |
where fa is the pseudo-frequency corresponding to the scale a, fc is the centre frequency of a wavelet, fs is the sampling frequency.
In this study, the complex Morlet wavelet was used. The complex Morlet wavelet has been widely used in EEG signal analysis because it has good time-frequency localization and its sinusoidal shape approximates the shape of EEG signal quite well (Li et al., 2005; Meyer, 1993). The complex Morlet wavelet is defined as follows:
| (10) |
Where: fc is the centre frequency of the wavelet and σ is the variance of the Gaussian window, and fb the bandwidth parameter of the wavelet.
The Fourier transformation of ψ (t) is a Gaussian function centered at fc with variance of 1/ fb:
| (11) |
2.5. Shannon Entropy of the Peak Frequency Shifting
To compute the Shannon entropy of the peak frequency shifting, the spectrum of signal x is divided into M sub-bands from the lowest frequency fmin to the highest frequency fmax, denoted as Sb1, Sb2 … SbM, with central frequencies fc1< fc2…< fcM. The CWT scales a1, a2… aM corresponding to these central frequencies are determined by (7). The peak frequency at any time point τ is defined as follows:
| (12) |
The SEPFS can be computed as follows:
| (13) |
Where: N is the length of the signal x, and ni is the frequencies that the fp (τ) is equal to fci.
2.6. Simulation
A frequency modulated sinusoid signal s1=sin(2π(1+5t)t) was simulated with 200 Hz sampling frequency (Fig. 1a). The power distribution of s1 on the time-frequency plane is shown in Fig. 1b.
Fig.1.
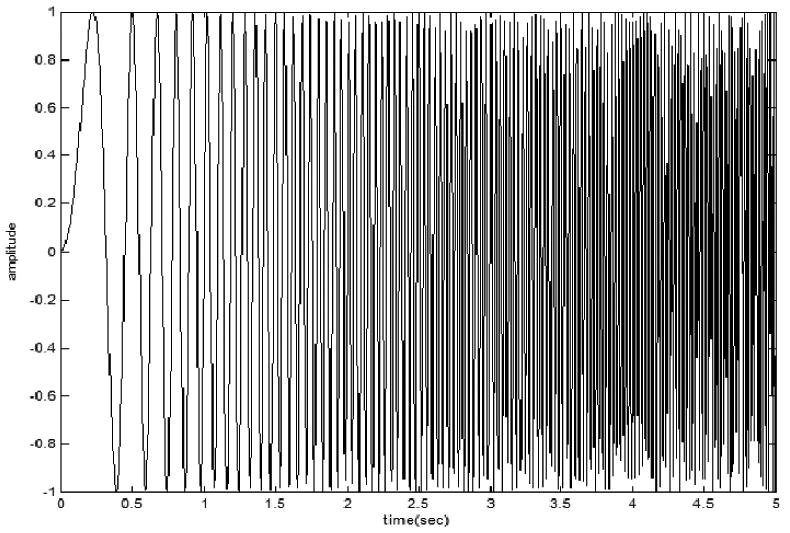
(a) frequency modulated sinusoid signal s1=sin(2π(1+5t)t) simulated with 200 Hz sampling frequency; (b) Power distribution of s1 on the time-frequency plane.
For comparison, another synthesized signal s2 was simulated. s2 is the summation of two signals x1 and x2: x1 is a 20 Hz sinusoid signal with linearly increasing magnitude: x1=0.1t sin(2π f1t), and x2 is a 40 Hz sinusoid signal with exponentially decreasing magnitude: x2= e−0.5t sin(2πf2t). The two components and the synthesized signal s2 = x1 + x2 are presented in Fig. 2a, the power distribution of s2 on the time-frequency plane is shown in Fig. 2b.
Fig.2.

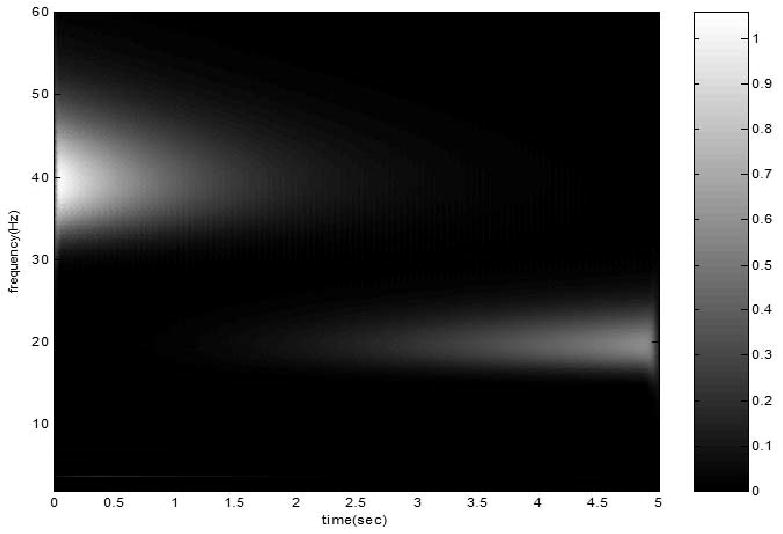
(a) The time course of two components (tom and middle) and the synthesized signal s2 = x1 +x2 (bottom): (b) the power distribution of the synthesized signal s2 on the time-frequency plane, the peak frequency shift from 40 Hz to 20 Hz.
The frequency band of 2 Hz to 50 Hz was equally divided into 75 sub-bands. The SEPFS of s1 and s2 was calculated according to (10) and (11) are 5.56 bit and 1.08 bit, respectively. The SEPFS of s1 is much higher than that of s2, because s1 can be considered as a combination of infinite number of transient sinusoid components with different frequencies and phases; whereas s2 is merely the summation of two amplitude modified sinusoid components. This result is in agreement with the simulation results of DS, SE and KLD using the same signals reported in Tong et al. (2007) study, that s1 has lower stationarity index at all frequencies other than s2. However, it should be noted that SEPFS can directly measure the nonstationarity of the whole signal rather than those at individual frequencies.
2.7. Time domain representation of EEG signal
For comparison, the nonstationarity of EEG signals obtain before after concussion in time-domain was also assessed by the variation of the standard deviation of EEG signal across epochs.
2.8. Statistical analysis
The SEPFS of the full frequency band on each channel was estimated. A two-way ANOVA (testing day, prior to and after concussion X EEG channel) with repeated measures within subjects' design was conducted and paired t-test at each channel was implemented, to assess significant effect of concussion on SEPFS. A paired t-test was conducted to assess statistically significant differences of SEPFS measures between groups and within normal control subjects at test-retest. The significance level for the statistic tests was set to p< 0.05.
3. Results
3.1. Neuropsychological Test Performance Data
Table 1 shows the neuropsychological data for the concussion (mTBI) and normal controls groups. As can be seen from the data presented in Table 1, no significant differences were observed between mTBI subjects and normal controls for all of the variables under NP testing. It should also be noted that no significant differences for all of the variables under NP testing were observed at baseline and those obtained on day 30 +/- 3 days post-injury (p > 0.05).
3.2. EEG (SEPFS) data
The ANOVA revealed a significant main effect of testing day and concussion, (F (1, 29) =12.5, p=0.001). The mean values of the SEPFS of each EEG channel before and after concussion are listed in Table 2, along with the corresponding t-scores and p-values. The distribution of the t-scores is also shown in Fig. 3.
Table 2.
The mean values of SEPFS before and after concussion and the t-score and p-values of paired t-test for each channel. * *denotes p < 0.01, * denotes p < 0.05, -- denotes p > 0.05***
| Electrodes | O2 | O1 | Pz | P4 | T4 | C4 | T3 | Cz | C3 | Fz |
|---|---|---|---|---|---|---|---|---|---|---|
| Before | 4.3977 | 4.4983 | 4.7415 | 4.6782 | 4.9920 | 4.9201 | 5.0211 | 4.9508 | 4.9451 | 4.9556 |
| After MTBI | 3.3767 | 3.4939 | 4.1589 | 4.1157 | 4.7247 | 4.6597 | 4.6477 | 4.6141 | 4.5354 | 4.7402 |
| t-score | 5.1831 | 5.9612 | 2.9244 | 3.1928 | 2.9919 | 2.7258 | 3.3704 | 3.3723 | 3.0638 | 2.7588 |
| p-value (%) | <0.001** | <0.001** | 0.72 | 0.38 | 0.62 | 1.15 | 0.24 | 0.24 | 0.52 | 1.07 |
| Electrodes | F4 | F8 | F3 | fp2 | f7 | fp1 | T6 | T5 | P3 | |
| Before | 4.9674 | 4.9234 | 4.9590 | 4.8581 | 4.8932 | 4.7630 | 4.5871 | 4.7628 | 4.7478 | |
| After MTBI | 4.7394 | 4.7020 | 4.7409 | 4.6393 | 4.7300 | 4.6145 | 3.8135 | 3.9501 | 4.0599 | |
| t-score | 2.8112 | 2.0982 | 2.9553 | 1.7383 | 1.9737 | 1.0598 | 4.4585 | 4.2178 | 3.6282 | |
| p-value (%) | 0.95 | 4.62 | 0.67 | 9.45 | 5.96 | 9.94 | 0.02** | 0.03** | 0.013** |
Fig.3.
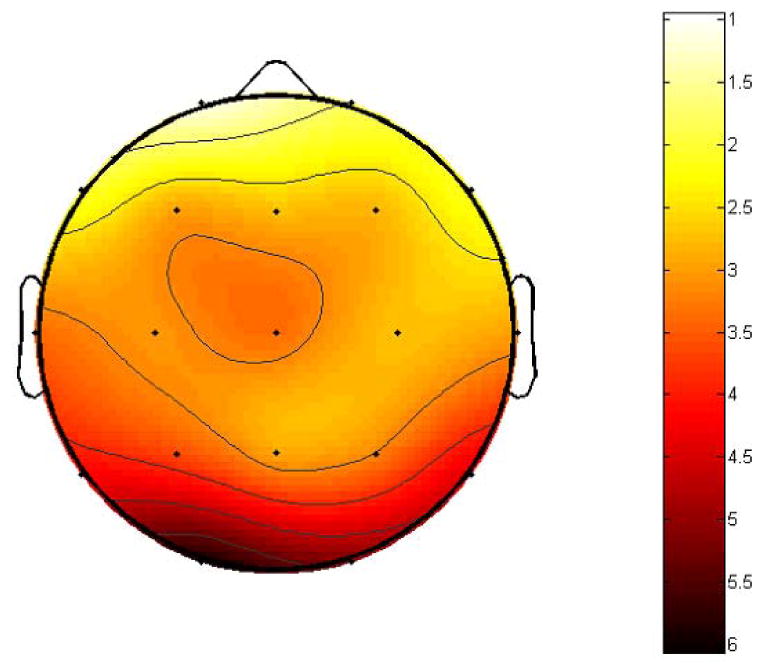
The scalp distribution of the t-scores of SEPFS difference before and after a concussive episode, note the most pronounced effect (e.g., significant decreased of SEPFS values) after concussion at electrode sites representing occipital, temporal and parietal regions.
As can been seen from Table 2 and Fig. 3, the SEPFS values significantly decreased after concussion. This effect was most pronounced at electrode sites representing occipital region, O1(t=5.18, d.f.=14, p<0.001), O2, (t=5.9612, d.f.=14, p<0.001), temporal region, T6(t=4.45,d.f.=14, p<0.001), T5(t=4.21,d.f.=14, p<0.001), and parietal regions, P4(t=3.19, d.f.=14, p<0.001), P3(t=3.62, d.f.=14, p<0.001). No other brain regions were affected by concussion as assessed by SEPFS.
The same EEG data set was subjected to traditional time domain nonstationarity analysis. The results are shown in Table 3. As can be seen they were similar to SEPFS trend indicating the reduction of nonstationarity values after concussion. This effect was most pronounced at the same brain regions (e.g., occipital, temporal, and parietal, p<0.05). It should be noted however, the effect of concussion was less significantly pronounced (statistically) compared to SEPFS.
Table 3.
The mean values of standard deviation (SD) variability across trials before and after concussion and the t-score and p-values of paired t-test for each channel. * *denotes p < 0.01, * denotes p < 0.05, -- denotes p > 0.05
| Electrodes | O2 | O1 | Pz | P4 | T4 | C4 | T3 | Cz | C3 | Fz |
|---|---|---|---|---|---|---|---|---|---|---|
| Before | 4.7441 | 4.7776 | 3.5309 | 3.5171 | 1.7155 | 2.4209 | 1.7404 | 2.6780 | 2.5130 | 2.4899 |
| After MTBI | 3.2137 | 3.5235 | 2.6003 | 2.5934 | 1.4309 | 1.9399 | 1.4311 | 2.1415 | 1.9309 | 2.0587 |
| t-score | 3.8718 | 2.9719 | 2.6711 | 2.5802 | 1.4486 | 1.8209 | 1.6407 | 1.8538 | 2.2802 | 1.4231 |
| p-value (%) | 0.05* | 0.05* | 1.2 | 1.5 | -- | -- | -- | -- | 3.01 | -- |
| Electrodes | F4 | F8 | F3 | fp2 | f7 | fp1 | T6 | T5 | P3 | |
| Before | 2.4220 | 2.3602 | 2.4151 | 3.2860 | 2.3444 | 3.1674 | 3.2595 | 2.9874 | 3.3621 | |
| After MTBI | 2.0849 | 2.1187 | 1.9678 | 2.5287 | 1.8991 | 2.5313 | 2.2603 | 2.1794 | 2.4090 | |
| t-score | 2.2802 | 0.5488 | 1.5611 | 0.9283 | 1.2589 | 0.7449 | 3.2668 | 2.5860 | 2.9913 | |
| p-value (%) | -- | -- | -- | -- | -- | -- | 0.27 | 1.5 | 0.056 |
Finally, no statistically significant differences for the SEPFS data were observed between normal controls as a function of testing days (p> 0.05). However, SEPFS data were significantly lower in mTBI subjects compared to age/sex matched normal controls at occipital (O1 & O2, p< 0.001), temporal (T5 & T6, p<0.05) and parietal (P3, p<0.05) electrode sites (see Table 4).
Table 4.
The mean values of SEPFS for mTBI subjects and age/day of testing-matched normal controls (NC), and the t-score and p-values of paired t-test for each channel. * *denotes p < 0.01, * denotes p < 0.05, -- denotes p > 0.05
| Electrodes | O2 | O1 | Pz | P4 | T4 | C4 | T3 | Cz | C3 | Fz |
|---|---|---|---|---|---|---|---|---|---|---|
| NC | 4.4945 | 4.5473 | 4.2047 | 4.6782 | 4.8320 | 4.8202 | 5.1211 | 4.7503 | 4.6151 | 4.8551 |
| mTBI | 3.3767 | 3.4939 | 4.1589 | 4.1157 | 4.7247 | 4.6597 | 4.6477 | 4.6141 | 4.5354 | 4.7402 |
| t-score | 5.2852 | 5.9112 | 2.5214 | 3.1328 | 2.9919 | 2.6958 | 3.3701 | 3.3821 | 3.0738 | 2.6988 |
| p-value (%) | <0.001** | <0.001** | 0.69 | 0.42 | 0.67 | 1.22 | 0.22 | 0.23 | 0.54 | 1.09 |
| Electrodes | F4 | F8 | F3 | fp2 | f7 | fp1 | T6 | T5 | P3 | |
| NC | 4.8654 | 4.7234 | 4.9510 | 4.7571 | 4.8777 | 4.7131 | 4.7891 | 4.9128 | 4.5478 | |
| mTBI | 4.7394 | 4.7020 | 4.7409 | 4.6393 | 4.7300 | 4.6145 | 3.8135 | 3.9501 | 4.0599 | |
| t-score | 2.7812 | 2.011 | 2.9533 | 1.6383 | 1.9831 | 1.0498 | 4.4989 | 4.2678 | 3.5682 | |
| p-value (%) | 0.99 | 4.57 | 0.66 | 9.36 | 5.96 | 28.64 | 0.02** | 0.03** | 0.013** |
4. Discussion
The major finding from this study is that EEG nonstationarity, as assessed by SEPFS values, was reduced in subjects after a single concussive episode. SEPFS has at least two unique merits. First, since it is based on the order statistic, it is less affected by the spectrum distortion of the higher-frequency component. It should be noted, however, that SEPFS values were consistent with those obtained using traditional time domain analysis of EEG nonstationarity. Overall, this indeed indicates the reduction of EEG nonstationarity in concussed individuals. Second, SEPFS has an interesting biological meaning of the EEG signal, since it is directly associated with the quality (peak frequency) rather than with the amplitude of EEG waveform.
The change in the quality of EEG waveforms is a more common phenomenon than change in the amplitude (Berger 1929). For example, as an individual moves from a relaxed state to one of stimulation and activity, the EEG does not increase in amplitude but rather changes in quality of the waveforms from alpha rhythm to beta rhythm. Moreover it has been reported recently that the positive shift in EEG frequency band during the life span may reflect the process of brain maturation. It is assumed that the better feedback loops become integrated and interconnected with other brain areas, the faster the frequency of EEG oscillations will be (Klimesh, 1999). In agreement with this assumption, impaired long-distance connection of the brain (Cao and Slobounov, 2010) and the shift in the mean frequency in the alpha (8-10 Hz) band towards the lower band (Tebano et al., 1988) were both observed in patients who suffered from mTBI. It should be noted that the shift in the mean frequency in the alpha band may be biased by temporal average. Therefore, details of how the frequency shifts over time may be biased and/or eliminated by the averaging procedure are important issues that are beyond the scope of this report.
The most pronounced alteration of SEPFS values after concussion was observed in the occipital, temporal and parietal areas. The localized reduction of EEG nonstationarity is similar to our previous observation that EEG abnormal features in concussed subjects are concentrated in occipital, temporal and parietal areas (Cao et al., 2008). Specifically, the non-supervised pattern recognition algorithm, the support vector machine (SVM), has been applied as a tool to classify concussed athletes residual functional deficits. Indeed, discriminative features were observed at theta, alpha and beta frequency bands. Most importantly, the EEG features selected for classification were linked to occipital and temporal areas.
Localized reduction of EEG nonstationarity observed in the present study is complementary to results of our most recent EEG wavelet entropy study (Slobounov et al., 2009). Indeed, the reduced EEG-IQ (information quality) within 30 days post-injury was observed at parietal, temporal and occipital areas in concussed athletes. This effect was most pronounced in subjects suffering from recurrent and multiple concussive episodes. The consistent appearance of EEG alterations within this spatial distribution may be explained by the fact that most subjects reported the concussive injury following impact to the side of their head. A recent report by Delaney et al. (2006) has also indicated that temporal impact of the head or helmet frequently results in a mechanism producing the mTBI.
Although SEPFS and EEG-IQ measures of concussed individuals yield similar results, they are conceptually different. SEPFS measures the spectral variability of the EEG signal over time (i.e. non-stationarity), whereas the EEG-IQ is an indicator of the spectral complexity of the EEG signal. Theoretically, nonstationarity can be considered as a factor of complexity but complexity may not necessarily indicate nonstationarity. A stationary signal with low SEPFS values can have a complex spectrum (high EEG-IQ).
As a final note, we would like to stress that this report has focused on method and clinical issues rather than aimed at addressing physiological meaning and conceptual aspects of EEG measures in general, and SEPFS in particular. That said, our results are in favor of a major concept in that different rhythmic properties of EEG may be linked to general brain functions and brain health status. Specifically, as we recorded EEG during the resting state (not in response to specific tasks, such as mental or physical activities), we could claim that different EEG rhythms are associated with the activity of various large-scale resting networks. This claim is consistent with notion that RSNs 1 (default) and 2 (dorsal attention) have a stronger relationship with alpha and beta rhythms, albeit in opposite directions, with RSN1 showing positive correlation and RSN2 showing negative correlations with alpha and beta rhythms. Moreover, RSNs 3 (visual network) may be linked to all rhythms with the exclusion of the gamma rhythm, whereas, RSN 4 (auditory network) may be linked to delta, theta, and beta rhythms. Finally, RSN 5 (somato-motor network) may be linked to beta rhythm, whereas the RSN 6 (self-referential network) to gamma rhythm (Mantini et al, 2007). We hypothesize that the entropy of the spectral peak reflects the fluctuations of the resting state networks and switching among various brain states, a concept that requires further experimentation.
In conclusion, this current report is complementary to our previous research indicating that both variability and complexity of the EEG signal may be altered as a result of mTBI. The major empirical finding from this study provides further evidence that residual brain dysfunction in a concussed individual may be detected in “asymptomatic” subjects via EEG SEPFS measures. The current findings further reveal that alteration of brain functions as a result of mTBI may not be detected using conventional assessment tools. Whether this alteration is relatively transient resulting in reallocation of neural processing resources during increased processing load (McAllister et al., 2001), or a long-term persistent residual brain dysfunction, is yet to be determined.
Acknowledgments
This study was supported by NIH Grant RO1 NS056227-01A2 “Identification of Athletes at Risk for Traumatic Brain Injury”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berger H. Uber das Elektrenkephalogramm des Menschen. Translated and reprinted: Electroencephalogr Clin Neurophysiol. 1969 28 [Google Scholar]
- Bigler E, Bazarian J. Diffusion tensor imaging: A biomarker for mild traumatic brain injury? Neurology. 2010 doi: 10.1212/WNL.0b013e3181d3e43a. e-Pub of print on January 27, 2010: www.neurology.org. [DOI] [PubMed]
- Bluml S, Brooks W. Magnetic resonance spectroscopy of traumatic brain injury and concussion. In: Slobounov S, Sebastianelli W, editors. Foundations of sport-related brain injuries. New York: Springer Press; 2006. pp. 197–220. [Google Scholar]
- Cantu R. Concussion classification: ongoing controversy. In: Slobounov S, Sebastianelli W, editors. Foundations of sport-related brain injuries. New York: Springer Press; 2006. pp. 87–111. [Google Scholar]
- Cao C, Tutwiler R, Slobounov S. Automatic classification of athletes with residual functional deficits following concussion by means of EEG signal using support vector machine. IEEE Trans Neural Syst Rehabil Eng. 2008;16(4):327–35. doi: 10.1109/TNSRE.2008.918422. [DOI] [PubMed] [Google Scholar]
- Cao C, Slobounov S. Alteration of cortical functional connectivity as a result of traumatic brain injury revealed by graph theory, ICA and s LORETA analyses of EEG signals. IEEE Trans Neural Sys Rehab Eng. 2010;118(1):11–9. doi: 10.1109/TNSRE.2009.2027704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JT, Guskiewicz KM, Stergiou S. A nonlinear dynamic approach for evaluating postural control: new directions for the management of sportrelated cerebral concussion. Sport Med. 2005a;35(11):935–50. doi: 10.2165/00007256-200535110-00002. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer VS, Stergiou N. Detecting altered postural control after cerebral concussion in athletes with normal postural stability. Br J Sports Med. 2005b;39(11):805–11. doi: 10.1136/bjsm.2004.015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Merser VS, Stergion N. Recovery of postural control after cerebral concussion: new insights using approximate entropy. J Athletic Train. 2006;41(3):305–13. [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A. Functional abnormalities in symptomatic concussed athletes: An fMRI study. Neuroimage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Delaney S, Puni J, Rouah F. Mechanisms of injury for concussions in university football, ice hockey, and soccer: a pilot study. Clin J Sport Med. 2006;16(2):162–5. doi: 10.1097/00042752-200603000-00013. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Holschneider DP, Leuchter AF. Beta activity in aging and dementia. 1995;8(2):169–80. doi: 10.1007/BF01199780. [DOI] [PubMed] [Google Scholar]
- Gabor D. Theory of communications. J Inst Electr Engin. 1946;93:429–457. [Google Scholar]
- Goel V, Brambrink A, Baykal A, Koehler R, Hanley D, Thakor N. Dominant frequency analysis of EEG reveals brain's response during injury and recovery. IEEE Trans Biomed Eng. 1996;43(11):1083–1092. doi: 10.1109/10.541250. [DOI] [PubMed] [Google Scholar]
- Grossman A, Morlet J. Decomposition of Hardy functions into square integrable wavelets of constant shape. SIAM J Math Anal. 1984;15:723–736. [Google Scholar]
- Goryunova A, Bazarnaya N, Sorokina E, Semenova N, Globa O, Semenova Z, Pinelis V, Roshal L, Maslove O. Glutamate receptor autoantibody concentrations in children with chronic posttraumatic headache. Neurosci Beh Physiol. 2007;37(8):761–764. doi: 10.1007/s11055-007-0079-3. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten–twenty electrode system of the International Federation. Electromyogr Clin Neurophysiol. 1958;10:371–5. [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999;29(2,3):169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Levin HS. Neuroplasticity following non-penetrating traumatic brain injury. Brain Inj. 2003;17(8):665–674. doi: 10.1080/0269905031000107151. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. PNAS. 2007;104(32):13170–1317. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Saykin AJ. Neuroimaging findings in mild traumatic brain injury. J Clin Exp Neuropsychol. 2001;23:775–91. doi: 10.1076/jcen.23.6.775.1026. [DOI] [PubMed] [Google Scholar]
- Li X, Kapiris PG, Polygiannakis J, Eftaxias KA, Yao X. Fractal spectral analysis of pre-epileptic seizures phase: in terms of criticality. J Neural Eng Journal. 2005;2:11–16. doi: 10.1088/1741-2560/2/2/002. [DOI] [PubMed] [Google Scholar]
- Meyer Y. Wavelets: Algorithms and Applications. S.I.A.M.; 1993. [Google Scholar]
- Milligen B, Sánchez E, Estrada T, Hidalgo C, Brañas B, Carreras B, Garcia L. Wavelet bicoherence: A new turbulence analysis tool. Phys Plasmas. 1995;2(8):3017–32. [Google Scholar]
- Nuwer M, Novda D, Schrader D, Vespa L. Routine and quantitative EEG in mild traumatic brain injury. Clin Neurophys. 2005;116(9):2001–2025. doi: 10.1016/j.clinph.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Ptito A, Chen JK, Johnston K. Contribution of functional magnetic resonance imaging (fMRI) to sport concussion evaluation. Neurorehab. 2007;22:217–227. [PubMed] [Google Scholar]
- Randolph C, Miller MH. EEG and Cognitive Performance following Closed Head Injury. Neuropsychobiol. 1988;50:43–50. doi: 10.1159/000118471. [DOI] [PubMed] [Google Scholar]
- Saab ME, Gotman J. A system to detect the onset of epileptic seizures in scalp EEG. Clin Neurophysiol. 2005;116(2):427–42. doi: 10.1016/j.clinph.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Sebastianelli W, Simon R. Neurophysiological and behavioral concomitants of mild brain injury in college athletes. Clin Neurophysiol. 2002;113:185–93. doi: 10.1016/s1388-2457(01)00737-4. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Newell K, Slobounov E. Application of virtual reality graphics in assessment of concussion. Cyberpsychol Behav. 2006a;9(2):188–91. doi: 10.1089/cpb.2006.9.188. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Sebastianelli W, Moss R. Alteration of posture-related cortical potentials in mild traumatic brain injury. Neurosci Lett. 2006b;383:251–5. doi: 10.1016/j.neulet.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Sebastianelli W, Tutwiler R, Slobounov E. Alteration of postural responses to visual field motion in mild traumatic brain injury. Neurosurgery. 2006c;59(1):134–9. doi: 10.1227/01.NEU.0000219197.33182.3F. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Sebastianelli W, Cao C, Slobounov E, Newell K. Differential rate of recovery in athletes after first versus and second concussion episodes. Neurosurgery. 2007;61(2):238–44. doi: 10.1227/01.NEU.0000280001.03578.FF. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Cao C, Sebastianelli W, Slobounov E, Newell K. Residual deficits from concussion as revealed by virtual time-to-contract measures of postural stability. Clin Neurophysiol. 2008;119:281–9. doi: 10.1016/j.clinph.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Sebastianelli W, editors. Foundations of sport-related brain injuries. New York: Springer Press; 2006. [Google Scholar]
- Slobounov S, Cao C, Sebastianelli W. Differential effect of single versus recurrent mild traumatic brain injuries on wavelet entropy measures of EEG. Clin Neurophysiol. 2009;120(5):862–867. doi: 10.1016/j.clinph.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov S, Zhang K, Pennell D, Ray W, Johnson B, Sebastianelli W. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: a functional MRI study. Exp Brain Res. 2010;202:341–354. doi: 10.1007/s00221-009-2141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SM, Hall JR. A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. Clin Neurophysiol. 2006;23(5):440–455. doi: 10.1097/01.wnp.0000221363.12503.78. [DOI] [PubMed] [Google Scholar]
- Tebano MT, Cameroni M, Gallozzi G, Palazzino G, Ricci GF. EEG spectral analysis after minor head injury in man. Electroencephalogr Clin Neurophysiol. 1988;70(2):185–9. doi: 10.1016/0013-4694(88)90118-6. [DOI] [PubMed] [Google Scholar]
- Thakor NV, Tong S. Advances in quantitative electroencephalogram analysis methods. Annu Rev Biomed Eng. 2004;6:453–95. doi: 10.1146/annurev.bioeng.5.040202.121601. [DOI] [PubMed] [Google Scholar]
- Tong S, Thakor N. Time-frequency complexity of EEG following hypoxic-ishemic brain injury. Proc 25th Annu Int Conf: IEEE Eng Med Biol Soc. 2003;3:2570–257. [Google Scholar]
- Tong S, Li Z, Zhu Y, Thakor N. Describing the Nonstationarity Level of Neurological Signals Based on Quantifications of Time–Frequency Representation. IEEE Trans Biomed Eng. 2007;54(10):129–134. doi: 10.1109/TBME.2007.893497. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Walker RA, Gerson I, Geisler FH. EEG discriminant analyses of mild head injury. Electromyogr Clin Neurophysiol. 1989;73:94–106. doi: 10.1016/0013-4694(89)90188-0. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier D, Subramony S, Wessel K, et al. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. J Neurol Sci. 1997;145:205–11. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]


